Perioperative Administration of Cystine and Theanine Suppresses Inflammation and Facilitates Early Rehabilitation and Recovery after Esophagectomy: A Randomized, Double-Blind, Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
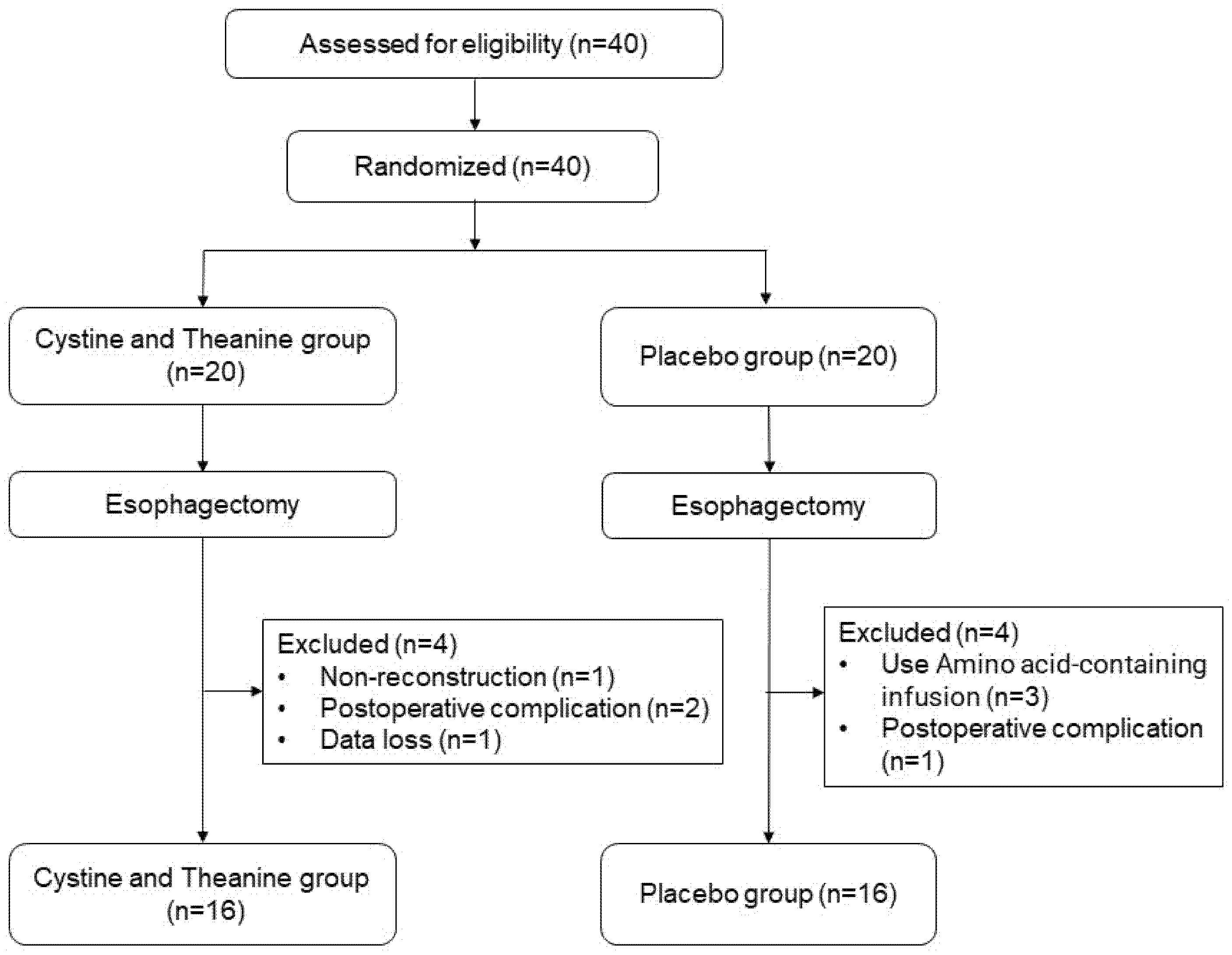
2.1. Trial Design and Randomization
2.2. Participants
2.2.1. Inclusion and Exclusion Criteria
2.2.2. Sample Size Calculation
2.3. Outcome Measures
2.3.1. Metabolic Equivalents and Exercise
2.3.2. Primary Endpoint
2.3.3. Secondary Endpoint
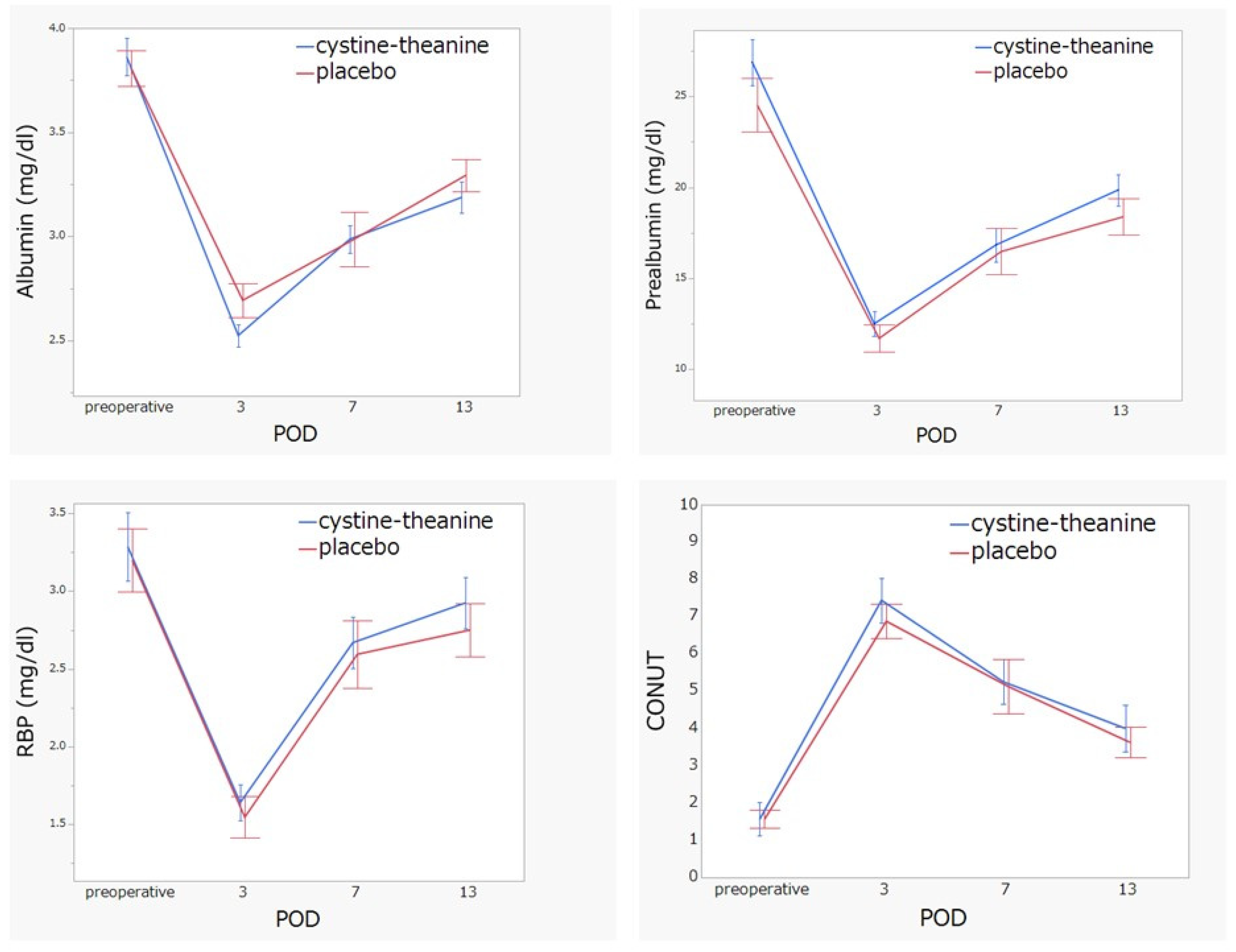
- White blood cell count, neutrophil count, lymphocyte count, C-reactive protein (CRP), serum albumin, total cholesterol, liver function, renal function, electrolytes, prealbumin, and retinol-binding protein (RBP) from the blood were also measured on the penultimate day of the surgery and POD 1–7, 10, and 13. The CONUT (controlling nutritional status) score is a well-established scale for estimating patients’ nutritional state, and it was calculated using albumin, lymphocyte count, and total cholesterol [19].
- We used the quality of recovery score (QoR-40) as a quality of life (QOL) questionnaire [20,21]. The questionnaire consists of 40 questions on the following subscales: Comfort, Emotions, Physical Independence, Patient Support, and Pain. Each item is rated on a five-point Likert scale. The patients completed the scale on the penultimate day of the surgery, POD 3, and POD 13. The tool was reported as a good objective measure of QoR after anesthesia and surgery [20] and had been used in various fields [22,23,24]. The QoR-40 has been shown to be superior among the 12 instruments in a meta-analysis for assessing QOL, and it was recommended for validation and application studies on short-term postoperative recovery [25]. Another quantitative systematic review included 3459 patients from 17 studies in nine countries to evaluate the validity, reliability, responsiveness, and clinical utility of QoR-40 and demonstrated that QoR-40 was a suitable measure of postoperative quality of recovery in a range of clinical and research situations [22].
2.3.4. Adverse Events
2.4. Study Procedure
2.4.1. Supplement and Placebo
2.4.2. Surgical Procedure
2.5. Data Analysis
3. Results
3.1. Baseline Characteristics of the Participants
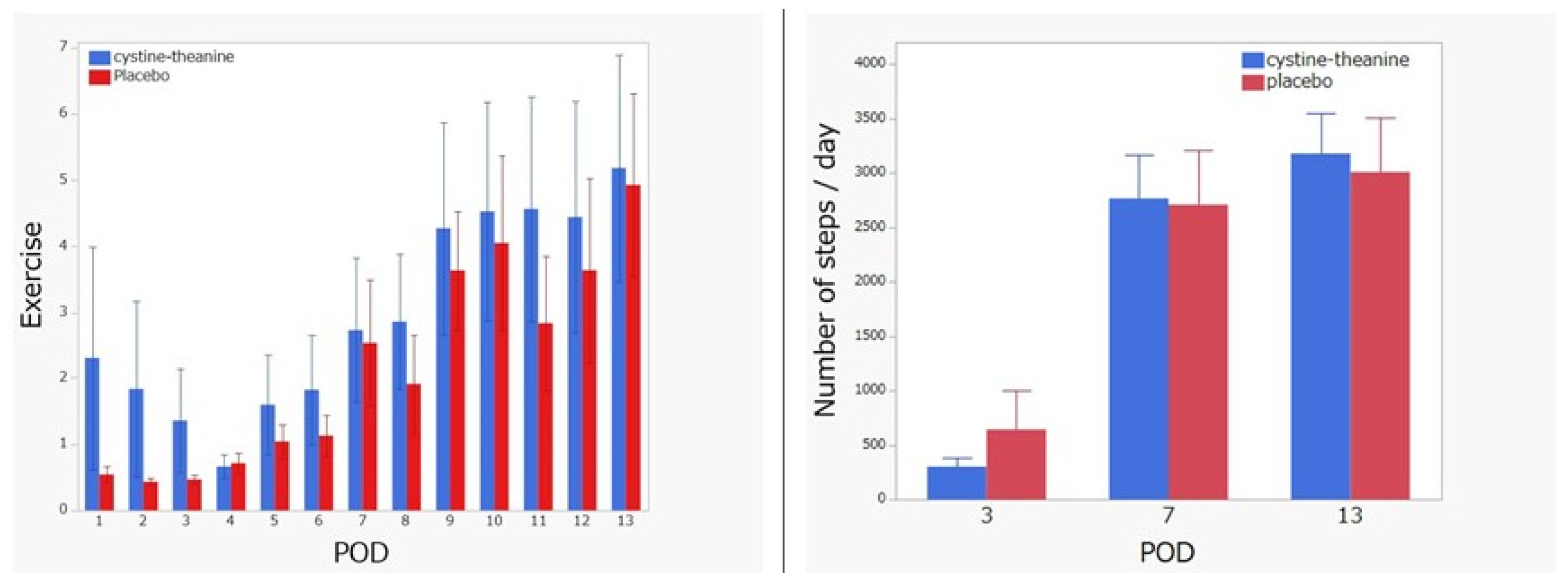
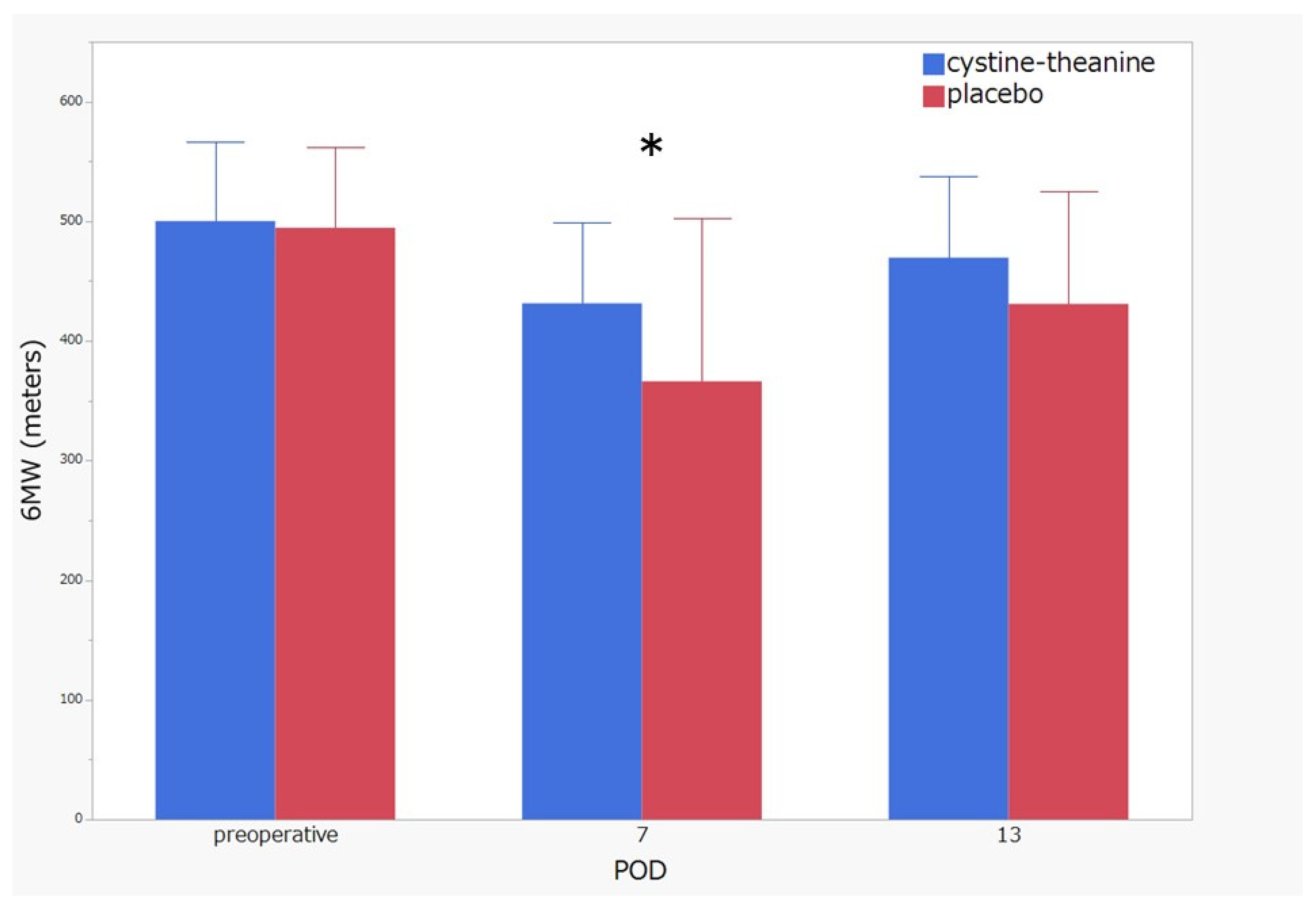
3.2. Primary Endpoint Comparisons between the CT and Placebo Groups on Physical Activity
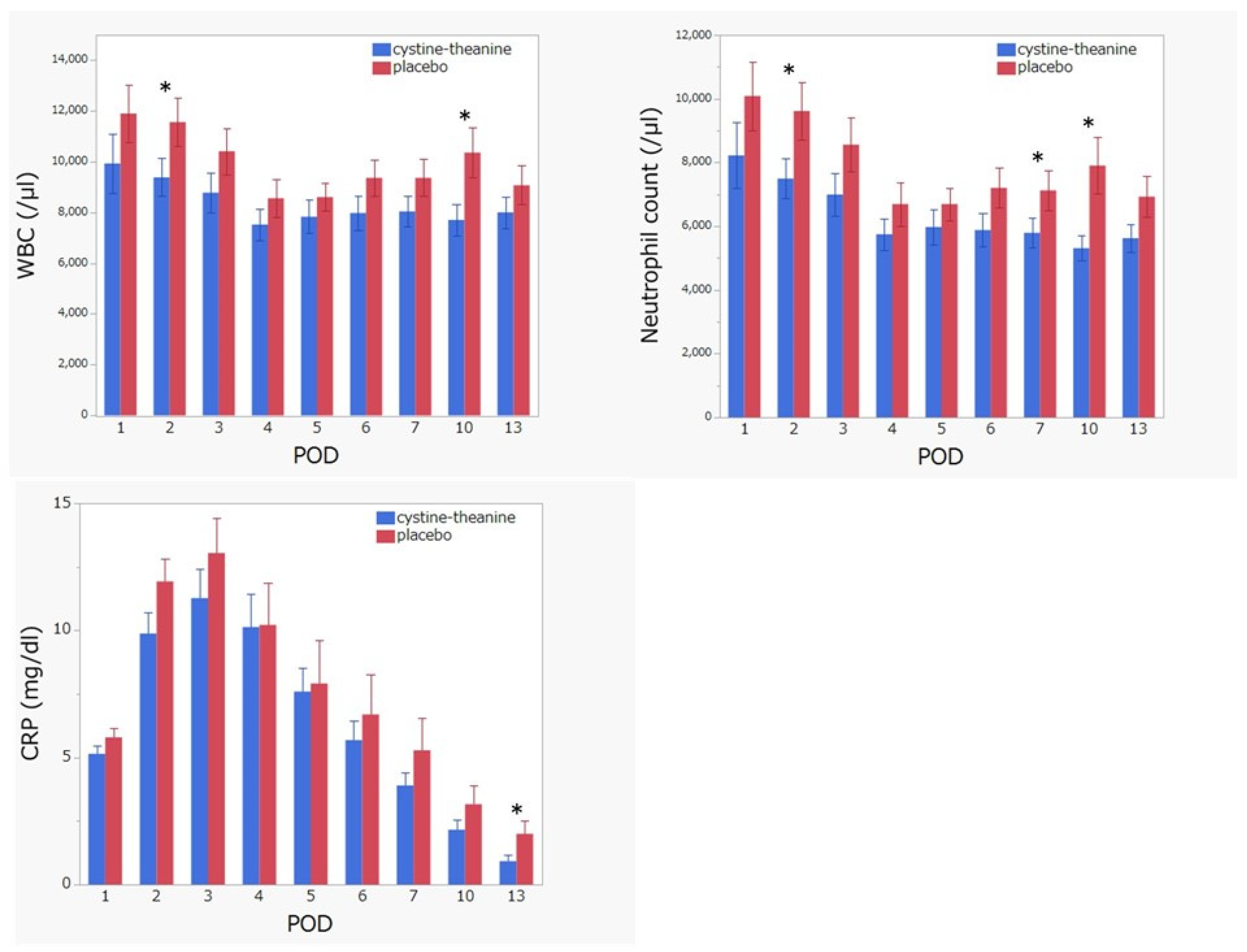
3.3. Secondary Endpoint Comparisons between the CT and Placebo Groups on Inflammation Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, M.; Tachimori, Y.; Oyama, T.; Toh, Y.; Matsubara, H.; Ueno, M.; Kono, K.; Uno, T.; Ishihara, R.; Muro, K.; et al. Comprehensive registry of esophageal cancer in Japan, 2013. Esophagus 2021, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.; Ljungqvist, O.; Von Meyenfeldt, M.; Revhaug, A.; Dejong, C.H.C.; Lassen, K.; Nygren, J.; Hausel, J.; Soop, M.; Andersen, J.; et al. Enhanced recovery after surgery: A consensus review of clinical care for patients undergoing colonic resection. Clin. Nutr. 2005, 24, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Kaibori, M.; Miyata, G.; Yoshii, K.; Fukushima, R. Perioperative management for gastrointestinal surgery after instituting interventions initiated by the Japanese Society of Surgical Metabolism and Nutrition. Asian J. Surg. 2020, 43, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, S.; Shibahara, S.; Arisaka, H.; Akiyama, Y. Enhancement of antigen-specific immunoglobulin G production in mice by co-administration of L-cystine and L-theanine. J. Vet. Med. Sci. 2007, 69, 1263–1270. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, S.; Shibakusa, T.; Tanaka, K.A. Cystine and theanine: Amino acids as oral immunomodulative nutrients. Springerplus 2013, 2, 635. [Google Scholar] [CrossRef] [Green Version]
- Shibakusa, T.; Mikami, T.; Kurihara, S.; Chiba, Y.; Tsuchiya, T.; Miyachi, T.; Oyama, A.; Tanaka, K.A.; Koyama, N. Enhancement of postoperative recovery by preoperative oral co-administration of the amino acids, cystine and theanine, in a mouse surgical model. Clin. Nutr. 2012, 31, 555–561. [Google Scholar] [CrossRef]
- Luo, J.L.; Hammarqvist, F.; Andersson, K.; Wernerman, J. Skeletal muscle glutathione after surgical trauma. Ann. Surg. 1996, 223, 420–427. [Google Scholar] [CrossRef]
- Margonis, K.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Douroudos, I.; Chatzinikolaou, A.; Mitrakou, A.; Mastorakos, G.; Papassotiriou, I.; Taxildaris, K.; et al. Oxidative stress biomarkers responses to physical overtraining: Implications for diagnosis. Free Radic. Biol. Med. 2007, 43, 901–910. [Google Scholar] [CrossRef]
- Murata, Y.; Shimamura, T.; Hamuro, J. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int. Immunol. 2002, 14, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Miyachi, T.; Tsuchiya, T.; Oyama, A.; Tsuchiya, T.; Abe, N.; Sato, A.; Chiba, Y.; Kurihara, S.; Shibakusa, T.; Mikami, T. Perioperative oral administration of cystine and theanine enhances recovery after distal gastrectomy: A prospective randomized trial. JPEN J. Parenter. Enteral Nutr. 2013, 37, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, C.; Yamasaki, S. Oesophagus including oesophagogastric junction. In International Union against Cancer TNM Classification of Malignant Tumours, 7th ed.; Sobin, L.H., Gospodarowicz, M.K., Wittekind, C., Eds.; Wiley-Blackwell: New York, NY, USA, 2009; pp. 66–72. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. Compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Yokoi, D.; Kobayashi, R.; Okada, H.; Kajita, Y.; Okuda, S. The relationships between three-axis accelerometer measures of physical activity and motor symptoms in patients with Parkinson’s disease: A single-center pilot study. BMC Neurol. 2020, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, K.; Oshima, Y.; Hikihara, Y.; Ishikawa-Takata, K.; Tabata, I.; Tanaka, S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br. J. Nutr. 2011, 105, 1681–1691. [Google Scholar] [CrossRef]
- Oshima, Y.; Kawaguchi, K.; Tanaka, S.; Ohkawara, K.; Hikihara, Y.; Ishikawa-Takata, K.; Tabata, I. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture 2010, 31, 370–374. [Google Scholar] [CrossRef]
- Ichinose, M.; Minakata, Y.; Motegi, T.; Ueki, J.; Gon, Y.; Seki, T.; Anzai, T.; Nakamura, S.; Hirata, K. Efficacy of tiotropium/olodaterol on lung volume, exercise capacity, and physical activity. Int. J. Chron. Obstruct. Pulm. Dis. 2018, 13, 1407–1419. [Google Scholar] [CrossRef] [Green Version]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Myles, P.S.; Weitkamp, B.; Jones, K.; Melick, J.; Hensen, S. Validity and reliability of a postoperative quality of recovery score: The QoR-40. Br. J. Anaesth. 2000, 84, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Wakita, T.; Fukuhara, S.; Nishiwada, M.; Inoue, S.; Kawaguchi, M.; Furuya, H. Validation of the Japanese version of the quality of recovery score QoR-40. J. Anaesth. 2011, 25, 509–515. [Google Scholar] [CrossRef]
- Gornall, B.F.; Myles, P.S.; Smith, C.L.; Burke, J.A.; Leslie, K.; Pereira, M.J.; Bost, J.E.; Kluivers, K.B.; Nilsson, U.G.; Tanaka, Y.; et al. Measurement of quality of recovery using the QoR-40: A quantitative systematic review. Br. J. Anaesth. 2013, 111, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, K.; Troedel, S.; Irwin, K.; Pearce, F.; Ugoni, A.; Gillies, R.; Pemberton, E.; Dharmage, S. Quality of recovery from anesthesia in neurosurgical patients. Anesthesiology 2003, 99, 1158–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myles, P.S.; Hunt, J.O.; Fletcher, H.; Solly, R.; Woodward, D.; Kelly, S. Relation between quality of recovery in hospital and quality of life at 3 months after cardiac surgery. Anesthesiology 2001, 95, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Kluivers, K.B.; Riphagen, I.; Vierhout, M.E.; Broölmann, H.A.; de Vet, H.C. Systematic review on recovery specific quality-of-life instruments. Surgery 2008, 143, 206–215. [Google Scholar] [CrossRef]
- Division of Cancer Treatment and Diagnosis NCI. Common Terminology Criteria for Adverse Events (CTCAE); Version v4.03; Cancer Therapy Evaluation Program: Bethesda, MD, USA, 2010. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Teshima, J.; Miyata, G.; Kamei, T.; Nakano, T.; Abe, S.; Katsura, K.; Taniyama, Y.; Sakurai, T.; Hikage, M.; Nakamura, T.; et al. Comparison of short-term outcomes between prone and lateral decubitus positions for thoracoscopic esophagectomy. Surg. Endosc. 2015, 29, 2756–2762. [Google Scholar] [CrossRef]
- Miyagawa, K.; Hayashi, Y.; Kurihara, S.; Maeda, A. Co-administration of l-cystine and l-theanine enhances efficacy of influenza vaccination in elderly persons: Nutritional status-dependent immunogenicity. Geriatr. Gerontol. Int. 2008, 8, 243–250. [Google Scholar] [CrossRef]
- Kurihara, S.; Hiraoka, T.; Akutsu, M.; Sukegawa, E.; Bannai, M.; Shibahara, S. Effects of (L)-cystine and (L)-theanine supplementation on the common cold: A randomized, double-blind, and placebo-controlled trial. J. Amino Acids 2010, 2010, 307475. [Google Scholar] [CrossRef] [Green Version]
- Kawada, S.; Kobayashi, K.; Ohtani, M.; Fukusaki, C. Cystine and theanine supplementation restores high-intensity resistance exercise-induced attenuation of natural killer cell activity in well-trained men. J. Strength Cond. Res. 2010, 24, 846–851. [Google Scholar] [CrossRef]
- Murakami, S.; Kurihara, S.; Koikawa, N.; Nakamura, A.; Aoki, K.; Yosigi, H.; Sawaki, K.; Ohtani, M. Effects of oral supplementation with cystine and theanine on the immune function of athletes in endurance exercise: Randomized, double-blind, placebo-controlled trial. Biosci. Biotechnol. Biochem. 2009, 73, 817–821. [Google Scholar] [CrossRef]
- Hayashi, K.; Yokoyama, Y.; Nakajima, H.; Nagino, M.; Inoue, T.; Nagaya, M.; Hattori, K.; Kadono, I.; Ito, S.; Nishida, Y. Preoperative 6-minute walk distance accurately predicts postoperative complications after operations for hepato-pancreato-biliary cancer. Surgery 2017, 161, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Schwartzman, K.; Carli, F.; Zavorsky, G.S.; Li, C.; Charlebois, P.; Stein, B.; Liberman, A.S.; Fried, G.M.; Feldman, L.S. The association of the distance walked in 6 min with pre-operative peak oxygen consumption and complications 1 month after colorectal resection. Anaesthesia 2013, 68, 811–816. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | CT * Group (n = 16) (%) | P * Group (n = 16) (%) | p-Value |
|---|---|---|---|
| Age (years) | 0.74 | ||
| Mean ± SE * | 68.4 ± 1.4 | 69.1 ± 1.4 | |
| (Range) | (58–78) | (52–78) | |
| Gender | 0.37 | ||
| Male | 14 (87.5) | 12 (75.0) | |
| Female | 2 (12.5) | 4 (25.0) | |
| Location | 0.051 | ||
| Upper third | 1 (6.3) | 3 (18.8) | |
| Middle third | 8 (50.0) | 7 (43.8) | |
| Lower third | 7 (43.8) | 2 (12.5) | |
| Abdominal | 0 (0.0) | 4 (25.0) | |
| Histological type | 0.69 | ||
| Squamous cell carcinoma | 13 (81.3) | 12 (75.0) | |
| Adenocarcinoma | 1 (6.3) | 3 (18.8) | |
| Other | 1 (6.3) | 1 (6.3) | |
| Clinical T | 0.65 | ||
| T1 | 4 (25.0) | 6 (37.5) | |
| T2 | 4 (25.0) | 2 (12.5) | |
| T3 | 8 (50.0) | 7 (43.8) | |
| T4a | 0 (0.0) | 1 (6.3) | |
| Clinical N | 0.43 | ||
| N0 | 5 (31.3) | 3 (18.8) | |
| N1 | 7 (43.8) | 11 (68.8) | |
| N2 | 4 (25.0) | 2 (12.5) | |
| Clinical Stage | 1 | ||
| Stage I | 4 (25.0) | 5 (31.3) | |
| Stage II | 5 (31.3) | 4 (25.0) | |
| Stage III | 7 (43.8) | 6 (37.5) | |
| Stage VI | 0 (0.0) | 1 (6.3) | |
| Pretreatment | 0.53 | ||
| None | 3 (18.8) | 5 (31.3) | |
| Chemotherapy | 10 (62.5) | 10 (62.5) | |
| Chemoradiotherapy | 3 (18.8) | 1 (6.3) | |
| Operation time (min) | 0.53 | ||
| Mean ± SE | 665 ± 19.0 | 682 ± 19.0 | |
| (Range) | (529–815) | (541–777) | |
| Blood loss (ml) | 0.074 | ||
| Mean ± SE | 463 ± 107 | 182 ± 107 | |
| (Range) | (16–2509) | (23–458) | |
| Complication (≥Grade 2) | |||
| Pneumonia | 2 (12.5) | 4 (25.0) | 0.37 |
| Anastomosis leakage | 2 (12.5) | 1 (6.3) | 0.54 |
| Colitis | 0 (0.0) | 2 (12.5) | 0.14 |
| Arrhythmia | 2 (12.5) | 0 (0.0) | 0.14 |
| Pyothorax | 0 (0.0) | 1 (6.3) | 0.31 |
| Chylothorax | 0 (0.0) | 1 (6.3) | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, H.; Taniyama, Y.; Sakurai, T.; Kodama, G.; Sato, C.; Fukutomi, T.; Ozawa, Y.; Ishida, H.; Koseki, K.; Yamauchi, T.; et al. Perioperative Administration of Cystine and Theanine Suppresses Inflammation and Facilitates Early Rehabilitation and Recovery after Esophagectomy: A Randomized, Double-Blind, Controlled Clinical Trial. Nutrients 2022, 14, 2319. https://doi.org/10.3390/nu14112319
Okamoto H, Taniyama Y, Sakurai T, Kodama G, Sato C, Fukutomi T, Ozawa Y, Ishida H, Koseki K, Yamauchi T, et al. Perioperative Administration of Cystine and Theanine Suppresses Inflammation and Facilitates Early Rehabilitation and Recovery after Esophagectomy: A Randomized, Double-Blind, Controlled Clinical Trial. Nutrients. 2022; 14(11):2319. https://doi.org/10.3390/nu14112319
Chicago/Turabian StyleOkamoto, Hiroshi, Yusuke Taniyama, Tadashi Sakurai, Gaku Kodama, Chiaki Sato, Toshiaki Fukutomi, Yohei Ozawa, Hirotaka Ishida, Ken Koseki, Takuro Yamauchi, and et al. 2022. "Perioperative Administration of Cystine and Theanine Suppresses Inflammation and Facilitates Early Rehabilitation and Recovery after Esophagectomy: A Randomized, Double-Blind, Controlled Clinical Trial" Nutrients 14, no. 11: 2319. https://doi.org/10.3390/nu14112319
APA StyleOkamoto, H., Taniyama, Y., Sakurai, T., Kodama, G., Sato, C., Fukutomi, T., Ozawa, Y., Ishida, H., Koseki, K., Yamauchi, T., Nakano, T., Unno, M., & Kamei, T. (2022). Perioperative Administration of Cystine and Theanine Suppresses Inflammation and Facilitates Early Rehabilitation and Recovery after Esophagectomy: A Randomized, Double-Blind, Controlled Clinical Trial. Nutrients, 14(11), 2319. https://doi.org/10.3390/nu14112319






