Association of Dietary Inflammatory Potential with Blood Inflammation: The Prospective Markers on Mild Cognitive Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive Assessment
2.3. Dietary Assessment
2.4. Calculation of DII
2.5. Laboratory Measurements
2.6. Statistical Analysis
3. Results
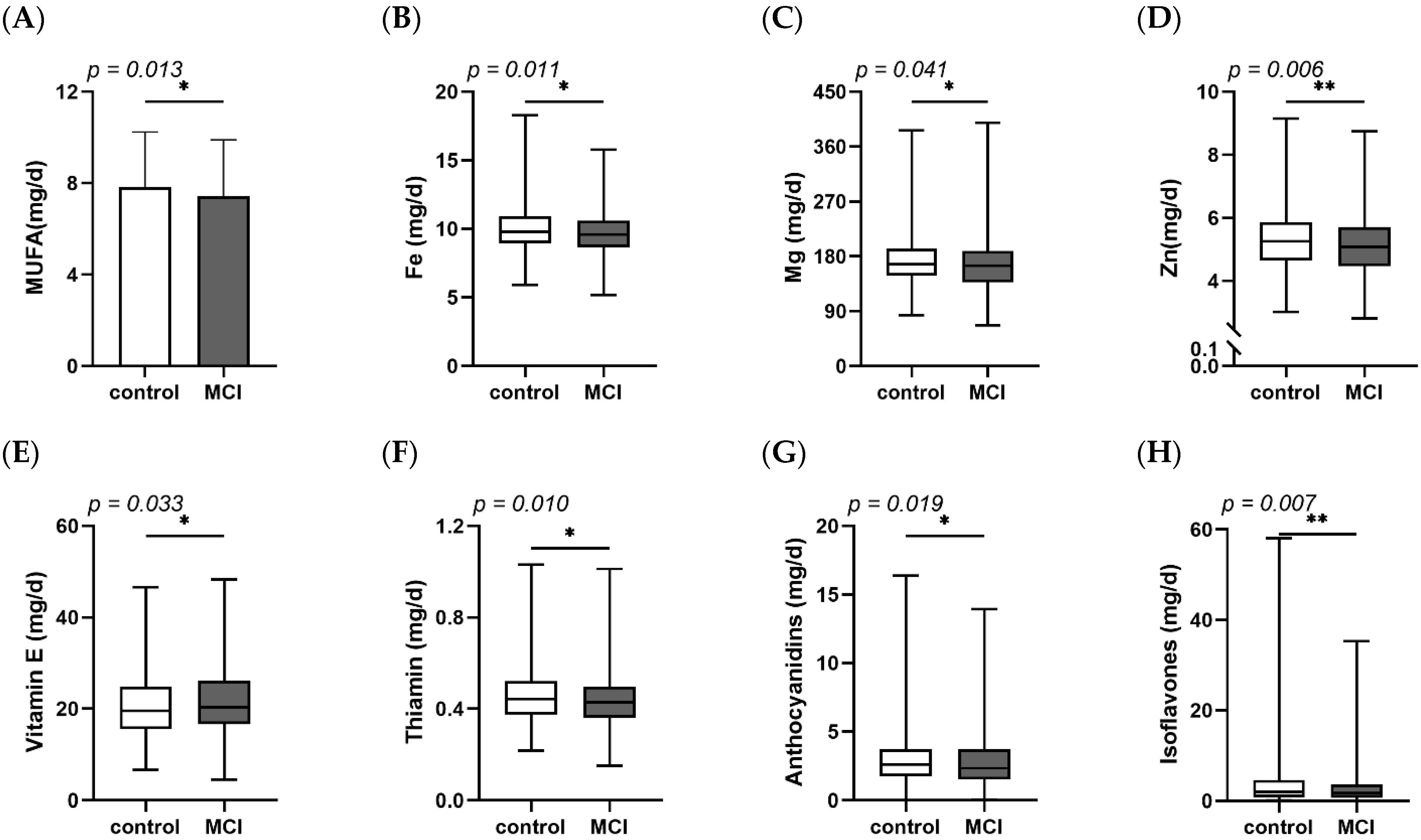
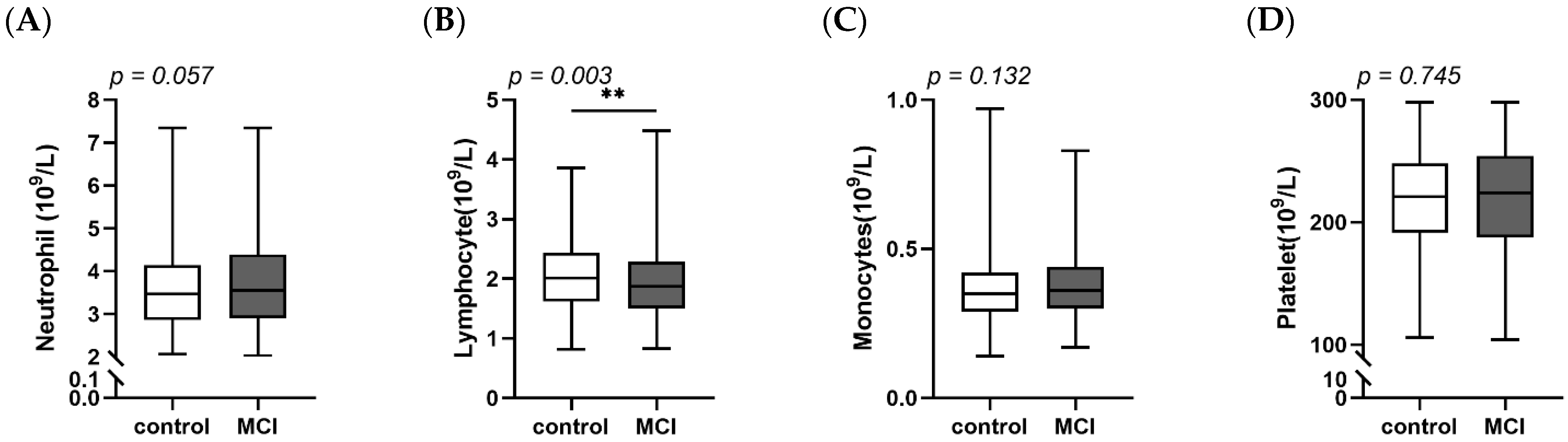
3.1. Demographic, Clinical Characteristics and Dietary Intake of Participants
3.2. Comparisons of DII, SII, SIRI between MCI and Controls
3.3. Correlation of DII, SII, SIRI with MoCA Score
3.4. Performance of DII on SIRI and SII in MCI Patients
3.5. The Roles of Inflammatory Markers on Suffering from MCI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Ageing 2019; United Nations: New York, NY, USA, 2019. [Google Scholar]
- United Nations. World Population Ageing 2017; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Rietzschel, E.R.; De Buyzere, M.L.; Langlois, M.; Debruyne, E.; Marcos, A.; Huybrechts, I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015, 113, 665–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, M.R.; Vitiello, M.V. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet. Neurol. 2019, 18, 296–306. [Google Scholar] [CrossRef]
- Aquilani, R.; Costa, A.; Maestri, R.; Cotta Ramusino, M.; Pierobon, A.; Dossena, M.; Solerte, S.B.; Condino, A.M.; Torlaschi, V.; Bini, P.; et al. Mini Nutritional Assessment May Identify a Dual Pattern of Perturbed Plasma Amino Acids in Patients with Alzheimer’s Disease: A Window to Metabolic and Physical Rehabilitation? Nutrients 2020, 12, 1845. [Google Scholar] [CrossRef]
- van’t Klooster, C.C.; van der Graaf, Y.; Ridker, P.M.; Westerink, J.; Hjortnaes, J.; Sluijs, I.; Asselbergs, F.W.; Bots, M.L.; Kappelle, L.J.; Visseren, F.L.J.; et al. The relation between healthy lifestyle changes and decrease in systemic inflammation in patients with stable cardiovascular disease. Atherosclerosis 2020, 301, 37–43. [Google Scholar] [CrossRef]
- Aquilani, R.; Costa, A.; Maestri, R.; Cotta Ramusino, M.; Perini, G.; Boselli, M.; Iadarola, P.; Buonocore, D.; Verri, M.; Dossena, M.; et al. Is the Brain Undernourished in Alzheimer’s Disease? Nutrients 2022, 14, 1872. [Google Scholar] [CrossRef]
- Watson, S.; Woodside, J.V.; Winning, L.; Wright, D.M.; Srinivasan, M.; McKenna, G. Associations between self-reported periodontal disease and nutrient intakes and nutrient-based dietary patterns in the UK Biobank. J. Clin. Periodontol. 2022, 49, 428–438. [Google Scholar] [CrossRef]
- Frith, E.; Shivappa, N.; Mann, J.R.; Hebert, J.R.; Wirth, M.D.; Loprinzi, P.D. Dietary inflammatory index and memory function: Population-based national sample of elderly Americans. Br. J. Nutr. 2018, 119, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Kwon, S.C.; Kim, M.H.; Lee, K.W.; Choi, S.Y.; Shivappa, N.; Hebert, J.R.; Chung, H.K. Inflammatory potential of diet is associated with cognitive function in an older adult Korean population. Nutrition 2018, 55–56, 56–62. [Google Scholar] [CrossRef]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary patterns and associations with biomarkers of inflammation in adults: A systematic review of observational studies. Nutr. J. 2021, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Egea Rodrigues, C.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Zabetian-Targhi, F.; Srikanth, V.K.; Smith, K.J.; Oddy Ph, D.W.; Beare, R.; Moran, C.; Wang, W.; Shivappa, N.; Hebert, J.R.; Breslin, M.; et al. Associations between the Dietary Inflammatory Index, Brain Volume, Small Vessel Disease, and Global Cognitive Function. J. Acad. Nutr. Diet. 2021, 121, 915–924.e3. [Google Scholar] [CrossRef] [PubMed]
- Trakarnwijitr, I.; Li, B.; Adams, H.; Layland, J.; Garlick, J.; Wilson, A. Age modulates the relationship between platelet-to-lymphocyte ratio and coronary artery disease. Int. J. Cardiol. 2017, 248, 349–354. [Google Scholar] [CrossRef]
- He, S.; Lei, W.; Li, J.; Yu, K.; Yu, Y.; Zhou, L.; Zhang, X.; He, M.; Guo, H.; Yang, H.; et al. Relation of Platelet Parameters with Incident Cardiovascular Disease (The Dongfeng-Tongji Cohort Study). Am. J. Cardiol. 2019, 123, 239–248. [Google Scholar] [CrossRef]
- Xu, M.; Chen, R.; Liu, L.; Liu, X.; Hou, J.; Liao, J.; Zhang, P.; Huang, J.; Lu, L.; Chen, L.; et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: The Dongfeng-Tongji cohort study. Atherosclerosis 2021, 323, 20–29. [Google Scholar] [CrossRef]
- Jin, Z.; Wu, Q.; Chen, S.; Gao, J.; Li, X.; Zhang, X.; Zhou, Y.; He, D.; Cheng, Z.; Zhu, Y.; et al. The Associations of Two Novel Inflammation Indexes, SII and SIRI with the Risks for Cardiovascular Diseases and All-Cause Mortality: A Ten-Year Follow-Up Study in 85,154 Individuals. J. Inflamm. Res. 2021, 14, 131–140. [Google Scholar] [CrossRef]
- An, Y.; Zhang, X.; Wang, Y.; Wang, Y.; Liu, W.; Wang, T.; Qin, Z.; Xiao, R. Longitudinal and nonlinear relations of dietary and Serum cholesterol in midlife with cognitive decline: Results from EMCOA study. Mol. Neurodegener. 2019, 14, 51. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Y.; Liu, W.; Wang, T.; Wang, L.; Hao, L.; Ju, M.; Xiao, R. Diet quality, gut microbiota, and microRNAs associated with mild cognitive impairment in middle-aged and elderly Chinese population. Am. J. Clin. Nutr. 2021, 114, 429–440. [Google Scholar] [CrossRef]
- Qin, H.; Zhu, B.; Hu, C.; Zhao, X. Later-Onset Hypertension Is Associated with Higher Risk of Dementia in Mild Cognitive Impairment. Front. Neurol. 2020, 11, 557977. [Google Scholar] [CrossRef]
- He, Y.; Ma, G.; Zhai, F.; Li, Y.; Hu, Y.; Feskens, E.J.; Yang, X. Dietary patterns and glucose tolerance abnormalities in Chinese adults. Diabetes Care 2009, 32, 1972–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.H.; Shen, X.N.; Xu, W.; Huang, Y.Y.; Li, H.Q.; Tan, L.; Tan, C.C.; Dong, Q.; Tan, L.; Yu, J.T.; et al. A panel of blood lipids associated with cognitive performance, brain atrophy, and Alzheimer’s diagnosis: A longitudinal study of elders without dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12041. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Wirth, M.D.; Hurley, T.G.; Hebert, J.R. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999–2002. Mol. Nutr. Food Res. 2017, 61, 1600630. [Google Scholar] [CrossRef]
- Ferreira, M.; Cronje, H.T.; van Zyl, T.; Bondonno, N.; Pieters, M. The association between an energy-adjusted dietary inflammatory index and inflammation in rural and urban Black South Africans. Public Health Nutr. 2021; 1–13, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoreishy, S.M.; Askari, G.; Mohammadi, H.; Campbell, M.S.; Khorvash, F.; Arab, A. Associations between potential inflammatory properties of the diet and frequency, duration, and severity of migraine headaches: A cross-sectional study. Sci. Rep. 2022, 12, 2878. [Google Scholar] [CrossRef] [PubMed]
- Kotemori, A.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shivappa, N.; Hebert, J.R.; Ishihara, J.; Inoue, M.; Tsugane, S. Dietary Inflammatory Index Is Associated with Inflammation in Japanese Men. Front. Nutr. 2021, 8, 604296. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, Y.; Zhang, Z.; Huang, S.; Jiao, C.; Zhang, Z.; Mao, L. Associations of dietary inflammatory potential with postpartum weight change and retention: Results from a cohort study. Obesity 2021, 29, 1689–1699. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Xu, Q.; Liu, W.; Wang, P.; Yao, J.; Zhao, A.; Chen, Y.; Wang, W. Higher dietary inflammation potential and certain dietary patterns are associated with polycystic ovary syndrome risk in China: A case-control study. Nutr. Res. 2022, 100, 1–18. [Google Scholar] [CrossRef]
- Yi, H.J.; Sung, J.H.; Lee, D.H. Systemic Inflammation Response Index and Systemic Immune-Inflammation Index Are Associated with Clinical Outcomes in Patients Treated with Mechanical Thrombectomy for Large Artery Occlusion. World Neurosurg. 2021, 153, e282–e289. [Google Scholar] [CrossRef]
- Hayden, K.M.; Beavers, D.P.; Steck, S.E.; Hebert, J.R.; Tabung, F.K.; Shivappa, N.; Casanova, R.; Manson, J.E.; Padula, C.B.; Salmoirago-Blotcher, E.; et al. The association between an inflammatory diet and global cognitive function and incident dementia in older women: The Women’s Health Initiative Memory Study. Alzheimer’s Dement. 2017, 13, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Tangestani Fard, M.; Stough, C. A Review and Hypothesized Model of the Mechanisms That Underpin the Relationship Between Inflammation and Cognition in the Elderly. Front. Aging. Neurosci. 2019, 11, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbe-Tuana, F.; Funchal, G.; Schmitz, C.R.R.; Maurmann, R.M.; Bauer, M.E. The interplay between immunosenescence and age-related diseases. Semin. Immunopathol. 2020, 42, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Armijos, R.X.; Xun, P.; Weigel, M.M. Dietary Inflammatory Index and Cardiometabolic Risk in Ecuadorian Women. Nutrients 2021, 13, 2640. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Wu, C.H.; Hsu, P.F.; Chen, S.C.; Huang, S.S.; Chan, W.L.; Lin, S.J.; Chou, C.Y.; Chen, J.W.; Pan, J.P.; et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur. J. Clin. Investig. 2020, 50, e13230. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Li, W.C.; Xia, S.H.; Xiang, T.; Tang, C.; Luo, J.L.; Lin, M.J.; Xia, X.W.; Wang, W.B. Predictive Value of the Systemic Immune Inflammation Index for Adverse Outcomes in Patients with Acute Ischemic Stroke. Front. Neurol. 2022, 13, 836595. [Google Scholar] [CrossRef]
- Qiu, T.; Zeng, Q.; Luo, X.; Xu, T.; Shen, Z.; Xu, X.; Wang, C.; Li, K.; Huang, P.; Li, X.; et al. Effects of Cigarette Smoking on Resting-State Functional Connectivity of the Nucleus Basalis of Meynert in Mild Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 755630. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Zhang, N.; Fu, P.; Jing, Z.; Yu, C.; Zhao, D.; Hao, W.; Zhou, C. Body mass index and mild cognitive impairment among rural older adults in China: The moderating roles of gender and age. BMC Psychiatry 2021, 21, 54. [Google Scholar] [CrossRef]
- van Loenhoud, A.C.; Groot, C.; Bocancea, D.I.; Barkhof, F.; Teunissen, C.; Scheltens, P.; van de Flier, W.M.; Ossenkoppele, R. Association of Education and Intracranial Volume with Cognitive Trajectories and Mortality Rates Across the Alzheimer Disease Continuum. Neurology 2022, 98, e1679–e1691. [Google Scholar] [CrossRef]
- Costagliola, G.; Nuzzi, G.; Spada, E.; Comberiati, P.; Verduci, E.; Peroni, D.G. Nutraceuticals in Viral Infections: An Overview of the Immunomodulating Properties. Nutrients 2021, 13, 2410. [Google Scholar] [CrossRef]
- Tsai, Y.W.; Lu, C.H.; Chang, R.C.; Hsu, Y.P.; Ho, L.T.; Shih, K.C. Palmitoleic acid ameliorates palmitic acid-induced proinflammation in J774A.1 macrophages via TLR4-dependent and TNF-alpha-independent signallings. Prostaglandins Leukot. Essent. Fat. Acids 2021, 169, 102270. [Google Scholar] [CrossRef] [PubMed]
- Amirani, E.; Aghadavod, E.; Shafabakhsh, R.; Asemi, Z.; Tabassi, Z.; Panahandeh, I.; Naderi, F.; Abed, A. Anti-inflammatory and antioxidative effects of thiamin supplements in patients with gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2022, 35, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Cenk, E.; Schmutz, C.; Pahlke, G.; Oertel, A.; Kollarova, J.; Mock, H.P.; Matros, A.; Marko, D. Immunomodulatory Properties of Blackberry Anthocyanins in THP-1 Derived Macrophages. Int. J. Mol. Sci. 2021, 22, 10483. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Li, F.; Ma, Q.; Li, L.; Zhang, L.; Yang, Z.; Huang, Y.; Han, Z.; Wang, R.; Tao, Z.; Zheng, Y.; et al. Alterations of inflammatory cytokines in super-acute stroke patients and the potential pathogenesis. J. Clin. Neurosci. 2022, 99, 35–43. [Google Scholar] [CrossRef]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Emran, T.B.; Rabaan, A.A.; Mutair, A.A.; Alawi, Z.A.; Alhumaid, S.; Dhama, K. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders-Advances and challenges. Hum. Vaccin. Immunother. 2022, 18, 2035117. [Google Scholar] [CrossRef]
- Weng, Y.; Zeng, T.; Huang, H.; Ren, J.; Wang, J.; Yang, C.; Pan, W.; Hu, J.; Sun, F.; Zhou, X.; et al. Systemic Immune-Inflammation Index Predicts 3-Month Functional Outcome in Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis. Clin. Interv. Aging 2021, 16, 877–886. [Google Scholar] [CrossRef]
- Li, J.; Yuan, Y.; Liao, X.; Yu, Z.; Li, H.; Zheng, J. Prognostic Significance of Admission Systemic Inflammation Response Index in Patients with Spontaneous Intracerebral Hemorrhage: A Propensity Score Matching Analysis. Front. Neurol. 2021, 12, 718032. [Google Scholar] [CrossRef]
- Dong, X.; Nao, J.; Gao, Y. Peripheral Monocyte Count Predicts Outcomes in Patients with Acute Ischemic Stroke Treated with rtPA Thrombolysis. Neurotox. Res. 2020, 37, 469–477. [Google Scholar] [CrossRef]
- Diaz, K.; Kohut, M.L.; Russell, D.W.; Stegemoller, E.L. Peripheral inflammatory cytokines and motor symptoms in persons with Parkinson’s disease. Brain Behav. Immun. Health 2022, 21, 100442. [Google Scholar] [CrossRef]
- Marx, W.; Veronese, N.; Kelly, J.T.; Smith, L.; Hockey, M.; Collins, S.; Trakman, G.L.; Hoare, E.; Teasdale, S.B.; Wade, A.; et al. The Dietary Inflammatory Index and Human Health: An Umbrella Review of Meta-Analyses of Observational Studies. Adv. Nutr. 2021, 12, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Assmann, K.E.; Andreeva, V.A.; Touvier, M.; Neufcourt, L.; Shivappa, N.; Hebert, J.R.; Wirth, M.D.; Hercberg, S.; Galan, P.; et al. Long-term association between the dietary inflammatory index and cognitive functioning: Findings from the SU.VI.MAX study. Eur. J. Nutr. 2017, 56, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
| Total | Group | p | ||
|---|---|---|---|---|
| Control | MCI | |||
| Demographic characteristics | ||||
| N | 1050 | 481 | 569 | |
| Age | 70 (67.73) | 70 (67.73) | 69 (67.73) | 0.759 |
| Female n (%) | 629 (59.9) | 313 (65.1) | 316 (55.5) | 0.002 ** |
| Smoking | 210 (28.0) | 75 (21.9) | 135 (33.2) | 0.001 ** |
| MoCA score | 21 (17, 23) | 22 (20, 25) | 19 (15, 22) | <0.001 ** |
| BMR (kcal) | 1258 (1154, 1387) | 1263 (1157, 1377) | 1254 (1149, 1395) | 0.839 |
| Education | <0.001 ** | |||
| Illiterate n (%) | 234 (22.3) | 165 (34.3) | 69 (12.1) | |
| Primary school n (%) | 353 (33.6) | 194 (40.3) | 159 (27.9) | |
| Junior high school n (%) | 376 (35.8) | 86 (17.9) | 290 (51.0) | |
| High school and above n (%) | 87 (8.3) | 36 (7.5) | 51 (9.0) | |
| BMI | 0.111 | |||
| Emaciation n (%) | 14 (1.3) | 4 (0.8) | 10 (1.8) | |
| Normal n (%) | 278 (26.5) | 120 (24.9) | 158 (27.8) | |
| Overweight n (%) | 458 (43.6) | 204 (42.4) | 254 (44.6) | |
| Obesity n (%) | 300 (28.6) | 153 (31.8) | 147 (25.8) | |
| Total | Group | p | ||
|---|---|---|---|---|
| Control | MCI | |||
| DII | 0.78 (−0.09, 1.45) | 0.80 (−0.07, 1.38) | 0.76 (−0.16, 1.52) | 0.726 |
| SIRI | 0.64 (0.46, 0.90) | 0.61 (0.44, 0.84) | 0.68 (0.48, 0.94) | <0.001 ** |
| SII | 396.63 (295.28, 527.68) | 367.89 (285.26, 497.78) | 412.87 (311.10, 544.06) | 0.001 ** |
| β | 95% CI | p | |
|---|---|---|---|
| Age | −0.048 | (−0.125, 0.028) | 0.215 |
| Gender | −0.346 | (−1.484, 0.793) | 0.551 |
| Education | 2.401 | (2.033, 2.768) | <0.001 ** |
| Smoking | −0.631 | (−1.440, 0.177) | 0.126 |
| BMI (kg/m2) | −0.019 | (−0.123, 0.085) | 0.719 |
| BMR (kcal) | 0.004 | (0.000, 0.007) | 0.028 * |
| DII | −0.363 | (−0.625, −0.101) | 0.007 ** |
| SIRI | −1.505 | (−2.265, −0.745) | <0.001 ** |
| β | 95% CI | p | |
|---|---|---|---|
| Age | −0.061 | (−0.140, 0.019) | 0.134 |
| Gender | −0.278 | (2.021, 2.798) | 0.640 |
| Education | 2.409 | (−1.662, −0.010) | <0.001 ** |
| Smoking | −0.836 | (−1.448, 0.891) | 0.047 * |
| BMI (kg/m2) | −0.019 | (0.000, 0.007) | 0.726 |
| BMR (kcal) | 0.003 | (−0.670, −0.117) | 0.067 |
| DII | −0.394 | (−0.005, −0.001) | 0.005 ** |
| SII | −0.003 | (−1.448, 0.891) | 0.001 ** |
| SIRI | SII | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| MCI | ||||||
| Age | 0.008 | (−0.003,0.019) | 0.147 | 1.424 | (−4.143, 6.991) | 0.615 |
| Gender | −0.202 | (−0.372, −0.033) | 0.019 * | 14.399 | (−74.559, 103.357) | 0.750 |
| Education | −0.075 | (−0.135, −0.016) | 0.013 * | −31.599 | (−63.730, 0.531) | 0.054 |
| Smoking | 0.116 | (0.006, 0.227) | 0.039 * | 29.550 | (−27.917, 87.016) | 0.312 |
| BMI (kg/m2) | 0.010 | (−0.005, 0.025) | 0.200 | −0.183 | (−8.310, 7.943) | 0.965 |
| BMR (kcal) | 0.000 | (−0.001, 0.000) | 0.541 | 0.032 | (−0.233, 0.296) | 0.814 |
| DII | 0.042 | (0.004, 0.079) | 0.031 * | 10.811 | (−9.611, 31.232) | 0.298 |
| MCI | ||
|---|---|---|
| PR (95% CI) | p | |
| DII effects | ||
| Q2(0~2) vs. Q1(<0) | 0.90 (0.77, 1.06) | 0.196 |
| Q3(>2) vs. Q1(<0) | 1.23 (1.03, 1.47) | 0.025 * |
| SIRI effects | ||
| Q2(0.5~0.6) vs. Q1(<0.5) | 1.02 (0.81, 1.28) | 0.872 |
| Q3(0.6~0.9) vs. Q1(<0.5) | 1.28 (1.04, 1.57) | 0.018 * |
| Q4(>0.9) vs. Q1(<0.5) | 1.31 (1.07, 1.60) | 0.010 * |
| SII effects | ||
| Q2(295~396) vs. Q1(<295) | 1.01 (0.81, 1.27) | 0.913 |
| Q3(396~528) vs. Q1(<295) | 1.11 (0.89, 1.38) | 0.340 |
| Q4(>528) vs. Q1(<295) | 1.29 (1.06, 1.57) | 0.013 * |
| MCI | ||
|---|---|---|
| PR (95% CI) | p | |
| Model1 | ||
| DII effects | ||
| Q2(0~2) vs. Q1(<0) | 0.9 (0.77, 1.06) | 0.217 |
| Q3(>2) vs. Q1(<0) | 1.22 (1.01, 1.48) | 0.041 * |
| SIRI effects | ||
| Q2(0.5~0.6) vs. Q1(<0.5) | 1.03 (0.82, 1.29) | 0.810 |
| Q3(0.6~0.9) vs. Q1(<0.5) | 1.28 (1.04, 1.57) | 0.018 * |
| Q4(>0.9) vs. Q1(<0.5) | 1.32 (1.08, 1.62) | 0.006 ** |
| Model2 | ||
| DII effects | ||
| Q2(0~2) vs. Q1(<0) | 0.91 (0.77, 1.08) | 0.266 |
| Q3(>2) vs. Q1(<0) | 1.25 (1.01, 1.54) | 0.041 * |
| SII effects | ||
| Q2(295~396) vs. Q1(<295) | 1.03 (0.83, 1.29) | 0.767 |
| Q3(396~528) vs. Q1(<295) | 1.1 (0.89, 1.37) | 0.381 |
| Q4(>528) vs. Q1(<295) | 1.32 (1.08, 1.62) | 0.007 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, T.; Li, H.; Li, D.; Wang, X.; Zhao, A.; Liang, W.; Xiao, R.; Xi, Y. Association of Dietary Inflammatory Potential with Blood Inflammation: The Prospective Markers on Mild Cognitive Impairment. Nutrients 2022, 14, 2417. https://doi.org/10.3390/nu14122417
Wang X, Li T, Li H, Li D, Wang X, Zhao A, Liang W, Xiao R, Xi Y. Association of Dietary Inflammatory Potential with Blood Inflammation: The Prospective Markers on Mild Cognitive Impairment. Nutrients. 2022; 14(12):2417. https://doi.org/10.3390/nu14122417
Chicago/Turabian StyleWang, Xuan, Tiantian Li, Hongrui Li, Dajun Li, Xianyun Wang, Ai Zhao, Wannian Liang, Rong Xiao, and Yuandi Xi. 2022. "Association of Dietary Inflammatory Potential with Blood Inflammation: The Prospective Markers on Mild Cognitive Impairment" Nutrients 14, no. 12: 2417. https://doi.org/10.3390/nu14122417
APA StyleWang, X., Li, T., Li, H., Li, D., Wang, X., Zhao, A., Liang, W., Xiao, R., & Xi, Y. (2022). Association of Dietary Inflammatory Potential with Blood Inflammation: The Prospective Markers on Mild Cognitive Impairment. Nutrients, 14(12), 2417. https://doi.org/10.3390/nu14122417






