Abstract
Fructose consumption, especially in food additives and sugar-sweetened beverages, has gained increasing attention due to its potential association with obesity and metabolic syndrome. The relationship between fructose and a high-salt diet, leading to hypertension and other deleterious cardiovascular parameters, has also become more evident, especially in preclinical studies. However, these studies have been modeled primarily on Western diets. The purpose of this review is to evaluate the dietary habits of individuals from China, Japan, and Korea, in light of the existing preclinical studies, to assess the potential relevance of existing data to East Asian societies. This review is not intended to be exhaustive, but rather to highlight the similarities and differences that should be considered in future preclinical, clinical, and epidemiologic studies regarding the impact of dietary fructose and salt on blood pressure and cardiovascular health worldwide.
1. Introduction
High blood pressure is a major risk factor for cardiovascular morbidity and mortality. The contribution of modifiable risk factors to the development and progression of hypertension has been extensively discussed in the literature, with one of the main contributors being diet [1,2,3,4,5]. Indeed, the presence of high salt, sugar, and fat—alone or in different permutations in the diet—has been shown to contribute to the development of cardiovascular, renal, and endocrine disorders [6,7,8,9,10]. Several preclinical studies have been conducted mimicking a Western-type diet [11,12,13,14,15,16,17,18,19]; however, there is a paucity of preclinical data on diets high in salt and moderate in sugar-sweetened beverages (SSB) consumed by individuals living in Asian countries, such as Japan, the Republic of Korea, the People’s Republic of China, and Taiwan. The aim of this review is to critically assess whether existing preclinical findings regarding fructose and salt consumption may be relevant to East Asian populations.
2. Materials and Methods
We searched PubMed (https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 2 June 2022)), the Web of Science Core Collection (https://www.library.ethz.ch/en/Resources/Databases/Web-of-Science-Core-Collection (accessed on 25 April 2022)), and the Cochrane Registry (http://www.cochranelibrary.com/about/central-landing-page.html (accessed on 25 April 2022)) from January 1980 to April 2022. The following search terms were used: fructose and blood pressure or hypertension; fructose and sodium; fructose and kidney; fructose and salt; fructose and insulin resistance; high-salt diet; fructose and salt and sympathetic nervous system; fructose and renal transporters; fructose and intestinal absorption; Asian population; fructose and China; fructose and Japan; fructose and Korea; sugar-sweetened beverages and China; sugar-sweetened beverages and Japan; sugar-sweetened beverages and Korea; salt or sodium diet and China; salt or sodium diet and Japan; and salt or sodium diet and Korea. Relevant human and animal studies that addressed fructose and salt consumption in East Asian populations were included. In addition, reviews regarding consumption of these nutrients by Western countries, as well as animal studies—primarily in rodents—on fructose and a high-salt diet, were used for comparison. The review is not intended to be exhaustive, but rather to be a critical appraisal and comparison of model diets and their relevancy to East Asian vs. Western populations. Although there were more than 20,000 items found regarding sodium and 1372 reports regarding fructose in East Asia, only 78 references were found when searching human studies on fructose AND sodium with respect to either China, Korea, or Japan. This highlights the paucity of articles regarding the combined dietary intakes in East Asian populations.
This review focuses on the impact of fructose and salt consumption on the development of hypertension, independent or preceding the full expression of metabolic syndrome, which may lead to heightened cardiovascular risk. The influence of fructose on metabolic syndrome is well recognized and reviewed in detail in several recent reviews [20,21,22,23].
3. Results
3.1. Susceptibility to Hypertension: Genetics
The factors influencing one’s susceptibility to high blood pressure may be related to genetic [24] or lifestyle characteristics [10]. Several genome-wide association studies (GWASs) revealed more than 200 genetic loci related to hypertension development in individuals of European ancestry [24,25,26,27,28]. Similar studies, albeit on a smaller scale, have been conducted in East Asian individuals [29,30,31]. Up until 2016, data regarding Asian individuals were more limited, which is important given that allele frequencies appear to be less common across ethnic and racial groups than within groups [24]. More recently, a large GWAS was conducted in East Asian populations and compared to Europeans, which revealed 19 new genetic loci and proposed a common ancestry-specific variant association model in these two cohorts [31]. Nevertheless, hypertension is more prevalent in people of East Asian ancestry compared to European ancestry. Thus, although some differences can be ascribed to genetic susceptibilities, one can argue that lifestyle factors, particularly the modifiable ones such as diet, have gained less attention. The higher hypertension prevalence in Asian populations may, in part, explain the higher morbidity and mortality rates due to stroke rather than coronary heart disease in these individuals, whereas the converse is true in Western countries [32]. Alternatively, the impact of hypertension on cerebrovascular versus cardiovascular systems may vary among different ethnicities, due to other genetic, epigenetic, environmental, and lifestyle factors.
3.2. Salt-Sensitive Blood Pressure
High salt intake has been described as one of the most prominent environmental factors contributing to hypertension [33,34,35]. Table 1 shows the reported salt intake for several large studies in East Asian populations. Individuals may display normal blood pressure but respond to a high-salt diet with an increase in blood pressure, termed salt-sensitive blood pressure (SSBP) [36]. Interestingly, SSBP is an independent risk factor for cardiovascular disease, whether the individual is normotensive or hypertensive at baseline [37]. The causal association between high salt intake and blood pressure has been unequivocally demonstrated by the INTERMAP (International Study of Macro- and Micro-nutrients and Blood Pressure) study, which included 52 study sites distributed throughout 32 countries, including Asian sites [38]. A recent cohort study conducted in Northern China found that among 2057 participants, SSBP prevalence is the same between hypertensive (28.5%) and normotensive (28.2%) groups, with daily salt intake being higher in the former (8.97 g/day) compared to the latter (6.87 g/day) [39]. Notably, these daily intakes are well above the upper limit of 1500 mg of sodium/day recommended by the American Heart Association, which equates to about 3.8 g of salt/day [40]. In addition to dietary factors (i.e., sodium intake), specific gene loci have been associated with SSBP. These occur in the genes encoding the proteins involved in pathways regulating salt balance. Among these are genes encoding for angiotensinogen [41], α-adducin [42], aldosterone synthase [43], and the G-protein coupled to the Na+-H+ exchanger [44]. One study compared the frequency of these alleles between Japanese and Caucasian cohorts, and found that they were all significantly higher in the former group [45]. The GenSalt study also identified genetic associations of SSBP with potassium-selective ion channels (Kir), cyclic GMP-dependent protein kinase genes, and the epithelial sodium channel (ENaC) among participants in rural China [46,47,48]. An association between SSBP and single nucleotide polymorphisms (SNPs) has also been found in Korean individuals who have emigrated to the United States [49].

Table 1.
Dietary sodium consumption in Asian populations.
Additional gene polymorphisms associated with hypertension have been reported in Asians [61], but these are not as clearly related to salt sensitivity. Given such genetic susceptibility to SSBP and the enhanced cardiovascular risk imparted by hypertension, it is reasonable that strategies to reduce sodium intake in East Asian populations would be worthwhile.
The guidelines for salt intake by the Japanese Society of Hypertension [62], the Dietary Guidelines for Chinese Residents [63], and the Korean Society of Hypertension [64] all recommend <6 g/d of dietary salt or 2.4 g/d of dietary sodium, which is comparable to the 2.3 g/d of sodium intake (equivalent to 5.85 g/d of salt) endorsed by the American College of Cardiology and American Heart Association [65]. Since many of the studies regarding dietary salt intake and various associated clinical parameters, including hypertension, depend on questionnaires or recall, it is noteworthy that verification by urinary sodium collection from the INTERMAP study showed agreement with the dietary recall of salt intake within 2.9 and 3.9 mmol/d for individuals from Japan, the United Kingdom, and the United States. In contrast, excretion of salt for the Chinese cohort averaged 54.0 mmol/d (~3.2 g/d of salt) greater than that reported by dietary recall. The estimated misclassification of salt intake ranged from 25.4% for the United Kingdom to 58.6% for China [57]. Thus, the reliability of dietary recall and questionnaires for salt intake (as well as other nutrients) should be viewed with some caution.
3.3. Fructose Intake, Blood Pressure, and Cardiovascular Risk
In Western societies, fructose ingestion has been correlated with higher blood pressures [66,67]; an interaction with sodium intake has also been demonstrated [6,68,69,70]. Despite its low glycemic index, the association between fructose, obesity and cardiovascular disease has been strong enough that Segal et al. [71] proposed adoption of a fructose index. The majority of reports address fructose in relation to obesity, diabetes, or metabolic syndrome. The results from the large Western CARDIA (Coronary Artery Risk Development in Young Adults; n = 240,508) cohort indicated that the risk of hypertension with consumption of SSB is 12% higher, even when controlled for sex, age, race, BMI and smoking history [72]. Sucrose, a disaccharide comprising fructose and glucose, can also induce elevations in blood pressure when paired with high salt intake [73]. The deleterious effects of fructose related primarily to the levels of fructose that can be achieved in the systemic and portal bloodstream. Notably, greater amounts of dietary sucrose are required to achieve the levels of fructose that result from consumption of high-fructose corn syrup [74]. Moreover, in a report by the Mayo Clinic, evaluating 24 prospective cohort studies comprising 624,128 unique individuals, total sugars and fructose were associated with cardiovascular mortality, but not incidence. Sucrose was not associated with either cardiovascular incidence or mortality [75]; hence, the focus of this review was on fructose and salt ingestion.
In general, the average fructose intake by individuals in East Asia tended to be lower (Table 2) than that observed in Western nations [8,62,66], where values averaged 49 g/d in adults, but can be as high as 75 g/d—especially in adolescents. The fructose in Western diets is ingested mostly in the form of high-fructose corn syrup found in beverages and food additives [76]. A superficial inspection of the publications regarding SSB consumption in Asian countries suggests that intake has increased over the last two decades, with the highest intakes in adolescents and males (Table 3), which mirrors the epidemiologic findings in Western societies [8,76]. The lack of a standard for reporting consumption of fructose and SSB, such as absolute caloric intake per day vs. percent of total caloric intake, makes comparisons among studies difficult. The problem is compounded by different definitions that constitute a serving of SSB, as well as variability in the caloric value (anywhere from 44 to 108 kcal/250 mL) of different SSB.

Table 2.
Dietary fructose intake in Asian populations.

Table 3.
Fructose intake as sugar-sweetened beverages in Asian populations.
There is a paucity of data regarding the impact, if any, of fructose intake, either alone or together with dietary sodium, on blood pressure exclusively in Asian populations. The INTERMAP study [69,85] that examined the relationship of blood pressure as a major risk factor for cardiovascular disease included individuals from Japan, the People’s Republic of China, the United Kingdom, and the United States. Unfortunately, the data on SSB (that included high-fructose corn syrup) showing a correlation with higher risk of cardiovascular events included samples from the United Kingdom and the United States only [86].
One randomized, double-blind, cross-over trial enrolled 18 healthy young Chinese adults of both sexes and examined the effect of drinking a 25% glucose or 25% fructose solution. Systolic blood pressure was significantly higher, as were aldose reductase and uric acid levels, whereas serum nitric oxide levels were lower with fructose at both 1 h and 3 h post-ingestion. The impact on diastolic blood pressure was similar between fructose and glucose trials. Consistent with its low glycemic index, fructose did not increase serum glucose or insulin levels [101]. Comparable large clinical trials or epidemiological studies regarding the impact of fructose, either with or without salt modulation, on blood pressure in Asian populations are lacking and are very much needed.
3.4. Preclinical Data on Dietary Fructose, High Salt, and Blood Pressure
Rodent models have used fructose diets providing >60% of total daily caloric intake to induce insulin-resistant hypertension [11,71,72]. More recent studies have modified the fructose content to 20% in order to better simulate the typical intake of the upper quintile of Western diets [15]. In short-term experiments (2–3 weeks), rats that were fed 20% fructose w/w in drinking water did not display elevated blood pressure compared with control rats given water or 20% glucose. However, when paired with a high-salt diet (4% NaCl), systolic blood pressure by tail-cuff measurements was significantly elevated (140 ± 2 mmHg) compared with the controls (122 ± 1 mmHg) or with rats fed fructose alone (128 ± 1 mmHg) [15]. Hypertension in rats fed fructose plus high-salt diets has also been reported using telemetry, albeit with more modest increases in blood pressure [102,103,104]. The fructose intake by Asian populations is generally less than that by individuals from Europe or America, but more recent findings suggest a troubling rise, particularly in SSB consumption by adolescents [91,94,95,98]. This would suggest that preclinical data regarding fructose, either with or without high-salt intake, will be more relevant to societies worldwide.
Fructose is absorbed predominantly via GLUT5, which has a low affinity for glucose but high affinity for fructose in the jejunum. When large amounts of fructose are ingested and absorbed, the conversion of fructose to pyruvate by the liver is exceeded. The excess fructose enters the circulation, where fructose can then both enhance intestinal sodium absorption and increase renal tubule sodium reabsorption, leading to extracellular volume expansion [16,19,70,102,103,104]. The putative anion transporter-1 (PAT1, also known as SCL26A6 in humans) is a chloride/base exchanger on the apical surface of intestinal cells. Jejunal PAT1 colocalizes with GLUT5; PAT1 increases with fructose feeding and is coupled with the intestinal Na/H exchanger-3 (NHE3) [102,105,106,107]. Consequently, sodium and chloride absorption by the gut are increased. Thus, when delivered concurrently with fructose, a rise in dietary sodium does not result in higher fecal sodium excretion in rats [15]. The maintenance of sodium homeostasis, therefore, depends upon renal excretion.
Urinary excretion of sodium was impaired in fructose-fed rats (Figure 1). Cumulative sodium balance is positive, which was not observed in glucose-fed rats [15,108]. Proximal tubule sodium reabsorption in vitro in a porcine cell line was enhanced in the presence of fructose [109]. Likewise, the activity of a proximal tubule Na/H exchanger, NHE3, isolated from rats fed 20% fructose in drinking water was augmented in a PKC-dependent manner when glucose was replaced with fructose in the test bath. Significantly, systolic blood pressure was 13 mmHg higher in the fructose-fed rats on a high-salt diet vs. rats fed a high-salt diet alone [110] or rats fed glucose and a high-salt diet [69]. Additional studies demonstrated that NHE3 activity was further potentiated by angiotensin II (Ang II) [16,104]. Although Na,K-ATPase activity did not appear to be altered by fructose [110], the proximal tubule expression of sodium-linked glucose transporters that are capable of co-transporting sodium and fructose, SGLT4 and SGLT5, was increased in fructose-fed rats, leading to greater reabsorption of both substances and decreased urinary sodium excretion [16]. In addition to augmentation of proximal tubule sodium reabsorptive processes, fructose feeding also appeared to increase the expression of sodium, potassium, and 2 chloride co-transporter (NKCC2) in the thick ascending limb of Henle [19].
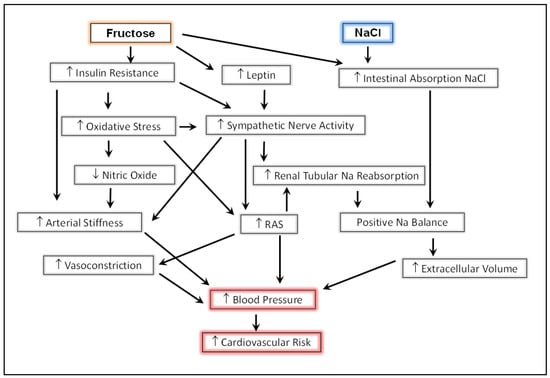
Figure 1.
Interactions of pathways resulting in hypertension and increased cardiovascular risk induced by excess fructose and salt consumption (see text). RAS, renin angiotensin system.
Fructose consumption impacts the renin–angiotensin–aldosterone system. Expansion of extracellular volume and elevated blood pressure are capable of independently suppressing renin secretion; however, the suppression of plasma renin activity (PRA) was reduced in rats fed 20% fructose and 4% salt [15]. Notably, the sustained levels of PRA and Ang II resulted from renal sympathetic nerve activity, which was also increased in rats fed fructose and a high-salt diet. Consistent with this mechanism, renal cryo-denervation produced a decrease in PRA and Ang II in this model [111]. Importantly, sympathetic inputs to the renal tubules also stimulated sodium reabsorption all along the nephron [112]. The potentiation of sodium transport by Ang II, along with the failure to optimally suppress PRA and Ang II, contributed to further positive sodium balance. Furthermore, the direct effect of Ang II to induce vasoconstriction, combined with an expanded vascular volume and impaired baroreflex sensitivity [113], resulted in hypertension.
Excess fructose consumption also contributed to the development of insulin resistance in preclinical models, despite the absence of frank hyperglycemia [108,114]. The mechanisms relating to fructose, insulin resistance, and hypertension have been elegantly reviewed by Tran et al. [11] and Xu [115]. In general, hyperinsulinemia and hyperleptinemia induced by fructose promoted sympathoexcitation, endothelial dysfunction, and metabolic derangements that led to oxidative stress, reduced nitric oxide, and the stimulation of cytokines and immune mechanisms [116,117,118]. Together, these conspire to decrease arterial compliance and increase vasoconstriction. Both insulin resistance and aortic stiffness can be ameliorated by free radical scavengers [114] and renal nerve ablation [111].
4. Conclusions
This overview of preclinical and clinical data is not meant to be exhaustive. Rather, we have highlighted some of the basic studies, mostly performed in rodents, that have demonstrated the potential deleterious impact that consumption of substantial amounts of fructose with a high-salt diet can exert on blood pressure, the sympathetic nervous system, and cardiovascular parameters. Clinical studies have clearly emphasized the association of hypertension, sympathoexcitation, and arterial stiffness with increased cardiovascular morbidity and mortality. In general, individuals living in Japan, Korea, and China tend to have higher salt intake and lower fructose consumption compared with the Western populations that the preclinical studies were designed to model. Additional preclinical investigations that model Asian dietary patterns, and more rigorous clinical and epidemiological studies that take into consideration the genetic and environmental nuances of those societies, will be needed.
Author Contributions
Conceptualization, N.F.R.; formal analysis, B.H.K. and N.F.R.; writing—original draft preparation, B.H.K., D.K. and N.F.R.; writing—N.F.R.; project administration, N.F.R.; funding acquisition, N.F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institutes of Health, Heart Lung and Blood Institute, grant number HL163844.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data in this review were obtained using the following databases: PubMed https://www.ncbi.nlm.nih.gov/pubmed/ (accessed 2 June 2022); the Web of Science Core Collection https://www.library.ethz.ch/en/Resources/Databases/Web-of-Science-Core-Collection (accessed on 25 April 2022); and the Cochrane Registry http://www.cochranelibrary.com/about/central-landing-page.html (accessed on 25 April 2022).
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Folkow, B.; Svanborg, A. Physiology of cardiovascular aging. Physiol. Rev. 1993, 73, 725–764. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Murphy, R.A.; Newman, A.B.; Bauer, D.C.; Harris, T.B.; Yang, Z.; Applegate, W.B.; Kritchevsky, S.B. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: The Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern. Med. 2015, 175, 410–419. [Google Scholar] [CrossRef]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart, A.; Obesity Committee of the Council on Nutrition, P.A.; Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All-Cause Mortality with Arterial Stiffness A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Eren, O.C.; Ortiz, A.; Afsar, B.; Covic, A.; Kuwabara, M.; Lanaspa, M.A.; Johnson, R.J.; Kanbay, M. Multilayered Interplay Between Fructose and Salt in Development of Hypertension. Hypertension 2019, 73, 265–272. [Google Scholar] [CrossRef] [PubMed]
- DeChristopher, L.R.; Auerbach, B.J.; Tucker, K.L. High fructose corn syrup, excess-free-fructose, and risk of coronary heart disease among African Americans- the Jackson Heart Study. BMC Nutr. 2020, 6, 70. [Google Scholar] [CrossRef]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef]
- Appel, L.J. The Effects of Dietary Factors on Blood Pressure. Cardiol. Clin. 2017, 35, 197–212. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Xiang, Y.B.; Yang, G.; Li, H.; Gao, Y.T.; Zheng, W.; Shu, X.O. Adherence to dietary guidelines and mortality: A report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am. J. Clin. Nutr. 2014, 100, 693–700. [Google Scholar] [CrossRef]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef]
- Park, J.H.; Ku, H.J.; Kim, J.K.; Park, J.W.; Lee, J.H. Amelioration of High Fructose-Induced Cardiac Hypertrophy by Naringin. Sci. Rep. 2018, 8, 9464. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Soares, E.; Fernandes, J.; Viana, S.; Carvalho, E.; Pereira, F.C.; Reis, F. Early cardiac changes in a rat model of prediabetes: Brain natriuretic peptide overexpression seems to be the best marker. Cardiovasc. Diabetol. 2013, 12, 44. [Google Scholar] [CrossRef]
- Komnenov, D.; Levanovich, P.E.; Rossi, N.F. Hypertension Associated with Fructose and High Salt: Renal and Sympathetic Mechanisms. Nutrients 2019, 11, 569. [Google Scholar] [CrossRef]
- Gordish, K.L.; Kassem, K.M.; Ortiz, P.A.; Beierwaltes, W.H. Moderate (20%) fructose-enriched diet stimulates salt-sensitive hypertension with increased salt retention and decreased renal nitric oxide. Physiol. Rep. 2017, 5, e13162. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Cabral, P.D.; Hong, N.J.; Asirwatham, J.; Saez, F.; Garvin, J.L. Fructose reabsorption by rat proximal tubules: Role of Na(+)-linked cotransporters and the effect of dietary fructose. Am. J. Physiol. Renal Physiol. 2019, 316, F473–F480. [Google Scholar] [CrossRef]
- Galipeau, D.; Verma, S.; McNeill, J.H. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2478–H2484. [Google Scholar] [CrossRef] [PubMed]
- Caldiz, C.I.; de Cingolani, G.E. Insulin resistance in adipocytes from spontaneously hypertensive rats: Effect of long-term treatment with enalapril and losartan. Metabolism 1999, 48, 1041–1046. [Google Scholar] [CrossRef]
- Ares, G.R.; Kassem, K.M.; Ortiz, P.A. Fructose acutely stimulates NKCC2 activity in rat thick ascending limbs by increasing surface NKCC2 expression. Am. J. Physiol. Renal Physiol. 2019, 316, F550–F557. [Google Scholar] [CrossRef] [PubMed]
- Coronati, M.; Baratta, F.; Pastori, D.; Ferro, D.; Angelico, F.; Del Ben, M. Added Fructose in Non-Alcoholic Fatty Liver Disease and in Metabolic Syndrome: A Narrative Review. Nutrients 2022, 14, 1127. [Google Scholar] [CrossRef]
- Stricker, S.; Rudloff, S.; Geier, A.; Steveling, A.; Roeb, E.; Zimmer, K.P. Fructose Consumption-Free Sugars and Their Health Effects. Dtsch. Arztebl. Int. 2021, 118, 71–78. [Google Scholar] [CrossRef]
- Student, J.; Sowers, J.; Lockette, W. THIRSTY FOR FRUCTOSE: Arginine Vasopressin, Fructose, and the Pathogenesis of Metabolic and Renal Disease. Front. Cardiovasc. Med. 2022, 9, 883365. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Packard, C.J.; Boren, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef]
- Ehret, G.B.; Ferreira, T.; Chasman, D.I.; Jackson, A.U.; Schmidt, E.M.; Johnson, T.; Thorleifsson, G.; Luan, J.; Donnelly, L.A.; Kanoni, S.; et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat. Genet. 2016, 48, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- International Consortium for Blood Pressure Genome-Wide Association, S; Ehret, G.B.; Munroe, P.B.; Rice, K.M.; Bochud, M.; Johnson, A.D.; Chasman, D.I.; Smith, A.V.; Tobin, M.D.; Verwoert, G.C.; et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011, 478, 103–109. [Google Scholar] [CrossRef]
- Surendran, P.; Drenos, F.; Young, R.; Warren, H.; Cook, J.P.; Manning, A.K.; Grarup, N.; Sim, X.; Barnes, D.R.; Witkowska, K.; et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat. Genet. 2016, 48, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kraja, A.T.; Smith, J.A.; Brody, J.A.; Franceschini, N.; Bis, J.C.; Rice, K.; Morrison, A.C.; Lu, Y.; Weiss, S.; et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat. Genet. 2016, 48, 1162–1170. [Google Scholar] [CrossRef]
- Warren, H.R.; Evangelou, E.; Cabrera, C.P.; Gao, H.; Ren, M.X.; Mifsud, B.; Ntalla, I.; Surendran, P.; Liu, C.Y.; Cook, J.P.; et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat. Genet. 2017, 49, 403–415. [Google Scholar] [CrossRef]
- Kato, N.; Loh, M.; Takeuchi, F.; Verweij, N.; Wang, X.; Zhang, W.; Kelly, T.N.; Saleheen, D.; Lehne, B.; Leach, I.M.; et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat. Genet. 2015, 47, 1282–1293. [Google Scholar] [CrossRef]
- Kanai, M.; Akiyama, M.; Takahashi, A.; Matoba, N.; Momozawa, Y.; Ikeda, M.; Iwata, N.; Ikegawa, S.; Hirata, M.; Matsuda, K.; et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat. Genet. 2018, 50, 390–400. [Google Scholar] [CrossRef]
- Takeuchi, F.; Akiyama, M.; Matoba, N.; Katsuya, T.; Nakatochi, M.; Tabara, Y.; Narita, A.; Saw, W.Y.; Moon, S.; Spracklen, C.N.; et al. Interethnic analyses of blood pressure loci in populations of East Asian and European descent. Nat. Commun. 2018, 9, 5052. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, H. Explanation for the Japanese paradox: Prevention of increase in coronary heart disease and reduction in stroke. J. Atheroscler. Thromb. 2007, 14, 278–286. [Google Scholar] [CrossRef]
- Cutler, J.A.; Follmann, D.; Allender, P.S. Randomized trials of sodium reduction: An overview. Am. J. Clin. Nutr. 1997, 65, 643S–651S. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Weinberger, M.H.; Fineberg, N.S.; Fineberg, S.E.; Weinberger, M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001, 37, 429–432. [Google Scholar] [CrossRef]
- Elijovich, F.; Weinberger, M.H.; Anderson, C.A.; Appel, L.J.; Bursztyn, M.; Cook, N.R.; Dart, R.A.; Newton-Cheh, C.H.; Sacks, F.M.; Laffer, C.L.; et al. Salt Sensitivity of Blood Pressure: A Scientific Statement from the American Heart Association. Hypertension 2016, 68, e7–e46. [Google Scholar] [CrossRef]
- Morimoto, A.; Uzu, T.; Fujii, T.; Nishimura, M.; Kuroda, S.; Nakamura, S.; Inenaga, T.; Kimura, G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 1997, 350, 1734–1737. [Google Scholar] [CrossRef]
- Stamler, J. The INTERSALT Study: Background, methods, findings, and implications. Am. J. Clin. Nutr. 1997, 65, 626S–642S. [Google Scholar] [CrossRef]
- Qi, H.; Liu, B.; Guo, C.; Liu, Z.; Cao, H.; Liu, K.; Sun, W.; Zhang, L. Effects of environmental and genetic risk factors for salt sensitivity on blood pressure in northern China: The systemic epidemiology of salt sensitivity (EpiSS) cohort study. BMJ 2018, 8, e023042. [Google Scholar] [CrossRef]
- Whelton, P.K.; Appel, L.J.; Sacco, R.L.; Anderson, C.A.; Antman, E.M.; Campbell, N.; Dunbar, S.B.; Frohlich, E.D.; Hall, J.E.; Jessup, M.; et al. Sodium, blood pressure, and cardiovascular disease: Further evidence supporting the American Heart Association sodium reduction recommendations. Circulation 2012, 126, 2880–2889. [Google Scholar] [CrossRef]
- Kamitani, A.; Rakugi, H.; Higaki, J.; Yi, Z.; Mikami, H.; Miki, T.; Ogihara, T. Association analysis of a polymorphism of the angiotensinogen gene with essential hypertension in Japanese. J. Hum. Hypertens 1994, 8, 521–524. [Google Scholar]
- Bianchi, G.; Tripodi, G.; Casari, G.; Salardi, S.; Barber, B.R.; Garcia, R.; Leoni, P.; Torielli, L.; Cusi, D.; Ferrandi, M.; et al. 2 Point Mutations within the Adducin Genes Are Involved in Blood-Pressure Variation. Proc. Natl. Acad. Sci. USA 1994, 91, 3999–4003. [Google Scholar] [CrossRef] [PubMed]
- Brand, E.; Chatelain, N.; Mulatero, P.; Fery, I.; Curnow, K.; Jeunemaitre, X.; Corvol, P.; Pascoe, L.; Soubrier, F. Structural analysis and evaluation of the aldosterone synthase gene in hypertension. Hypertension 1998, 32, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Siffert, W.; Rosskopf, D.; Siffert, G.; Busch, S.; Moritz, A.; Erbel, R.; Sharma, A.M.; Ritz, E.; Wichmann, H.E.; Jakobs, K.H.; et al. Association of a human G-protein beta3 subunit variant with hypertension. Nat. Genet. 1998, 18, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, T.; Ishikawa, K.; Sugimoto, K.; Rakugi, H.; Ogihara, T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens. Res. 2003, 26, 521–525. [Google Scholar] [CrossRef]
- Gong, X.; Han, X.; Lu, X.; Chen, J.; Huang, J.; Kelly, T.N.; Chen, C.S.; He, J.; Gu, D.; Chen, S. Association of Kir genes with blood pressure responses to dietary sodium intervention: The GenSalt study. Hypertens. Res. 2018, 41, 1045–1053. [Google Scholar] [CrossRef]
- Han, C.; Hu, Z.; Liu, F.; Yang, X.; Kelly, T.N.; Chen, J.; Huang, J.; Chen, C.S.; He, J.; Chen, S.; et al. Genetic variants of cGMP-dependent protein kinase genes and salt sensitivity of blood pressure: The GenSalt study. J. Hum. Hypertens. 2019, 33, 62–68. [Google Scholar] [CrossRef]
- Gu, X.; Gu, D.; He, J.; Rao, D.C.; Hixson, J.E.; Chen, J.; Li, J.; Huang, J.; Wu, X.; Rice, T.K.; et al. Resequencing Epithelial Sodium Channel Genes Identifies Rare Variants Associated with Blood Pressure Salt-Sensitivity: The GenSalt Study. Am. J. Hypertens. 2018, 31, 205–211. [Google Scholar] [CrossRef]
- Ko, J.; Lee, M.; Patel, D.I.; Nguyen, V.; Wang, J. Examining the Potential Effect of a Salt Sensitivity Biomarker in Korean American Immigrants: A Pilot Study. J. Immigr. Minor. Health 2021. [Google Scholar] [CrossRef]
- Lee, H.S.; Duffey, K.J.; Popkin, B.M. Sodium and potassium intake patterns and trends in South Korea. J. Hum. Hypertens. 2013, 27, 298–303. [Google Scholar] [CrossRef]
- Song, E.K.; Moser, D.K.; Kang, S.M.; Lennie, T.A. Self-reported Adherence to a Low-Sodium Diet and Health Outcomes in Patients with Heart Failure. J. Cardiovasc. Nurs. 2016, 31, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Chang, J.H.; Kim, J.H.; Kang, J.W. Is high sodium intake associated with hearing impairment? The association between spot urine sodium concentration and hearing threshold in Korean adolescents. Asia Pac. J. Clin. Nutr. 2018, 27, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, S.H.; Shim, Y.S. Association of sodium intake with insulin resistance in Korean children and adolescents: The Korea National Health and Nutrition Examination Survey 2010. J. Pediatr. Endocrinol. Metab. 2018, 31, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, K.; Lee, B.K.; Ahn, J. Association of the Healthy Eating Index with Estimated Cardiovascular Age in Adults from the KNHANES 2013–2017. Nutrients 2020, 12, 2912. [Google Scholar] [CrossRef]
- Uechi, K.; Sugimoto, M.; Kobayashi, S.; Sasaki, S. Urine 24-Hour Sodium Excretion Decreased between 1953 and 2014 in Japan, but Estimated Intake Still Exceeds the WHO Recommendation. J. Nutr. 2017, 147, 390–397. [Google Scholar] [CrossRef]
- Okuda, M.; Sasaki, S. Assessment of Foods Associated with Sodium and Potassium Intake in Japanese Youths Using the Brief-Type Self-Administered Diet History Questionnaire. Nutrients 2021, 13, 2345. [Google Scholar] [CrossRef]
- Wen, X.; Zhou, L.; Stamler, J.; Chan, Q.; Van Horn, L.; Daviglus, M.L.; Dyer, A.R.; Elliott, P.; Ueshima, H.; Miura, K.; et al. Agreement between 24-h dietary recalls and 24-h urine collections for estimating sodium intake in China, Japan, UK, USA: The International Study of Macro- and Micro-nutrients and Blood Pressure. J. Hypertens. 2019, 37, 814–819. [Google Scholar] [CrossRef]
- Tan, M.; He, F.J.; Wang, C.; MacGregor, G.A. Twenty-Four-Hour Urinary Sodium and Potassium Excretion in China: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012923. [Google Scholar] [CrossRef]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef]
- Zhu, Z.; Cui, X.; Wei, X.; Zang, J.; Feng, J.; Wang, Z.; Shi, Z. Dietary Sodium Intake Is Positively Associated with Sugar-Sweetened Beverage Consumption in Chinese Children and Adolescents. Nutrients 2021, 13, 3949. [Google Scholar] [CrossRef]
- Xi, B.; Shen, Y.; Reilly, K.H.; Wang, X.; Mi, J. Recapitulation of four hypertension susceptibility genes (CSK, CYP17A1, MTHFR, and FGF5) in East Asians. Metabolism 2013, 62, 196–203. [Google Scholar] [CrossRef]
- Tsuchihashi, T. Dietary salt intake in Japan—Past, present, and future. Hypertens. Res. 2022, 45, 748–757. [Google Scholar] [CrossRef]
- Wang, S.S.; Lay, S.; Yu, H.N.; Shen, S.R. Dietary Guidelines for Chinese Residents (2016): Comments and comparisons. J. Zhejiang Univ. Sci. B 2016, 17, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Shin, J.; Kim, G.H.; Park, S.; Ihm, S.H.; Kim, H.C.; Kim, K.I.; Kim, J.H.; Lee, J.H.; Park, J.M.; et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: Part II-diagnosis and treatment of hypertension. Clin. Hypertens. 2019, 25, 20. [Google Scholar] [CrossRef]
- Bakris, G.; Ali, W.; Parati, G. ACC/AHA Versus ESC/ESH on Hypertension Guidelines: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 73, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Ferder, L.; Ferder, M.D.; Inserra, F. The role of high-fructose corn syrup in metabolic syndrome and hypertension. Curr. Hypertens. Rep. 2010, 12, 105–112. [Google Scholar] [CrossRef]
- Madero, M.; Perez-Pozo, S.E.; Jalal, D.; Johnson, R.J.; Sanchez-Lozada, L.G. Dietary fructose and hypertension. Curr. Hypertens. Rep. 2011, 13, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.J.; Stamler, J.; Van Horn, L.; Robertson, C.E.; Chan, Q.N.; Dyer, A.R.; Huang, C.C.; Rodriguez, B.L.; Zhao, L.C.; Daviglus, M.L.; et al. Sugar-Sweetened Beverage, Sugar Intake of Individuals, and Their Blood Pressure International Study of Macro/Micronutrients and Blood Pressure. Hypertension 2011, 57, 695–701. [Google Scholar] [CrossRef]
- Chan, Q.; Stamler, J.; Griep, L.M.; Daviglus, M.L.; Horn, L.V.; Elliott, P. An Update on Nutrients and Blood Pressure. J. Atheroscler. Thromb. 2016, 23, 276–289. [Google Scholar] [CrossRef]
- Soleimani, M. Dietary fructose, salt absorption and hypertension in metabolic syndrome: Towards a new paradigm. Acta Physiol. 2011, 201, 55–62. [Google Scholar] [CrossRef]
- Segal, M.S.; Gollub, E.; Johnson, R.J. Is the fructose index more relevant with regards to cardiovascular disease than the glycemic index? Eur. J. Nutr. 2007, 46, 406–417. [Google Scholar] [CrossRef]
- Jayalath, V.H.; de Souza, R.J.; Ha, V.; Mirrahimi, A.; Blanco-Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; Wolever, T.M.; Beyene, J.; et al. Sugar-sweetened beverage consumption and incident hypertension: A systematic review and meta-analysis of prospective cohorts. Am. J. Clin. Nutr. 2015, 102, 914–921. [Google Scholar] [CrossRef]
- Johnson, M.D.; Zhang, H.Y.; Kotchen, T.A. Sucrose does not raise blood pressure in rats maintained on a low salt intake. Hypertension 1993, 21, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Knapka, J.J.; MacArthy, P.; Yousufi, A.K.; Sabnis, S.G.; Antonovych, T.T. High sucrose diets increase blood pressure of both salt-sensitive and salt-resistant rats. Am. J. Hypertens. 1992, 5, 585–591. [Google Scholar] [CrossRef]
- Khan, T.A.; Tayyiba, M.; Agarwal, A.; Mejia, S.B.; de Souza, R.J.; Wolever, T.M.S.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of Total Sugars, Sucrose, Fructose, and Added Sugars with the Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Mayo Clin. Proc. 2019, 94, 2399–2414. [Google Scholar] [CrossRef] [PubMed]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228S–1235S. [Google Scholar] [CrossRef]
- Takeichi, H.; Taniguchi, H.; Fukinbara, M.; Tanaka, N.; Shikanai, S.; Sarukura, N.; Hsu, T.F.; Wong, Y.; Yamamoto, S. Sugar intakes from snacks and beverages in Japanese children. J. Nutr. Sci. Vitaminol. 2012, 58, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Uchida, K.; Ohnaka, K.; Morita, M.; Toyomura, K.; Kono, S.; Ueki, T.; Tanaka, M.; Kakeji, Y.; Maehara, Y.; et al. Sugars, sucrose and colorectal cancer risk: The Fukuoka colorectal cancer study. Scand. J. Gastroenterol. 2014, 49, 581–588. [Google Scholar] [CrossRef]
- Fujiwara, A.; Murakami, K.; Asakura, K.; Uechi, K.; Sugimoto, M.; Wang, H.C.; Masayasu, S.; Sasaki, S. Estimation of Starch and Sugar Intake in a Japanese Population Based on a Newly Developed Food Composition Database. Nutrients 2018, 10, 1474. [Google Scholar] [CrossRef]
- Yamakawa, M.; Wada, K.; Koda, S.; Mizuta, F.; Uji, T.; Oba, S.; Nagata, C. High Intake of Free Sugars, Fructose, and Sucrose Is Associated with Weight Gain in Japanese Men. J. Nutr. 2020, 150, 322–330. [Google Scholar] [CrossRef]
- Cho, H.; Budhathoki, S.; Kanehara, R.; Goto, A.; Yamaji, T.; Kakugawa, Y.; Saito, Y.; Matsuda, T.; Iwasaki, M.; Tsugane, S. Association between dietary sugar intake and colorectal adenoma among cancer screening examinees in Japan. Cancer Sci. 2020, 111, 3862–3872. [Google Scholar] [CrossRef] [PubMed]
- Edo, A.; Pertiwi, Y.D.; Hirooka, K.; Masuda, S.; Kamaruddin, M.I.; Yanagi, M.; Nagao, A.; Ohno, H.; Yoneda, M.; Kiuchi, Y. Association of Dietary Nutrient Intake with Early Age-Related Macular Degeneration in Japanese-Americans. Metabolites 2021, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, S.; Koung Ry, L.; Takeichi, H.; Emiko, S.; San, P.; Sarukura, N.; Kamoshita, S.; Yamamoto, S. Sugar intake and body weight in Cambodian and Japanese children. J. Med. Investig. 2014, 61, 72–78. [Google Scholar] [CrossRef]
- Pang, S.; Song, P.; Sun, X.; Qi, W.; Yang, C.; Song, G.; Wang, Y.; Zhang, J. Dietary fructose and risk of metabolic syndrome in Chinese residents aged 45 and above: Results from the China National Nutrition and Health Survey. Nutr. J. 2021, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.; Elliott, P.; Dennis, B.; Dyer, A.R.; Kesteloot, H.; Liu, K.; Ueshima, H.; Zhou, B.F.; Group, I.R. INTERMAP: Background, aims, design, methods, and descriptive statistics (nondietary). J. Hum. Hypertens. 2003, 17, 591–608. [Google Scholar] [CrossRef]
- Shay, C.M.; Stamler, J.; Dyer, A.R.; Brown, I.J.; Chan, Q.; Elliott, P.; Zhao, L.; Okuda, N.; Miura, K.; Daviglus, M.L.; et al. Nutrient and food intakes of middle-aged adults at low risk of cardiovascular disease: The international study of macro-/micronutrients and blood pressure (INTERMAP). Eur. J. Nutr. 2012, 51, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kwon, S.O.; Lee, Y. Weight Status and Dietary Factors Associated with Sugar-Sweetened Beverage Intake among Korean Children and Adolescents—Korea National Health and Nutrition Examination Survey, 2008–2011. Clin. Nutr. Res. 2013, 2, 135–142. [Google Scholar] [CrossRef][Green Version]
- Bae, J.; Chun, B.Y.; Park, P.S.; Choi, B.Y.; Kim, M.K.; Shin, M.H.; Lee, Y.H.; Shin, D.H.; Kim, S.K. Higher consumption of sugar-sweetened soft drinks increases the risk of hyperuricemia in Korean population: The Korean Multi-Rural Communities Cohort Study. Semin. Arthritis Rheum. 2014, 43, 654–661. [Google Scholar] [CrossRef]
- Ha, K.; Chung, S.; Lee, H.S.; Kim, C.I.; Joung, H.; Paik, H.Y.; Song, Y. Association of Dietary Sugars and Sugar-Sweetened Beverage Intake with Obesity in Korean Children and Adolescents. Nutrients 2016, 8, 31. [Google Scholar] [CrossRef]
- Song, H.J.; Paek, Y.J.; Choi, M.K.; Yoo, K.B.; Kang, J.H.; Lee, H.J. Gender Differences in the relationship between carbonated sugar-sweetened beverage intake and the likelihood of hypertension according to obesity. Int. J. Public Health 2017, 62, 573–581. [Google Scholar] [CrossRef]
- Lim, H.; Lee, H.J.; Choue, R.; Wang, Y. Trends in Fast-Food and Sugar-Sweetened Beverage Consumption and Their Association with Social Environmental Status in South Korea. J. Acad. Nutr. Diet. 2018, 118, 1228–1236.e1221. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Jo, G.; Chung, H.K.; Shin, M.J. Association between sugar-sweetened beverage consumption and incident hypertension in Korean adults: A prospective study. Eur. J. Nutr. 2019, 58, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Nakamura, K.; Miura, K.; Takamura, T.; Yoshita, K.; Nagasawa, S.Y.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; et al. Sugar-sweetened beverage and diet soda consumption and the 7-year risk for type 2 diabetes mellitus in middle-aged Japanese men. Eur. J. Nutr. 2014, 53, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Chan, T.F.; Huang, H.L.; Lee, C.Y.; Tsai, S.; Wu, P.W.; Yang, Y.C.; Wang, T.N.; Lee, C.H. Fructose-Rich Beverage Intake and Central Adiposity, Uric Acid, and Pediatric Insulin Resistance. J. Pediatr. 2016, 171, 90–96.e91. [Google Scholar] [CrossRef]
- Li, D.; Yu, D.; Zhao, L. Trend of sugar-sweetened beverage consumption and intake of added sugar in China nine provinces among adults. Wei Sheng Yan Jiu 2014, 43, 70–72. [Google Scholar]
- Gui, Z.H.; Zhu, Y.N.; Cai, L.; Sun, F.H.; Ma, Y.H.; Jing, J.; Chen, Y.J. Sugar-Sweetened Beverage Consumption and Risks of Obesity and Hypertension in Chinese Children and Adolescents: A National Cross-Sectional Analysis. Nutrients 2017, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, M.; Yang, C.; Zheng, H.; Zhu, Y. Association of sugar-sweetened beverage intake with risk of metabolic syndrome among children and adolescents in urban China. Public Health Nutr. 2020, 23, 2770–2780. [Google Scholar] [CrossRef]
- Gan, Q.; Xu, P.; Yang, T.; Cao, W.; Xu, J.; Li, L.; Pan, H.; Zhao, W.; Zhang, Q. Sugar-Sweetened Beverage Consumption Status and Its Association with Childhood Obesity among Chinese Children Aged 6–17 Years. Nutrients 2021, 13, 2211. [Google Scholar] [CrossRef]
- Gui, Z.; Huang, S.; Chen, Y.; Zhao, Y.; Jiang, N.; Zhang, S.; Chen, Y. Association between Sugar-Sweetened Beverage Consumption and Executive Function in Children. Nutrients 2021, 13, 4563. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, C.; Qu, S.; Wei, X.; Feng, J.; Zhang, S.; Wang, Y.; Su, J. Effects of School-Based Interventions on Reducing Sugar-Sweetened Beverage Consumption among Chinese Children and Adolescents. Nutrients 2021, 13, 1862. [Google Scholar] [CrossRef]
- Cai, W.; Li, J.; Shi, J.; Yang, B.; Tang, J.; Truby, H.; Li, D. Acute metabolic and endocrine responses induced by glucose and fructose in healthy young subjects: A double-blinded, randomized, crossover trial. Clin. Nutr. 2018, 37, 459–470. [Google Scholar] [CrossRef]
- Singh, A.K.; Amlal, H.; Haas, P.J.; Dringenberg, U.; Fussell, S.; Barone, S.L.; Engelhardt, R.; Zuo, J.; Seidler, U.; Soleimani, M. Fructose-induced hypertension: Essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int. 2008, 74, 438–447. [Google Scholar] [CrossRef]
- Soleimani, M.; Alborzi, P. The role of salt in the pathogenesis of fructose-induced hypertension. Int. J. Nephrol. 2011, 2011, 392708. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Hong, N.J.; Yang, N.; Cabral, P.D.; Berthiaume, J.M.; Dominici, F.P.; Garvin, J.L. Dietary Fructose Increases the Sensitivity of Proximal Tubules to Angiotensin II in Rats Fed High-Salt Diets. Nutrients 2018, 10, 1244. [Google Scholar] [CrossRef]
- Petrovic, S.; Wang, Z.; Ma, L.; Seidler, U.; Forte, J.G.; Shull, G.E.; Soleimani, M. Colocalization of the apical Cl-/HCO3- exchanger PAT1 and gastric H-K-ATPase in stomach parietal cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G1207–G1216. [Google Scholar] [CrossRef]
- Petrovic, S.; Wang, Z.; Ma, L.; Soleimani, M. Regulation of the apical Cl-/HCO-3 exchanger pendrin in rat cortical collecting duct in metabolic acidosis. Am. J. Physiol. Renal Physiol. 2003, 284, F103–F112. [Google Scholar] [CrossRef]
- Dudeja, P.K.; Rao, D.D.; Syed, I.; Joshi, V.; Dahdal, R.Y.; Gardner, C.; Risk, M.C.; Schmidt, L.; Bavishi, D.; Kim, K.E.; et al. Intestinal distribution of human Na+/H+ exchanger isoforms NHE-1, NHE-2, and NHE-3 mRNA. Am. J. Physiol. 1996, 271, G483–G493. [Google Scholar] [CrossRef]
- Levanovich, P.E.; Chung, C.S.; Komnenov, D.; Rossi, N.F. Fructose plus High-Salt Diet in Early Life Results in Salt-Sensitive Cardiovascular Changes in Mature Male Sprague Dawley Rats. Nutrients 2021, 13, 3129. [Google Scholar] [CrossRef]
- Queiroz-Leite, G.D.; Crajoinas, R.O.; Neri, E.A.; Bezerra, C.N.; Girardi, A.C.; Reboucas, N.A.; Malnic, G. Fructose acutely stimulates NHE3 activity in kidney proximal tubule. Kidney Blood Press. Res. 2012, 36, 320–334. [Google Scholar] [CrossRef]
- Cabral, P.D.; Hong, N.J.; Hye Khan, M.A.; Ortiz, P.A.; Beierwaltes, W.H.; Imig, J.D.; Garvin, J.L. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension 2014, 63, e68–e73. [Google Scholar] [CrossRef]
- Soncrant, T.; Komnenov, D.; Beierwaltes, W.H.; Chen, H.; Wu, M.; Rossi, N.F. Bilateral renal cryodenervation decreases arterial pressure and improves insulin sensitivity in fructose-fed Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R529–R538. [Google Scholar] [CrossRef]
- Johns, E.J.; Kopp, U.C.; DiBona, G.F. Neural control of renal function. Compr. Physiol. 2011, 1, 731–767. [Google Scholar] [CrossRef]
- Chen, H.H.; Chu, C.H.; Wen, S.W.; Lai, C.C.; Cheng, P.W.; Tseng, C.J. Excessive Fructose Intake Impairs Baroreflex Sensitivity and Led to Elevated Blood Pressure in Rats. Nutrients 2019, 11, 2581. [Google Scholar] [CrossRef]
- Komnenov, D.; Levanovich, P.E.; Perecki, N.; Chung, C.S.; Rossi, N.F. Aortic Stiffness and Diastolic Dysfunction in Sprague Dawley Rats Consuming Short-Term Fructose Plus High Salt Diet. Integr. Blood Press. Control 2020, 13, 111–124. [Google Scholar] [CrossRef]
- Xu, C.; Yu, J. Pathophysiological mechanisms of hypertension development induced by fructose consumption. Food Funct. 2022, 13, 1702–1717. [Google Scholar] [CrossRef]
- Kamata, K.; Yamashita, K. Insulin resistance and impaired endothelium-dependent renal vasodilatation in fructose-fed hypertensive rats. Res. Commun. Mol. Pathol. Pharmacol. 1999, 103, 195–210. [Google Scholar]
- Verma, S.; Bhanot, S.; Yao, L.; McNeill, J.H. Vascular insulin resistance in fructose-hypertensive rats. Eur. J. Pharmacol. 1997, 322, R1–R2. [Google Scholar] [CrossRef]
- Zenner, Z.P.; Gordish, K.L.; Beierwaltes, W.H. Free radical scavenging reverses fructose-induced salt-sensitive hypertension. Integr. Blood Press. Control 2018, 11, 1–9. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).