Swimming Suppresses Cognitive Decline of HFD-Induced Obese Mice through Reversing Hippocampal Inflammation, Insulin Resistance, and BDNF Level
Abstract
:1. Introduction
2. Materials and Methods
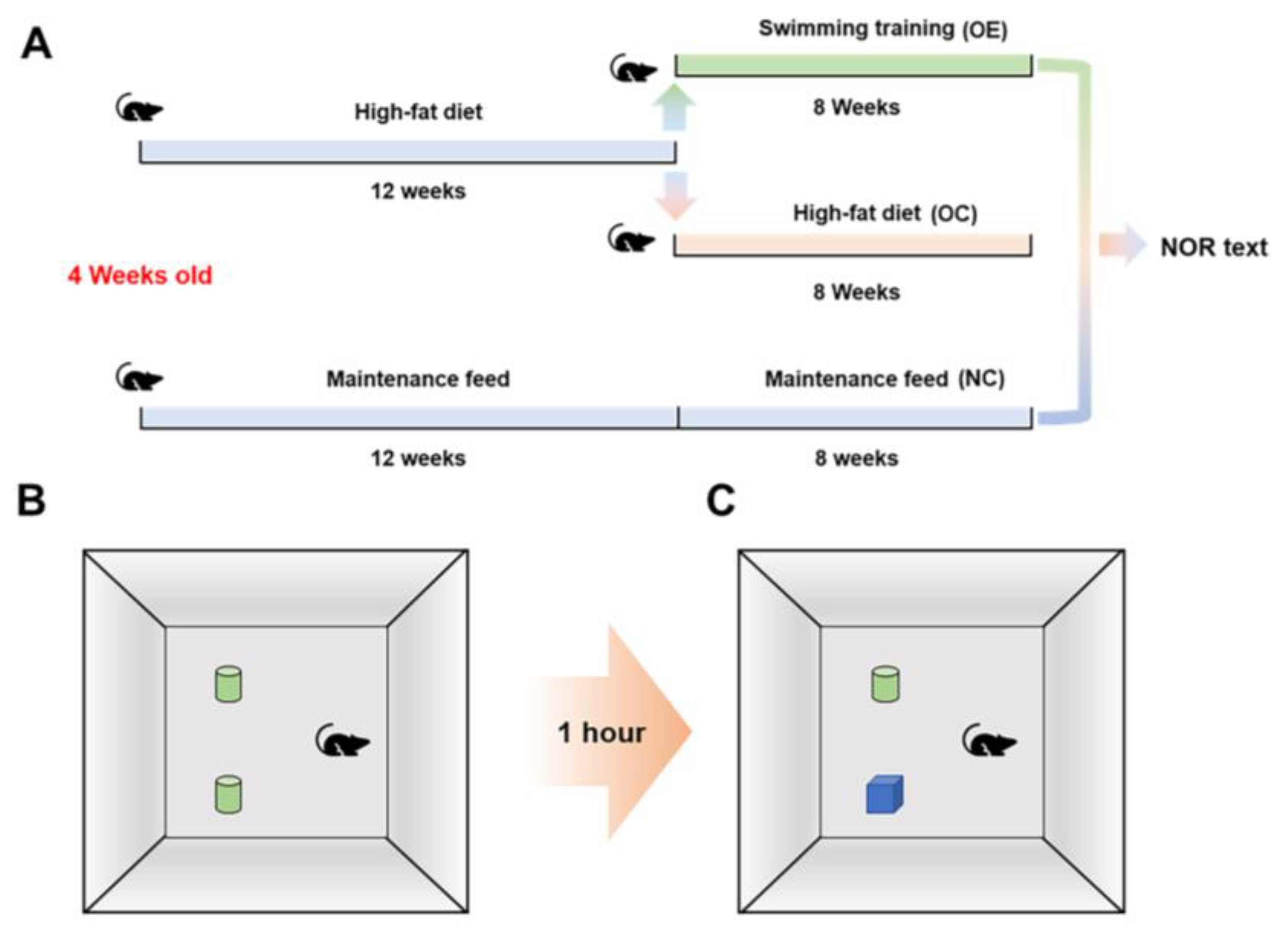
2.1. Animals, Experimental Design, and Ethics
2.2. Swimming Training Protocol
2.3. New Object Recognition Test
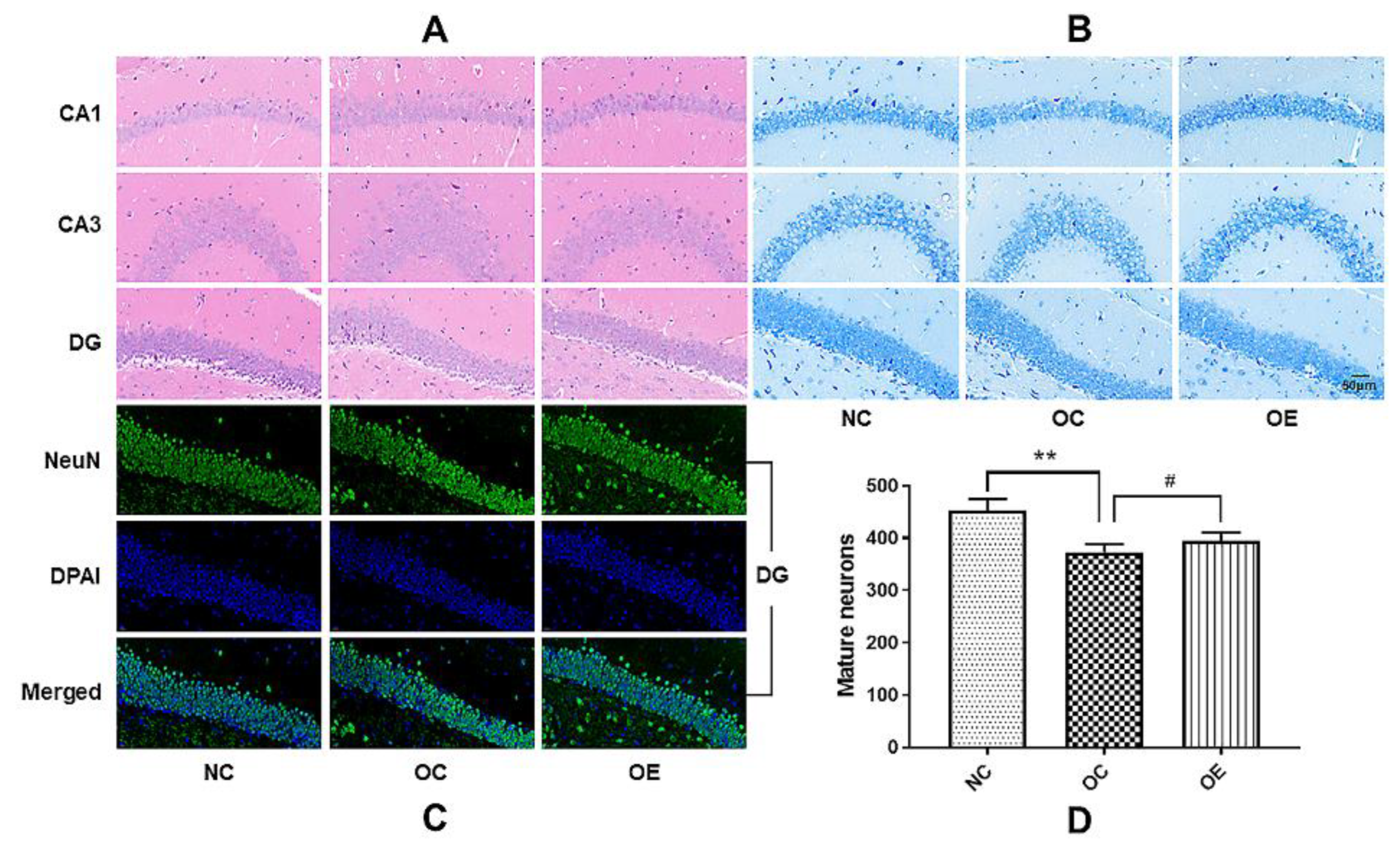
2.4. Histological Examination of Hippocampal Tissues
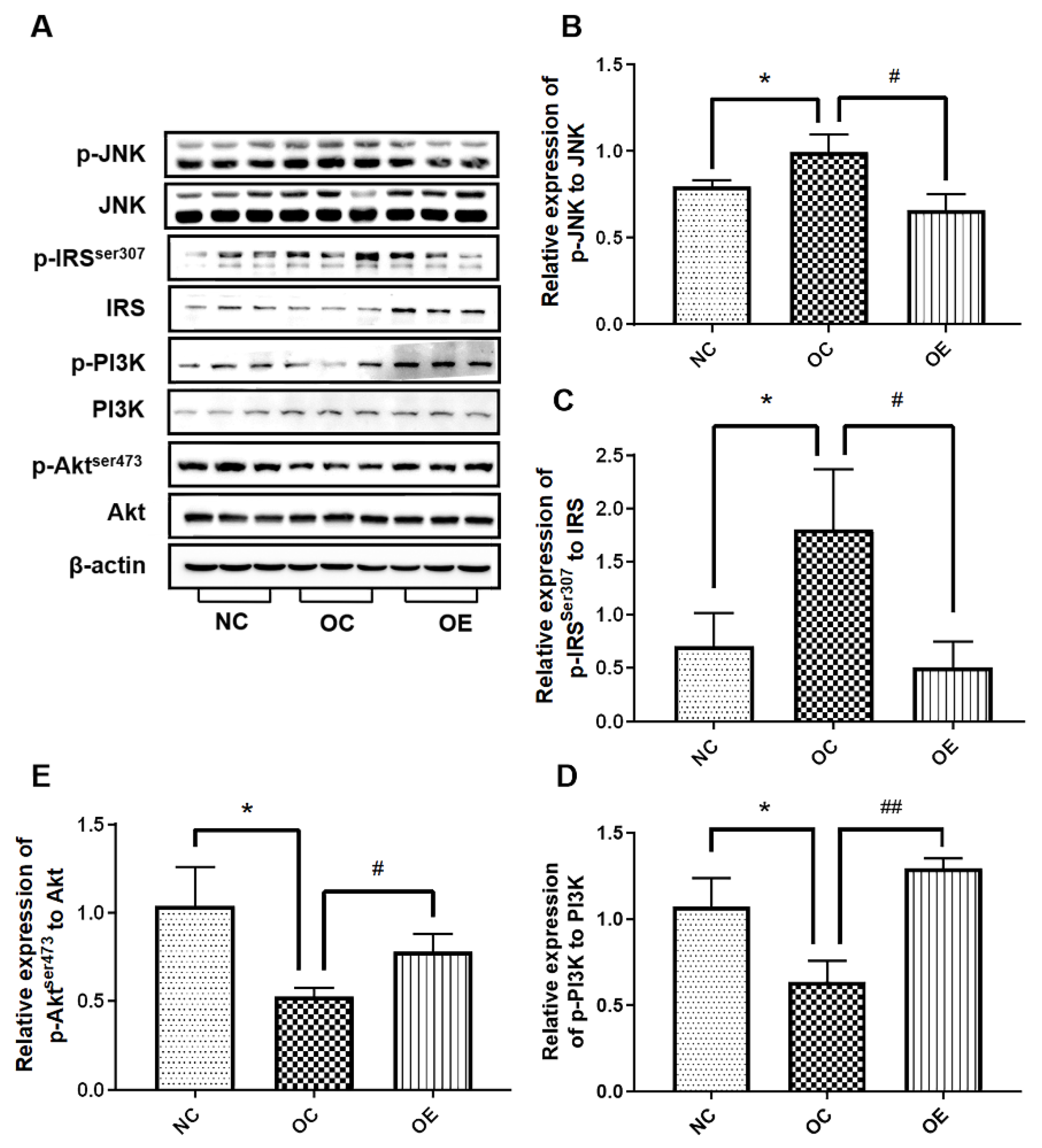
2.5. Western Blot
2.6. Statistical Analysis
3. Results
3.1. Swimming Reduced Lee’s Index and Body Weight of HFD-Induced Obese Mice
3.2. Swimming Intervention Enhanced Learning and Memory Capacity of Obese Mice
3.3. Swimming Suppressed Hippocampal Neuronal Degeneration of Obese Mice
3.4. Swimming Suppressed Hippocampal Neuroinflammation of Obese Mice
3.5. Swimming Activated Hippocampal Insulin Signaling in Obese Mice
3.6. Swimming Up-Regulated The Proteins Associated with Neurotrophic Factors and Synaptic Plasticity in the Hippocampal Tissue of Obese Mice
3.7. Swimming Inhibited Hippocampal Neuronal Apoptosis in Obese Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef]
- Reichelt, A.C.; Rank, M.M. The impact of junk foods on the adolescent brain. Birth Defects Res. 2017, 109, 1649–1658. [Google Scholar] [CrossRef]
- Tran, D.M.D.; Westbrook, R.F. A high-fat high-sugar diet-induced impairment in place-recognition memory is reversible and training-dependent. Appetite 2017, 110, 61–71. [Google Scholar] [CrossRef]
- Mohorko, N.; Černelič-Bizjak, M.; Poklar-Vatovec, T.; Grom, G.; Kenig, S.; Petelin, A.; Jenko-Pražnikar, Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr. Res. 2019, 62, 64–77. [Google Scholar] [CrossRef]
- Gunstad, J.; Strain, G.; Devlin, M.J.; Wing, R.; Cohen, R.A.; Paul, R.H.; Crosby, R.D.; Mitchell, J.E. Improved memory function 12 weeks after bariatric surgery. Surg. Obes. Relat. Dis. 2011, 7, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Crispino, M.; Trinchese, G.; Penna, E.; Cimmino, F.; Catapano, A.; Villano, I.; Perrone-Capano, C.; Mollica, M.P. Interplay between peripheral and central inflammation in obesity-promoted disorders: The impact on synaptic mitochondrial functions. Int. J. Mol. Sci. 2020, 21, 5964. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Fan, R.; Qiao, Q.; Sun, Y.; Gao, Y.; Liu, X. EGCG ameliorates high-fat- and high-fructose-induced cognitive defects by regulating the IRS/AKT and ERK/CREB/BDNF signaling pathways in the CNS. FASEB J. 2017, 31, 4998–5011. [Google Scholar] [CrossRef] [Green Version]
- Rivera, P.; Martos-Moreno, G.; Barrios, V.; Suárez, J.; Pavón, F.J.; Chowen, J.A.; de Fonseca, F.R.; Argente, J. A combination of circulating chemokines as biomarkers of obesity-induced insulin resistance at puberty. Pediatr. Obes. 2021, 16, e12711. [Google Scholar] [CrossRef]
- Mulati, A.; Ma, S.; Zhang, H.; Ren, B.; Zhao, B.; Wang, L.; Liu, X.; Zhao, T.; Kamanova, S.; Sair, A.T.; et al. Sea-buckthorn flavonoids alleviate high-fat and high-fructose diet-induced cognitive impairment by inhibiting insulin resistance and neuroinflammation. J. Agric. Food Chem. 2020, 68, 5835–5846. [Google Scholar] [CrossRef]
- Zhuang, J.; Lu, J.; Wang, X.; Wang, X.; Hu, W.; Hong, F.; Zhao, X.X.; Zheng, Y.L. Purple sweet potato color protects against high-fat diet-induced cognitive deficits through AMPK-mediated autophagy in mouse hippocampus. J. Nutr. Biochem. 2019, 65, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Patil, I.Y.; Jiang, T.; Sancheti, H.; Walsh, J.P.; Stiles, B.L.; Yin, F.; Cadenas, E. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS ONE 2015, 10, e0128274. [Google Scholar]
- Khabour, O.F.; Alzoubi, K.H.; Alomari, M.A.; Alzubi, M.A. Changes in spatial memory and BDNF expression to simultaneous dietary restriction and forced exercise. Brain Res. Bull. 2013, 90, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Tsan, L.; Décarie-Spain, L.; Noble, E.E.; Kanoski, S.E. Westerndiet consumption during development: Setting thestage for neurocognitive dysfunction. Front. Neurosci. 2021, 15, 632312. [Google Scholar] [CrossRef]
- Zhuang, H.; Yao, X.; Li, H.; Li, Q.; Yang, C.; Wang, C.; Xu, D.; Xiao, Y.; Gao, Y.; Gao, J.; et al. Long-term high-fat diet consumption by mice throughout adulthood induces neurobehavioral alterations and hippocampal neuronal remodeling accompanied by augmented microglial lipid accumulation. Brain Behav. Immun. 2022, 100, 155–171. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef] [Green Version]
- Fusco, S.; Spinelli, M.; Cocco, S.; Ripoli, C.; Mastrodonato, A.; Natale, F.; Rinaudo, M.; Livrizzi, G.; Grassi, C. Maternal insulin resistance multigenerationally impairs synaptic plasticity and memory via gametic mechanisms. Nat. Commun. 2019, 10, 4799. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Cho, H.; Skim, T.W. Physical exercise promotes memory capability by enhancing hippocampal mitochondrial functions and inhibiting apoptosis in obesity-induced insulin resistance by high fat diet. Metab. Brain Dis. 2018, 33, 283–292. [Google Scholar] [CrossRef]
- Kevin, F.; Joanne, M.C.; Harrison, D.E. The mouse in biomedical research. In American College of Laboratory Animal Medicine, American, 2nd ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 637–672. [Google Scholar]
- Mäkinen, E.; Lensu, S.; Honkanen, M.; Laitinen, P.; Wikgren, J.; Koch, L.G.; Britton, S.L.; Kainulainen, H.; Pekkala, S.; Nokia, M.S. Rats bred for low intrinsic aerobic exercise capacity link obesity with brain inflammation and reduced structural plasticity of the hippocampus. Brain Behav. Immun. 2021, 97, 250–259. [Google Scholar] [CrossRef]
- Davidson, T.L.; Hargrave, S.L.; Swithers, S.E.; Sample, C.H.; Fu, X.; Kinzig, K.P.; Zheng, W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience 2013, 253, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ge, Q.; Wu, Y.; Zhang, J.; Gu, Q.; Han, J. Impairment of long-term memory by a short-term high-fat diet via hippocampal oxidative stress and alterations in synaptic plasticity. Neuroscience 2020, 424, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Attuquayefio, T.; Stevenson, R.J.; Oaten, M.J.; Francis, H.M. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS ONE 2017, 12, e0172645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baym, C.L.; Khan, N.A.; Monti, J.M.; Raine, L.B.; Drollette, E.S.; Moore, R.D.; Scudder, M.R.; Kramer, A.F.; Hillman, C.H.; Cohen, N.J. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am. J. Clin. Nutr. 2014, 99, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Picolo, V.L.; Quadros, V.A.; Canzian, J.; Grisolia, C.K.; Goulart, J.T.; Pantoja, C.; de Bem, A.F.; Rosemberg, D.B. Short-term high-fat diet induces cognitive decline, aggression, and anxiety-like behavior in adult zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110288. [Google Scholar] [CrossRef]
- Setkowicz, Z.; Gaździńska, A.; Osoba, J.J.; Karwowska, K.; Majka, P.; Orzeł, J.; Kossowski, B.; Bogorodzki, P.; Janeczko, K.; Wyleżoł, M.; et al. Does long-term high fat diet always lead to smaller hippocampi volumes, metabolite concentrations, and worse learning and memory? A magnetic resonance and behavioral study in wistar rats. PLoS ONE 2015, 10, e0139987. [Google Scholar] [CrossRef]
- Abbasnejad, Z.; Nasseri, B.; Zardooz, H.; Ghasemi, R. Time-course study of high fat diet induced alterations in spatial memory, hippocampal JNK, P38, ERK and Akt activity. Metab. Brain Dis. 2019, 34, 659–673. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-induced neuroinflammation: Beyond the hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Wu, Q.; Liu, Y.; Chen, H.; Guo, C. The association between obesity and lower working memory is mediated by inflammation: Findings from a nationally representative dataset of U.S. adults. Brain Behav. Immun. 2020, 84, 173–179. [Google Scholar] [CrossRef]
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Layé, S.; Ferreira, G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav. Immun. 2014, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Nakandakari, S.; Muñoz, V.R.; Kuga, G.K.; Gaspar, R.C.; Sant’Ana, M.R.; Pavan, I.C.B.; da Silva, L.G.S.; Morelli, A.P.; Simabuco, F.M.; da Silva, A.S.R.; et al. Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain Behav. Immun. 2019, 79, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Shields, G.S.; Deer, L.K.; Hastings, P.D.; Hostinar, C. Adiposity, inflammation, and working memory: Evidence for a vicious cycle. Brain Behav. Immun. 2021, 13, 100202. [Google Scholar] [CrossRef] [PubMed]
- de Paula, G.C.; Brunetta, H.S.; Engel, D.F.; Gaspar, J.M.; Velloso, L.A.; Engblom, D.; de Oliveira, J.; de Bem, A.F. Hippocampal function is impaired by a short-term high-fat diet in mice: Increased blood-brain barrier permeability and neuroinflammation as triggering events. Front. Neurosci. 2021, 15, 734158. [Google Scholar] [CrossRef]
- Nie, J.; Chang, Y.; Li, Y.; Zhou, Y.; Qin, J.; Sun, Z.; Li, H. Caffeic acid phenethyl ester (propolis extract) ameliorates insulin resistance by inhibiting JNK and NF-κB inflammatory pathways in diabetic mice and HepG2 cell models. J. Agric. Food Chem. 2017, 65, 9041–9053. [Google Scholar] [CrossRef]
- Fan, R.; Hua, Y.; Shen, J.; Xiao, R.; Ma, W. Dietary fatty acids affect learning and memory ability via regulating inflammatory factors in obese mice. J. Nutr. Biochem. 2022, 103, 108959. [Google Scholar] [CrossRef]
- Mokhtari-Zaer, A.; Hosseini, M.; Roshan, N.M.; Boskabady, M.H. Treadmill exercise ameliorates memory deficits and hippocampal inflammation in ovalbumin-sensitized juvenile rats. Brain Res. Bull. 2020, 165, 40–47. [Google Scholar] [CrossRef]
- Cheng, M.; Cong, J.; Wu, Y.; Xie, J.; Wang, S.; Zhao, Y.; Zang, X. Chronic Swimming exercise ameliorates low-soybean-oil diet-induced spatial memory impairment by enhancing BDNF-mediated synaptic potentiation in developing spontaneously Hypertensive Rats. Neurochem. Res. 2018, 43, 1047–1057. [Google Scholar] [CrossRef]
- Ko, Y.J.; Ko, I.G. Voluntary wheel running improves spatial learning memory by suppressing inflammation and apoptosis via inactivation of nuclear factor kappa B in brain inflammation rats. Int. Neurourol. J. 2020, 24, 96–103. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Denver, P.; Gault, V.A.; McClean, P.L. Sustained high-fat diet modulates inflammation, insulin signalling and cognition in mice and a modified xenin peptide ameliorates neuropathology in a chronic high-fat model. Diabetes Obes. Metab. 2018, 20, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Mucellini, A.B.; Fonseca, N.; Manfro, G.G.; Silveira, P.P. Hippocampal insulin resistance and altered food decision-making as players on obesity risk. Neurosci. Biobehav. Rev. 2017, 77, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, S.; Ou, B.; Liu, Q.; Dai, J.; Ji, C.; Zhou, H.; Huang, H.; Ma, Y. The role of JNK signaling pathway in obesity-driven insulin resistance. Diabetes Metab. Syndr. Obes. 2020, 13, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kang, E.B. Effects of treadmill exercise on PI3K/AKT/GSK-3β pathway and tau protein in high-fat diet-fed rats. J. Exerc. Nutr. Biochem. 2018, 22, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Z.; Yu, J.; Li, S.; Tang, M.; Zeng, L.; Wang, H.; Xie, H.; Peng, L.; Huang, H. Quantitative proteomic analysis reveals synaptic dysfunction in the amygdala of rats susceptible to chronic mild stress. Neuroscience 2018, 376, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Baek, K.W.; Yu, H.S.; Ko, I.G.; Hwang, L.; Park, J.J. High-intensity exercise improves cognitive function and hippocampal brain-derived neurotrophic factor expression in obese mice maintained on high-fat diet. J. Exerc. Rehabil. 2020, 16, 124–131. [Google Scholar] [CrossRef]
- Lizarbe, B.; Soares, A.F.; Larsson, S.; Duarte, J.M.N. Neurochemical modifications in the hippocampus, cortex and hypothalamus of mice exposed to long-term high-fat diet. Front. Neurosci. 2018, 12, 985. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, L.; Han, S.; Qin, L.; Chen, N.; Wan, Z. Treadmill running and rutin reverse high fat diet induced cognitive impairment in diet induced obese mice. J. Nutr. Health Aging 2016, 20, 503–508. [Google Scholar] [CrossRef]
- Fernandes, J.; Soares, J.C.; do Amaral Baliego, L.G.; Arida, R.M. A single bout of resistance exercise improves memory consolidation and increases the expression of synaptic proteins in the hippocampus. Hippocampus 2016, 26, 1096–1103. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The gut microbiome and type 2 diabetes mellitus: Discussing a complex relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Ko, I.G.; Jin, J.J.; Hwang, L.; Baek, S.S. Treadmill exercise ameliorates impairment of spatial learning memory in pups born to old and obese mother rats. J. Exerc. Rehabil. 2021, 17, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, C.; Yin, Y.; Yang, Z.; Xue, H.; Mu, N.; Wang, Y.; Liu, M.; Ma, H. Aerobic interval training regulated SIRT3 attenuates high-fat-diet-associated cognitive dysfunction. Biomed. Res. Int. 2018, 2018, 2708491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pipatpiboon, N.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 2012, 153, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Dominy, J.J.; Sikalidis, A.K.; Hirschberger, L.L.; Wang, W.; Stipanuk, M.H. HepG2/C3A cells respond to cysteine deprivation by induction of the amino acid deprivation/integrated stress response pathway. Physiol. Genom. 2008, 33, 218–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liang, J.-L.; Wu, Q.-Y.; Li, J.-X.; Liu, Y.; Wu, L.-W.; Huang, J.-L.; Wu, X.-W.; Wang, M.-H.; Chen, N. Swimming Suppresses Cognitive Decline of HFD-Induced Obese Mice through Reversing Hippocampal Inflammation, Insulin Resistance, and BDNF Level. Nutrients 2022, 14, 2432. https://doi.org/10.3390/nu14122432
Zhang H, Liang J-L, Wu Q-Y, Li J-X, Liu Y, Wu L-W, Huang J-L, Wu X-W, Wang M-H, Chen N. Swimming Suppresses Cognitive Decline of HFD-Induced Obese Mice through Reversing Hippocampal Inflammation, Insulin Resistance, and BDNF Level. Nutrients. 2022; 14(12):2432. https://doi.org/10.3390/nu14122432
Chicago/Turabian StyleZhang, Hu, Ji-Ling Liang, Qiu-Yue Wu, Jin-Xiu Li, Ya Liu, Liang-Wen Wu, Jie-Lun Huang, Xiao-Wen Wu, Ming-Hui Wang, and Ning Chen. 2022. "Swimming Suppresses Cognitive Decline of HFD-Induced Obese Mice through Reversing Hippocampal Inflammation, Insulin Resistance, and BDNF Level" Nutrients 14, no. 12: 2432. https://doi.org/10.3390/nu14122432
APA StyleZhang, H., Liang, J.-L., Wu, Q.-Y., Li, J.-X., Liu, Y., Wu, L.-W., Huang, J.-L., Wu, X.-W., Wang, M.-H., & Chen, N. (2022). Swimming Suppresses Cognitive Decline of HFD-Induced Obese Mice through Reversing Hippocampal Inflammation, Insulin Resistance, and BDNF Level. Nutrients, 14(12), 2432. https://doi.org/10.3390/nu14122432






