The Role of Obesity, Inflammation and Sphingolipids in the Development of an Abdominal Aortic Aneurysm
Abstract
1. Introduction
2. Materials and Methods
3. Obesity as a Risk Factor of AAA
4. Matrix Metalloproteinase in AAA
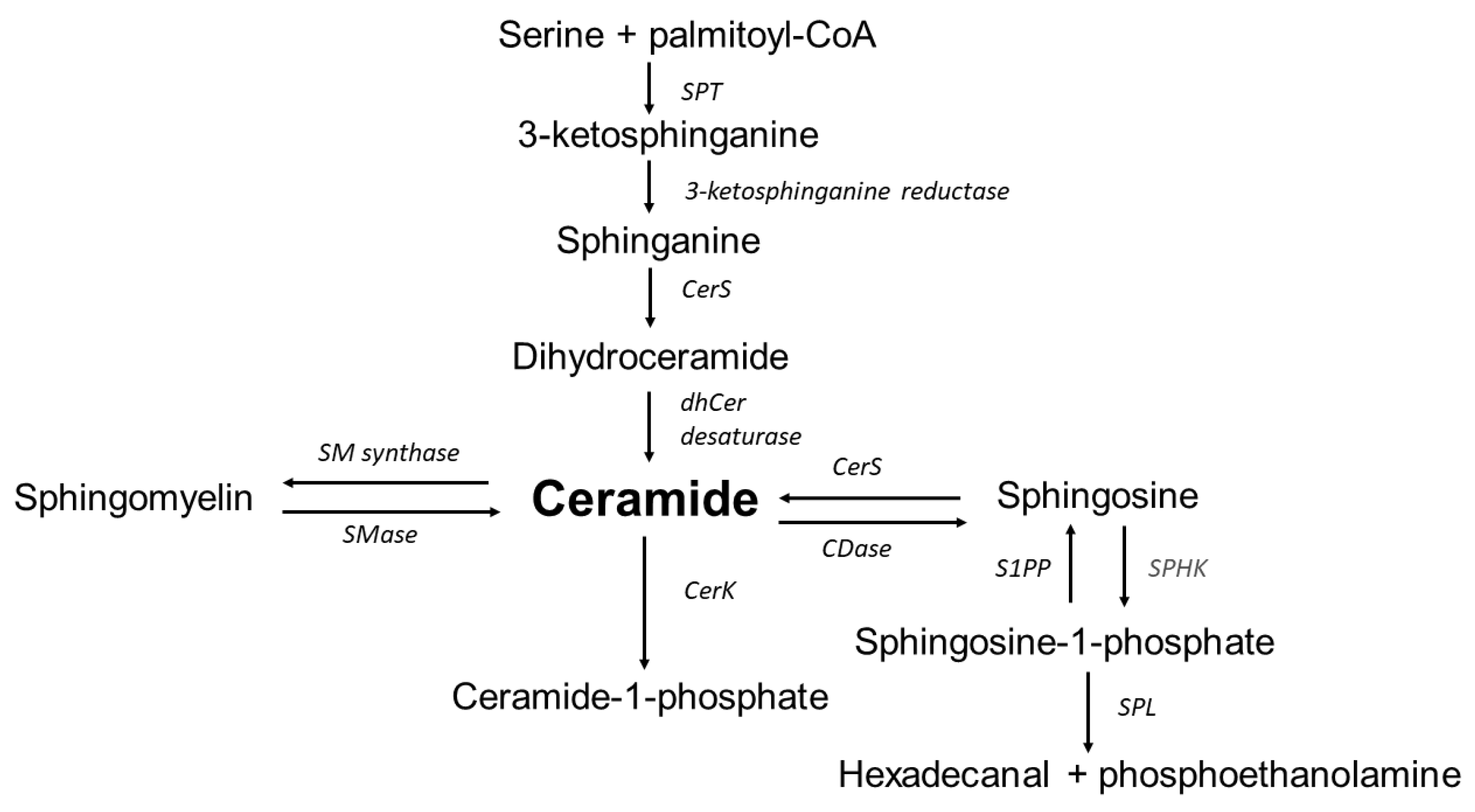
5. Sphingolipids Implication in the Pathogenesis of Abdominal Aortic Aneurysms
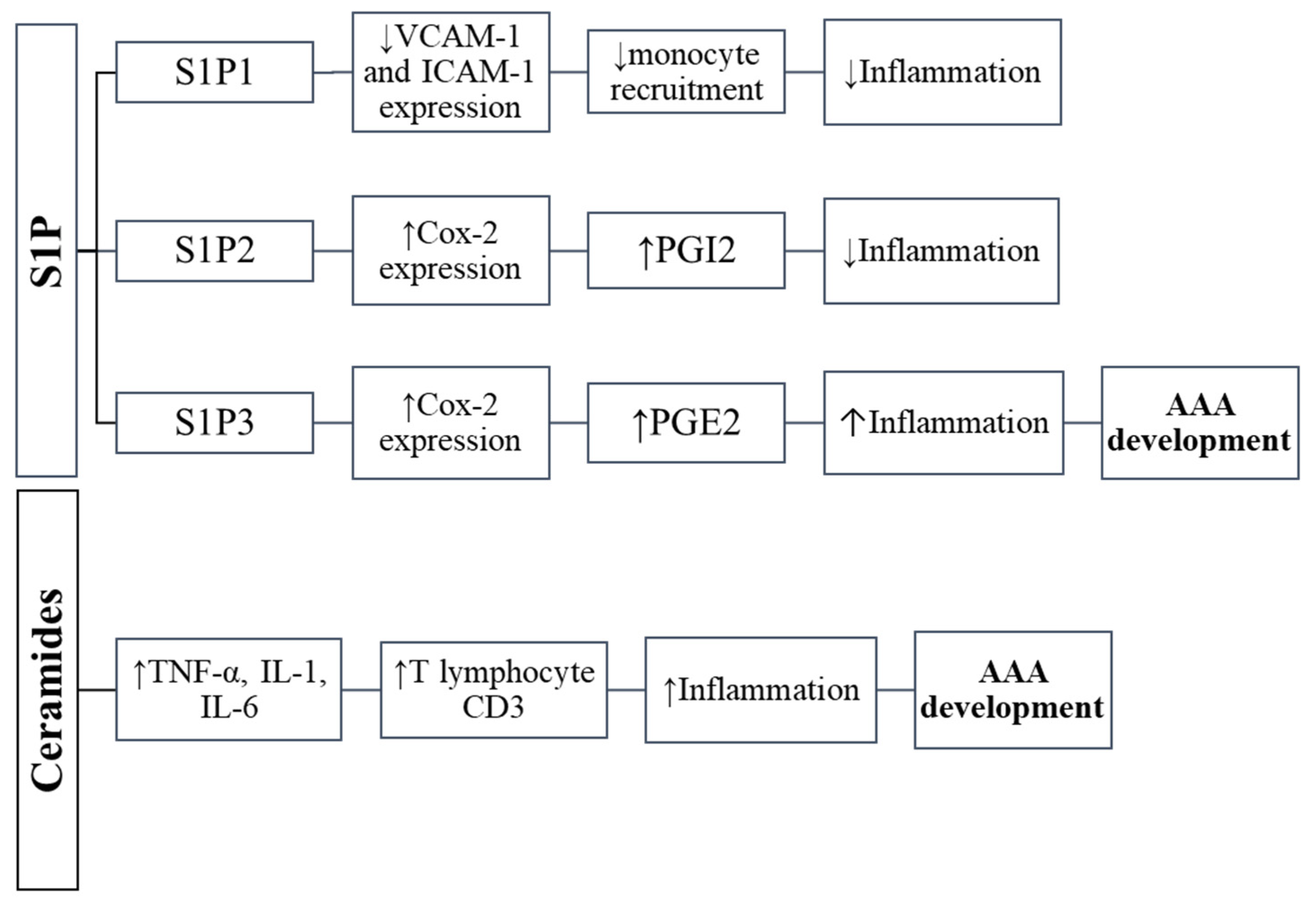
6. Sphingosine-1-Phosphate
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Altobelli, E.; Rapacchietta, L.; Profeta, V.F.; Fagnano, R. Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2805. [Google Scholar] [CrossRef] [PubMed]
- Kuivaniemi, H.; Ryer, E.J.; Elmore, J.R.; Tromp, G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev. Cardiovasc. Ther. 2015, 13, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Reimerink, J.J.; van der Laan, M.J.; Koelemay, M.J.; Balm, R.; Legemate, D.A. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br. J. Surg. 2013, 100, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, H.H.; Maegdefessel, L. Linking obesity with abdominal aortic aneurysm development. Eur. Heart J. 2020, 41, 2469–2471. [Google Scholar] [CrossRef]
- Golledge, J.; Clancy, P.; Jamrozik, K.; Norman, P.E. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation 2007, 116, 2275–2279. [Google Scholar] [CrossRef]
- Folkesson, M.; Vorkapic, E.; Gulbins, E.; Japtok, L.; Kleuser, B.; Welander, M.; Länne, T.; Wågsäter, D. Inflammatory cells, ceramides, and expression of proteases in perivascular adipose tissue adjacent to human abdominal aortic aneurysms. J. Vasc. Surg. 2017, 65, 1171–1179.e1171. [Google Scholar] [CrossRef]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, R.; Oo, A.Y.; Xiao, Q. Matrix Metalloproteinase in Abdominal Aortic Aneurysm and Aortic Dissection. Pharmaceuticals 2019, 12, 118. [Google Scholar] [CrossRef]
- Stillwell, W. An Introduction to Biological Membranes, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Schubert, K.M.; Scheid, M.P.; Duronio, V. Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J. Biol. Chem. 2000, 275, 13330–13335. [Google Scholar] [CrossRef]
- Li, P.L.; Zhang, Y. Cross talk between ceramide and redox signaling: Implications for endothelial dysfunction and renal disease. Handb. Exp. Pharmacol. 2013, 216, 171–197. [Google Scholar] [CrossRef]
- Qu, Z.; Cheuk, B.L.; Cheng, S.W. Differential expression of sphingosine-1-phosphate receptors in abdominal aortic aneurysms. Mediat. Inflamm. 2012, 2012, 643609. [Google Scholar] [CrossRef][Green Version]
- López-Vales, R.; David, S. Bioactive Lipids in Inflammation After Central Nervous System Injury. Adv. Exp. Med. Biol. 2019, 1127, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Muñoz, A.; Presa, N.; Gomez-Larrauri, A.; Rivera, I.G.; Trueba, M.; Ordoñez, M. Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate. Prog. Lipid Res. 2016, 61, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Pralhada Rao, R.; Vaidyanathan, N.; Rengasamy, M.; Mammen Oommen, A.; Somaiya, N.; Jagannath, M.R. Sphingolipid metabolic pathway: An overview of major roles played in human diseases. J. Lipids 2013, 2013, 178910. [Google Scholar] [CrossRef]
- Lahiri, S.; Futerman, A.H. The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol. Life Sci. 2007, 64, 2270–2284. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Heegaard, N.H.; Vammen, S.; Fasting, H.; Henneberg, E.W.; Heickendorff, L. Smoking, but not lipids, lipoprotein(a) and antibodies against oxidised LDL, is correlated to the expansion of abdominal aortic aneurysms. Eur. J. Vasc. EndoVasc. Surg. 2001, 21, 51–56. [Google Scholar] [CrossRef]
- Stackelberg, O.; Björck, M.; Sadr-Azodi, O.; Larsson, S.C.; Orsini, N.; Wolk, A. Obesity and abdominal aortic aneurysm. Br. J. Surg. 2013, 100, 360–366. [Google Scholar] [CrossRef]
- Police, S.B.; Thatcher, S.E.; Charnigo, R.; Daugherty, A.; Cassis, L.A. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arter. Thromb. Vasc. Biol. 2009, 29, 1458–1464. [Google Scholar] [CrossRef]
- Thanigaimani, S.; Golledge, J. Role of Adipokines and Perivascular Adipose Tissue in Abdominal Aortic Aneurysm: A Systematic Review and Meta-Analysis of Animal and Human Observational Studies. Front. Endocrinol. 2021, 12, 618434. [Google Scholar] [CrossRef]
- Kazi, M.; Thyberg, J.; Religa, P.; Roy, J.; Eriksson, P.; Hedin, U.; Swedenborg, J. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J. Vasc. Surg. 2003, 38, 1283–1292. [Google Scholar] [CrossRef]
- Rossi, C.; Santini, E.; Chiarugi, M.; Salvati, A.; Comassi, M.; Vitolo, E.; Madec, S.; Solini, A. The complex P2 × 7 receptor/inflammasome in perivascular fat tissue of heavy smokers. Eur. J. Clin. Investig. 2014, 44, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Sakaue, T.; Suzuki, J.; Hamaguchi, M.; Suehiro, C.; Tanino, A.; Nagao, T.; Uetani, T.; Aono, J.; Nakaoka, H.; Kurata, M.; et al. Perivascular Adipose Tissue Angiotensin II Type 1 Receptor Promotes Vascular Inflammation and Aneurysm Formation. Hypertension 2017, 70, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Kugo, H.; Moriyama, T.; Zaima, N. The role of perivascular adipose tissue in the appearance of ectopic adipocytes in the abdominal aortic aneurysmal wall. Adipocyte 2019, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Lee, W.J.; Funahashi, T.; Tanaka, S.; Matsuzawa, Y.; Chao, C.L.; Chen, C.L.; Tai, T.Y.; Chuang, L.M. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J. Clin. Endocrinol. Metab. 2001, 86, 3815–3819. [Google Scholar] [CrossRef] [PubMed]
- Blachnio-Zabielska, A.U.; Koutsari, C.; Tchkonia, T.; Jensen, M.D. Sphingolipid content of human adipose tissue: Relationship to adiponectin and insulin resistance. Obesity 2012, 20, 2341–2347. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Hady, H.R.; Markowski, A.R.; Kurianiuk, A.; Karwowska, A.; Górski, J.; Zabielski, P. Inhibition of Ceramide De Novo Synthesis Affects Adipocytokine Secretion and Improves Systemic and Adipose Tissue Insulin Sensitivity. Int. J. Mol. Sci. 2018, 19, 3995. [Google Scholar] [CrossRef]
- Grycel, S.; Markowski, A.R.; Hady, H.R.; Zabielski, P.; Kojta, I.; Imierska, M.; Górski, J.; Blachnio-Zabielska, A.U. Metformin treatment affects adipocytokine secretion and lipid composition in adipose tissues of diet-induced insulin-resistant rats. Nutrition 2019, 63–64, 126–133. [Google Scholar] [CrossRef]
- Yoshida, S.; Fuster, J.J.; Walsh, K. Adiponectin attenuates abdominal aortic aneurysm formation in hyperlipidemic mice. Atherosclerosis 2014, 235, 339–346. [Google Scholar] [CrossRef]
- Wågsäter, D.; Vorkapic, E.; van Stijn, C.M.; Kim, J.; Lusis, A.J.; Eriksson, P.; Tangirala, R.K. Elevated Adiponectin Levels Suppress Perivascular and Aortic Inflammation and Prevent AngII-induced Advanced Abdominal Aortic Aneurysms. Sci. Rep. 2016, 6, 31414. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Xu, G.; Zhou, C.; Sun, S. Crocetin attenuates palmitate-induced insulin insensitivity and disordered tumor necrosis factor-alpha and adiponectin expression in rat adipocytes. Br. J. Pharmacol. 2007, 151, 610–617. [Google Scholar] [CrossRef]
- Tao, M.; Yu, P.; Nguyen, B.T.; Mizrahi, B.; Savion, N.; Kolodgie, F.D.; Virmani, R.; Hao, S.; Ozaki, C.K.; Schneiderman, J. Locally applied leptin induces regional aortic wall degeneration preceding aneurysm formation in apolipoprotein E-deficient mice. Arter. Thromb. Vasc. Biol. 2013, 33, 311–320. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, K.; Kong, J.; Xu, L.; An, G.; Qin, W.; Li, J.; Zhang, Y. Deletion of resistin-like molecule-beta attenuates angiotensin II-induced abdominal aortic aneurysm. Oncotarget 2017, 8, 104171–104181. [Google Scholar] [CrossRef] [PubMed]
- Goodall, S.; Crowther, M.; Hemingway, D.M.; Bell, P.R.; Thompson, M.M. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation 2001, 104, 304–309. [Google Scholar] [CrossRef] [PubMed]
- He, C.M.; Roach, M.R. The composition and mechanical properties of abdominal aortic aneurysms. J. Vasc. Surg. 1994, 20, 6–13. [Google Scholar] [CrossRef]
- Sumner, D.S.; Hokanson, D.E.; Strandness, D.E., Jr. Stress-strain characteristics and collagen-elastin content of abdominal aortic aneurysms. Surg. Gynecol. Obstet. 1970, 130, 459–466. [Google Scholar]
- Campa, J.S.; Greenhalgh, R.M.; Powell, J.T. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis 1987, 65, 13–21. [Google Scholar] [CrossRef]
- Baxter, B.T.; Davis, V.A.; Minion, D.J.; Wang, Y.P.; Lynch, T.G.; McManus, B.M. Abdominal aortic aneurysms are associated with altered matrix proteins of the nonaneurysmal aortic segments. J. Vasc. Surg. 1994, 19, 797–802; discussion 803. [Google Scholar] [CrossRef][Green Version]
- Brophy, C.M.; Reilly, J.M.; Smith, G.J.; Tilson, M.D. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann. Vasc. Surg. 1991, 5, 229–233. [Google Scholar] [CrossRef]
- Koch, A.E.; Haines, G.K.; Rizzo, R.J.; Radosevich, J.A.; Pope, R.M.; Robinson, P.G.; Pearce, W.H. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am. J. Pathol. 1990, 137, 1199–1213. [Google Scholar]
- Aimes, R.T.; Quigley, J.P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.; Goodall, S.; Jones, J.L.; Bell, P.R.; Thompson, M.M. Localization of matrix metalloproteinase 2 within the aneurysmal and normal aortic wall. Br. J. Surg. 2000, 87, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.; Goodall, S.; Jones, J.L.; Bell, P.R.; Thompson, M.M. Increased matrix metalloproteinase 2 expression in vascular smooth muscle cells cultured from abdominal aortic aneurysms. J. Vasc. Surg. 2000, 32, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.S. Aortic aneurysmal disease. A generalized dilating diathesis. Arch. Surg. 1992, 127, 990–991. [Google Scholar] [CrossRef]
- Freestone, T.; Turner, R.J.; Coady, A.; Higman, D.J.; Greenhalgh, R.M.; Powell, J.T. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arter. Thromb. Vasc. Biol. 1995, 15, 1145–1151. [Google Scholar] [CrossRef]
- Thompson, R.W.; Holmes, D.R.; Mertens, R.A.; Liao, S.; Botney, M.D.; Mecham, R.P.; Welgus, H.G.; Parks, W.C. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J. Clin. Investig. 1995, 96, 318–326. [Google Scholar] [CrossRef]
- Chan, C.Y.; Chan, Y.C.; Cheuk, B.L.; Cheng, S.W. Clearance of matrix metalloproteinase-9 is dependent on low-density lipoprotein receptor-related protein-1 expression downregulated by microRNA-205 in human abdominal aortic aneurysm. J. Vasc. Surg. 2017, 65, 509–520. [Google Scholar] [CrossRef]
- Bown, M.J.; Jones, G.T.; Harrison, S.C.; Wright, B.J.; Bumpstead, S.; Baas, A.F.; Gretarsdottir, S.; Badger, S.A.; Bradley, D.T.; Burnand, K.; et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am. J. Hum. Genet. 2011, 89, 619–627. [Google Scholar] [CrossRef]
- Wild, J.B.; Stather, P.W.; Sylvius, N.; Choke, E.; Sayers, R.D.; Bown, M.J. Low density lipoprotein receptor related protein 1 and abdominal aortic aneurysms. Eur. J. Vasc. EndoVasc. Surg. 2012, 44, 127–132. [Google Scholar] [CrossRef]
- Herz, J.; Hamann, U.; Rogne, S.; Myklebost, O.; Gausepohl, H.; Stanley, K.K. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988, 7, 4119–4127. [Google Scholar] [CrossRef]
- Hussain, M.M. Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front. BioSci. 2001, 6, D417–D428. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Kowal, R.C.; Goldstein, J.L.; Brown, M.S. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 1990, 9, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Strickland, D.K. LRP: A multifunctional scavenger and signaling receptor. J. Clin. Investig. 2001, 108, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Lillis, A.P.; Van Duyn, L.B.; Murphy-Ullrich, J.E.; Strickland, D.K. LDL receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 2008, 88, 887–918. [Google Scholar] [CrossRef]
- Tucker, T.A.; Williams, L.; Koenig, K.; Kothari, H.; Komissarov, A.A.; Florova, G.; Mazar, A.P.; Allen, T.C.; Bdeir, K.; Mohan Rao, L.V.; et al. Lipoprotein receptor-related protein 1 regulates collagen 1 expression, proteolysis, and migration in human pleural mesothelial cells. Am. J. Respir. Cell Mol. Biol. 2012, 46, 196–206. [Google Scholar] [CrossRef]
- Rogalski, P.; Rogalska-Plonska, M.; Wroblewski, E.; Kostecka-Roslen, I.; Dabrowska, M.; Swidnicka-Siergiejko, A.; Wasielica-Berger, J.; Cydzik, M.; Hirnle, T.; Dobrzycki, S.; et al. Blood platelet function abnormalities in cirrhotic patients with esophageal varices in relation to the variceal bleeding history. Scand. J. Gastroenterol. 2019, 54, 311–318. [Google Scholar] [CrossRef]
- Hahn-Dantona, E.; Ruiz, J.F.; Bornstein, P.; Strickland, D.K. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J. Biol. Chem. 2001, 276, 15498–15503. [Google Scholar] [CrossRef]
- Kotze, C.W.; Ahmed, I.G. Etiology and Pathogenesis of Aortic Aneurysm. Available online: https://www.intechopen.com/books/484 (accessed on 12 May 2022).
- Sakalihasan, N.; Delvenne, P.; Nusgens, B.V.; Limet, R.; Lapière, C.M. Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J. Vasc. Surg. 1996, 24, 127–133. [Google Scholar] [CrossRef]
- Emonard, H.; Bellon, G.; de Diesbach, P.; Mettlen, M.; Hornebeck, W.; Courtoy, P.J. Regulation of matrix metalloproteinase (MMP) activity by the low-density lipoprotein receptor-related protein (LRP). A new function for an “old friend”. Biochimie 2005, 87, 369–376. [Google Scholar] [CrossRef]
- Rogalski, P.; Rogalska-Plonska, M.; Wroblewski, E.; Kostecka-Roslen, I.; Dabrowska, M.; Swidnicka-Siergiejko, A.; Wasielica-Berger, J.; Cydzik, M.; Hirnle, T.; Flisiak, R.; et al. Laboratory evidence for hypercoagulability in cirrhotic patients with history of variceal bleeding. Thromb. Res. 2019, 178, 41–46. [Google Scholar] [CrossRef]
- Haimovitz-Friedman, A.; Cordon-Cardo, C.; Bayoumy, S.; Garzotto, M.; McLoughlin, M.; Gallily, R.; Edwards, C.K., 3rd; Schuchman, E.H.; Fuks, Z.; Kolesnick, R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J. Exp. Med. 1997, 186, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Błachnio-Zabielska, A.U.; Pułka, M.; Baranowski, M.; Nikołajuk, A.; Zabielski, P.; Górska, M.; Górski, J. Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. J. Cell Physiol. 2012, 227, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Książek, M.; Baranowska, U.; Chabowski, A.; Baranowski, M. Arteriovenous Sphingosine-1-Phosphate Differences Across Selected Organs of the Rat. Cell Physiol. Biochem. 2018, 45, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.C.; Paultre, F.; Pearson, T.A.; Reed, R.G.; Francis, C.K.; Lin, M.; Berglund, L.; Tall, A.R. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arter. Thromb. Vasc. Biol. 2000, 20, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- Kasumov, T.; Li, L.; Li, M.; Gulshan, K.; Kirwan, J.P.; Liu, X.; Previs, S.; Willard, B.; Smith, J.D.; McCullough, A. Ceramide as a mediator of non-alcoholic Fatty liver disease and associated atherosclerosis. PLoS ONE 2015, 10, e0126910. [Google Scholar] [CrossRef] [PubMed]
- Kolak, M.; Westerbacka, J.; Velagapudi, V.R.; Wågsäter, D.; Yetukuri, L.; Makkonen, J.; Rissanen, A.; Häkkinen, A.M.; Lindell, M.; Bergholm, R.; et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 2007, 56, 1960–1968. [Google Scholar] [CrossRef]
- Xu, J.; Yeh, C.H.; Chen, S.; He, L.; Sensi, S.L.; Canzoniero, L.M.; Choi, D.W.; Hsu, C.Y. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell death. J. Biol. Chem. 1998, 273, 16521–16526. [Google Scholar] [CrossRef]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef]
- Kolesnick, R.N.; Haimovitz-Friedman, A.; Fuks, Z. The sphingomyelin signal transduction pathway mediates apoptosis for tumor necrosis factor, Fas, and ionizing radiation. Biochem. Cell Biol. 1994, 72, 471–474. [Google Scholar] [CrossRef]
- Meyer, S.G.; de Groot, H. Cycloserine and threo-dihydrosphingosine inhibit TNF-alpha-induced cytotoxicity: Evidence for the importance of de novo ceramide synthesis in TNF-alpha signaling. Biochim. Biophys. Acta 2003, 1643, 1–4. [Google Scholar] [CrossRef]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Li, P.L. Physiological and pathophysiological aspects of ceramide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R11–R26. [Google Scholar] [CrossRef] [PubMed]
- Augé, N.; Maupas-Schwalm, F.; Elbaz, M.; Thiers, J.C.; Waysbort, A.; Itohara, S.; Krell, H.W.; Salvayre, R.; Nègre-Salvayre, A. Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation 2004, 110, 571–578. [Google Scholar] [CrossRef]
- Rabkin, S.W. The Role Matrix Metalloproteinases in the Production of Aortic Aneurysm. Prog. Mol. Biol. Transl. Sci. 2017, 147, 239–265. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Han, F.; Gong, L.; Lv, Y.; Wan, Z.; Liu, H.; Ren, L.; Yang, S.; Zhang, W.; Li, T.; et al. Ceramide induces MMP-9 expression through JAK2/STAT3 pathway in airway epithelium. Lipids Health Dis. 2020, 19, 196. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Caretti, A.; Campisi, G.M.; Brizzolari, A.; Abad, J.L.; Paroni, R.; Signorelli, P.; Ghidoni, R. Inhibitors of ceramide de novo biosynthesis rescue damages induced by cigarette smoke in airways epithelia. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 753–759. [Google Scholar] [CrossRef]
- Buisson-Legendre, N.; Emonard, H.; Bernard, P.; Hornebeck, W. Relationship between cell-associated matrix metalloproteinase 9 and psoriatic keratinocyte growth. J. Investig. Dermatol. 2000, 115, 213–218. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Pilane, C.M.; LaBelle, E.F. NO induced apoptosis of vascular smooth muscle cells accompanied by ceramide increase. J. Cell Physiol. 2004, 199, 310–315. [Google Scholar] [CrossRef]
- Loidl, A.; Claus, R.; Ingolic, E.; Deigner, H.P.; Hermetter, A. Role of ceramide in activation of stress-associated MAP kinases by minimally modified LDL in vascular smooth muscle cells. Biochim. Biophys. Acta 2004, 1690, 150–158. [Google Scholar] [CrossRef]
- Liao, L.; Zhou, Q.; Song, Y.; Wu, W.; Yu, H.; Wang, S.; Chen, Y.; Ye, M.; Lu, L. Ceramide mediates Ox-LDL-induced human vascular smooth muscle cell calcification via p38 mitogen-activated protein kinase signaling. PLoS ONE 2013, 8, e82379. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N. A Rheostat of Ceramide and Sphingosine-1-Phosphate as a Determinant of Oxidative Stress-Mediated Kidney Injury. Int. J. Mol. Sci. 2022, 23, 4010. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Hsu, M.J.; Sheu, J.R.; Lee, L.W.; Hsieh, C.Y. Andrographolide, a Novel NF- κ B Inhibitor, Induces Vascular Smooth Muscle Cell Apoptosis via a Ceramide-p47phox-ROS Signaling Cascade. Evid. Based Complement. Altern. Med. 2013, 2013, 821813. [Google Scholar] [CrossRef] [PubMed]
- Pettus, B.J.; Bielawska, A.; Subramanian, P.; Wijesinghe, D.S.; Maceyka, M.; Leslie, C.C.; Evans, J.H.; Freiberg, J.; Roddy, P.; Hannun, Y.A.; et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J. Biol. Chem. 2004, 279, 11320–11326. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Stahelin, R.V.; Szulc, Z.; Bielawska, A.; Cho, W.; Chalfant, C.E. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. J. Biol. Chem. 2005, 280, 17601–17607. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Mitsutake, S.; Date, T.; Yokota, H.; Sugiura, M.; Kohama, T.; Igarashi, Y. Ceramide kinase deficiency improves diet-induced obesity and insulin resistance. FEBS Lett 2012, 586, 1300–1305. [Google Scholar] [CrossRef]
- Baudiß, K.; Ayata, C.K.; Lazar, Z.; Cicko, S.; Beckert, J.; Meyer, A.; Zech, A.; Vieira, R.P.; Bittman, R.; Gómez-Muñoz, A.; et al. Ceramide-1-phosphate inhibits cigarette smoke-induced airway inflammation. Eur. Respir. J. 2015, 45, 1669–1680. [Google Scholar] [CrossRef]
- Ordoñez, M.; Rivera, I.G.; Presa, N.; Gomez-Muñoz, A. Implication of matrix metalloproteinases 2 and 9 in ceramide 1-phosphate-stimulated macrophage migration. Cell. Signal. 2016, 28, 1066–1074. [Google Scholar] [CrossRef]
- Rosen, H.; Gonzalez-Cabrera, P.J.; Sanna, M.G.; Brown, S. Sphingosine 1-phosphate receptor signaling. Annu. Rev. Biochem. 2009, 78, 743–768. [Google Scholar] [CrossRef]
- Yang, L.; Yatomi, Y.; Miura, Y.; Satoh, K.; Ozaki, Y. Metabolism and functional effects of sphingolipids in blood cells. Br. J. Haematol. 1999, 107, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Seto, S.W.; Moxon, J.V.; Rush, C.; Walker, P.J.; Norman, P.E.; Golledge, J. Fenofibrate increases high-density lipoprotein and sphingosine 1 phosphate concentrations limiting abdominal aortic aneurysm progression in a mouse model. Am. J. Pathol. 2012, 181, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Moxon, J.V.; Liu, D.; Wong, G.; Weir, J.M.; Behl-Gilhotra, R.; Bradshaw, B.; Kingwell, B.A.; Meikle, P.J.; Golledge, J. Comparison of the serum lipidome in patients with abdominal aortic aneurysm and peripheral artery disease. Circ. Cardiovasc. Genet. 2014, 7, 71–79. [Google Scholar] [CrossRef]
- Payne, S.G.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate: Dual messenger functions. FEBS Lett. 2002, 531, 54–57. [Google Scholar] [CrossRef]
- Sanchez, T.; Hla, T. Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 2004, 92, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Wu, C.B.; Sun, C.C.; Liao, C.H.; Lau, Y.T.; Yang, C.M. Sphingosine-1-phosphate induces COX-2 expression via PI3K/Akt and p42/p44 MAPK pathways in rat vascular smooth muscle cells. J. Cell. Physiol. 2006, 207, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Hla, T.; Sekiguchi, M.; Kawahito, Y.; Yoshimura, R.; Miyazawa, K.; Iwasaki, T.; Sano, H.; Saba, J.D.; Tam, Y.Y. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: Regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006, 54, 742–753. [Google Scholar] [CrossRef]
- Lee, M.J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999, 99, 301–312. [Google Scholar] [CrossRef]
- Ryu, Y.; Takuwa, N.; Sugimoto, N.; Sakurada, S.; Usui, S.; Okamoto, H.; Matsui, O.; Takuwa, Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ. Res. 2002, 90, 325–332. [Google Scholar] [CrossRef]
- Davaille, J.; Gallois, C.; Habib, A.; Li, L.; Mallat, A.; Tao, J.; Levade, T.; Lotersztajn, S. Antiproliferative properties of sphingosine 1-phosphate in human hepatic myofibroblasts. A cyclooxygenase-2 mediated pathway. J. Biol. Chem. 2000, 275, 34628–34633. [Google Scholar] [CrossRef]
- Kim, J.I.; Jo, E.J.; Lee, H.Y.; Cha, M.S.; Min, J.K.; Choi, C.H.; Lee, Y.M.; Choi, Y.A.; Baek, S.H.; Ryu, S.H.; et al. Sphingosine 1-phosphate in amniotic fluid modulates cyclooxygenase-2 expression in human amnion-derived WISH cells. J. Biol. Chem. 2003, 278, 31731–31736. [Google Scholar] [CrossRef] [PubMed]
- Nodai, A.; Machida, T.; Izumi, S.; Hamaya, Y.; Kohno, T.; Igarashi, Y.; Iizuka, K.; Minami, M.; Hirafuji, M. Sphingosine 1-phosphate induces cyclooxygenase-2 via Ca2+-dependent, but MAPK-independent mechanism in rat vascular smooth muscle cells. Life Sci. 2007, 80, 1768–1776. [Google Scholar] [CrossRef]
- Zankl, A.R.; Schumacher, H.; Krumsdorf, U.; Katus, H.A.; Jahn, L.; Tiefenbacher, C.P. Pathology, natural history and treatment of abdominal aortic aneurysms. Clin. Res. Cardiol. 2007, 96, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Damirin, A.; Tomura, H.; Komachi, M.; Tobo, M.; Sato, K.; Mogi, C.; Nochi, H.; Tamoto, K.; Okajima, F. Sphingosine 1-phosphate receptors mediate the lipid-induced cAMP accumulation through cyclooxygenase-2/prostaglandin I2 pathway in human coronary artery smooth muscle cells. Mol. Pharmacol. 2005, 67, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- González-Díez, M.; Rodríguez, C.; Badimon, L.; Martínez-González, J. Prostacyclin induction by high-density lipoprotein (HDL) in vascular smooth muscle cells depends on sphingosine 1-phosphate receptors: Effect of simvastatin. Thromb. Haemost. 2008, 100, 119–126. [Google Scholar] [CrossRef]
- Fetalvero, K.M.; Martin, K.A.; Hwa, J. Cardioprotective prostacyclin signaling in vascular smooth muscle. Prostaglandins OTher. Lipid Mediat. 2007, 82, 109–118. [Google Scholar] [CrossRef]
- Cheuk, B.L.; Cheng, S.W. Differential secretion of prostaglandin E(2), thromboxane A(2) and interleukin-6 in intact and ruptured abdominal aortic aneurysms. Int. J. Mol. Med. 2007, 20, 391–395. [Google Scholar] [CrossRef][Green Version]
- Girkontaite, I.; Sakk, V.; Wagner, M.; Borggrefe, T.; Tedford, K.; Chun, J.; Fischer, K.D. The sphingosine-1-phosphate (S1P) lysophospholipid receptor S1P3 regulates MAdCAM-1+ endothelial cells in splenic marginal sinus organization. J. Exp. Med. 2004, 200, 1491–1501. [Google Scholar] [CrossRef]
- Masuko, K.; Murata, M.; Nakamura, H.; Yudoh, K.; Nishioka, K.; Kato, T. Sphingosine-1-phosphate attenuates proteoglycan aggrecan expression via production of prostaglandin E2 from human articular chondrocytes. BMC Musculoskelet. Disord. 2007, 8, 29. [Google Scholar] [CrossRef]
- Keul, P.; Sattler, K.; Levkau, B. HDL and its sphingosine-1-phosphate content in cardioprotection. Heart Fail. Rev. 2007, 12, 301–306. [Google Scholar] [CrossRef]
- Mulders, A.C.; Hendriks-Balk, M.C.; Mathy, M.J.; Michel, M.C.; Alewijnse, A.E.; Peters, S.L. Sphingosine kinase-dependent activation of endothelial nitric oxide synthase by angiotensin II. Arter. Thromb. Vasc. Biol. 2006, 26, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Karliner, J.S. Sphingosine kinase and sphingosine 1-phosphate in cardioprotection. J. Cardiovasc. Pharmacol. 2009, 53, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Levine, Y.C.; Li, G.K.; Michel, T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J. Biol. Chem. 2007, 282, 20351–20364. [Google Scholar] [CrossRef]
- Kimura, T.; Tomura, H.; Sato, K.; Ito, M.; Matsuoka, I.; Im, D.S.; Kuwabara, A.; Mogi, C.; Itoh, H.; Kurose, H.; et al. Mechanism and role of high density lipoprotein-induced activation of AMP-activated protein kinase in endothelial cells. J. Biol. Chem. 2010, 285, 4387–4397. [Google Scholar] [CrossRef]
- McKeage, K.; Keating, G.M. Fenofibrate: A review of its use in dyslipidaemia. Drugs 2011, 71, 1917–1946. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Granath, F.; Swedenborg, J.; Hultgren, R. More patients are treated for nonruptured abdominal aortic aneurysms, but the proportion of women remains unchanged. J. Vasc. Surg. 2008, 48, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Thelin, S.; Ståhle, E.; Ekbom, A.; Granath, F. Thoracic aortic aneurysm and dissection: Increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006, 114, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okrzeja, J.; Karwowska, A.; Błachnio-Zabielska, A. The Role of Obesity, Inflammation and Sphingolipids in the Development of an Abdominal Aortic Aneurysm. Nutrients 2022, 14, 2438. https://doi.org/10.3390/nu14122438
Okrzeja J, Karwowska A, Błachnio-Zabielska A. The Role of Obesity, Inflammation and Sphingolipids in the Development of an Abdominal Aortic Aneurysm. Nutrients. 2022; 14(12):2438. https://doi.org/10.3390/nu14122438
Chicago/Turabian StyleOkrzeja, Jakub, Alicja Karwowska, and Agnieszka Błachnio-Zabielska. 2022. "The Role of Obesity, Inflammation and Sphingolipids in the Development of an Abdominal Aortic Aneurysm" Nutrients 14, no. 12: 2438. https://doi.org/10.3390/nu14122438
APA StyleOkrzeja, J., Karwowska, A., & Błachnio-Zabielska, A. (2022). The Role of Obesity, Inflammation and Sphingolipids in the Development of an Abdominal Aortic Aneurysm. Nutrients, 14(12), 2438. https://doi.org/10.3390/nu14122438





