Comparison of the Effects of Intermittent Energy Restriction and Continuous Energy Restriction among Adults with Overweight or Obesity: An Overview of Systematic Reviews and Meta-Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Deviations from the Registered Protocol
2.3. Inclusion and Exclusion Criteria
2.3.1. Type of Included Reviews
- A systematic search strategy was used to guide literature retrieval;
- The criteria for included studies were explicit;
- More than two databases were searched;
- The outcomes of data extraction and quality assessment of included studies were finished and presented.
2.3.2. Type of Intervention
- 5:2 diet [28]: It consists of 2 days (consecutive or non-consecutive) of complete fasting or lower calorie intake than needed plus ad libitum eating on the other days per week;
- Alternate-day fasting, ADF [17]: It involves alternating ad libitum feeding days with fasting days. On fasting days, one is allowed to have a lower calorie intake than needed or complete fasting;
- Time-restricted feeding, TRF [17]: It involves following the same eating routine each day, with a certain number of hours designated as the fasting window and the remaining hours as the feeding window.
2.3.3. Participants
2.3.4. Outcomes
- Bodyweight (kg);
- Body mass index (kg/m2);
- Waist circumference (cm);
- Fat mass (kg);
- Fat-free mass (kg).
2.4. Search Methods
2.5. Selection and Data Extraction
2.6. Quality Assessment
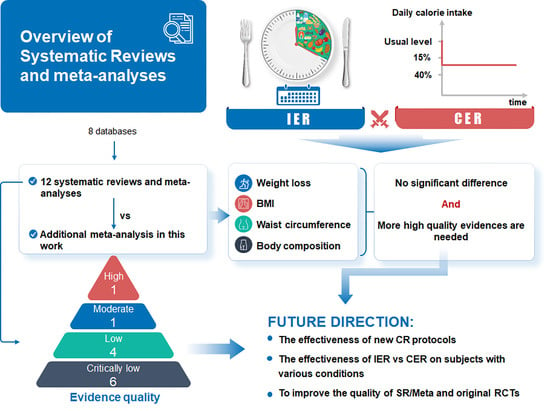
3. Results
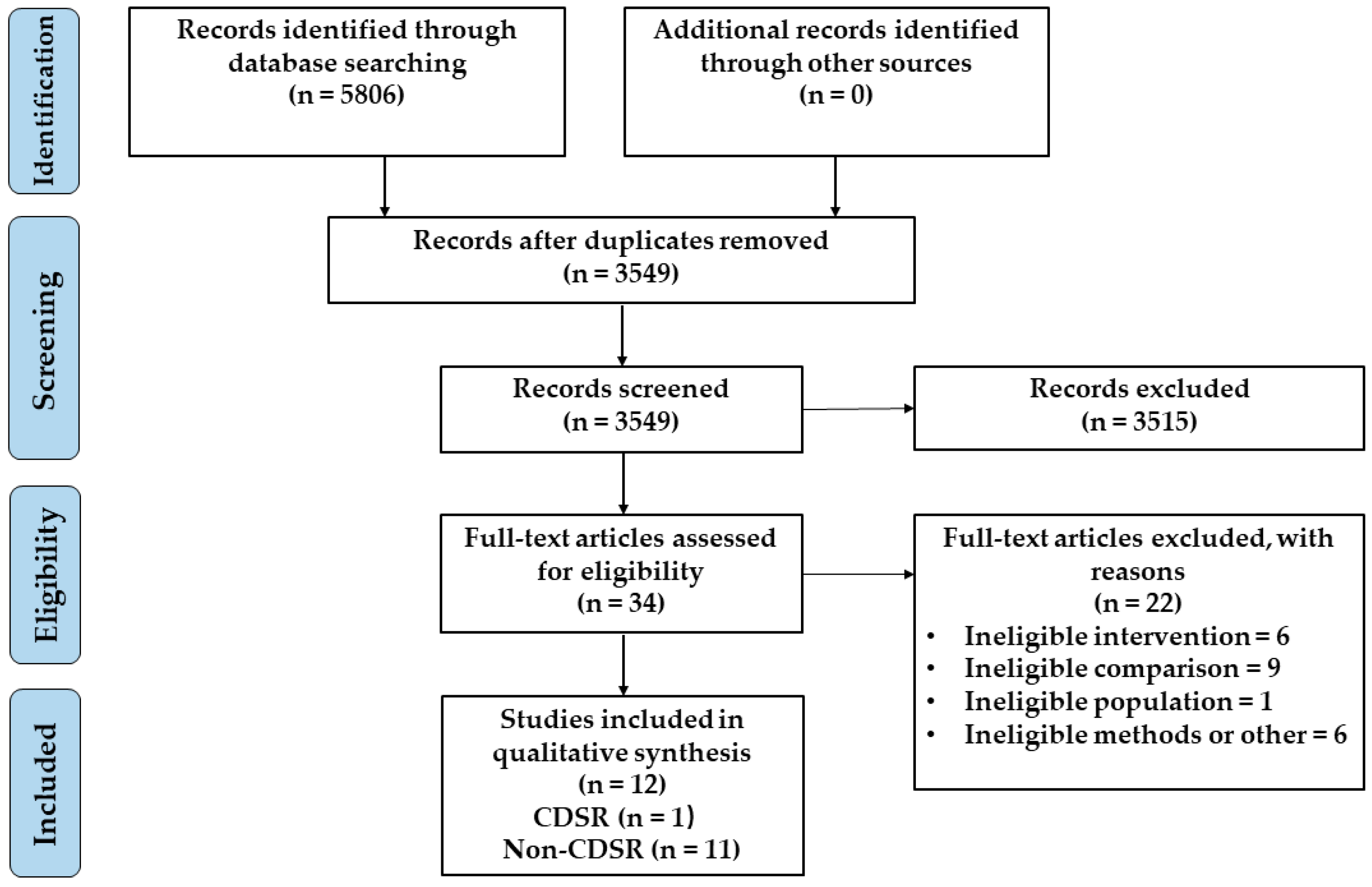
3.1. Results of the Search
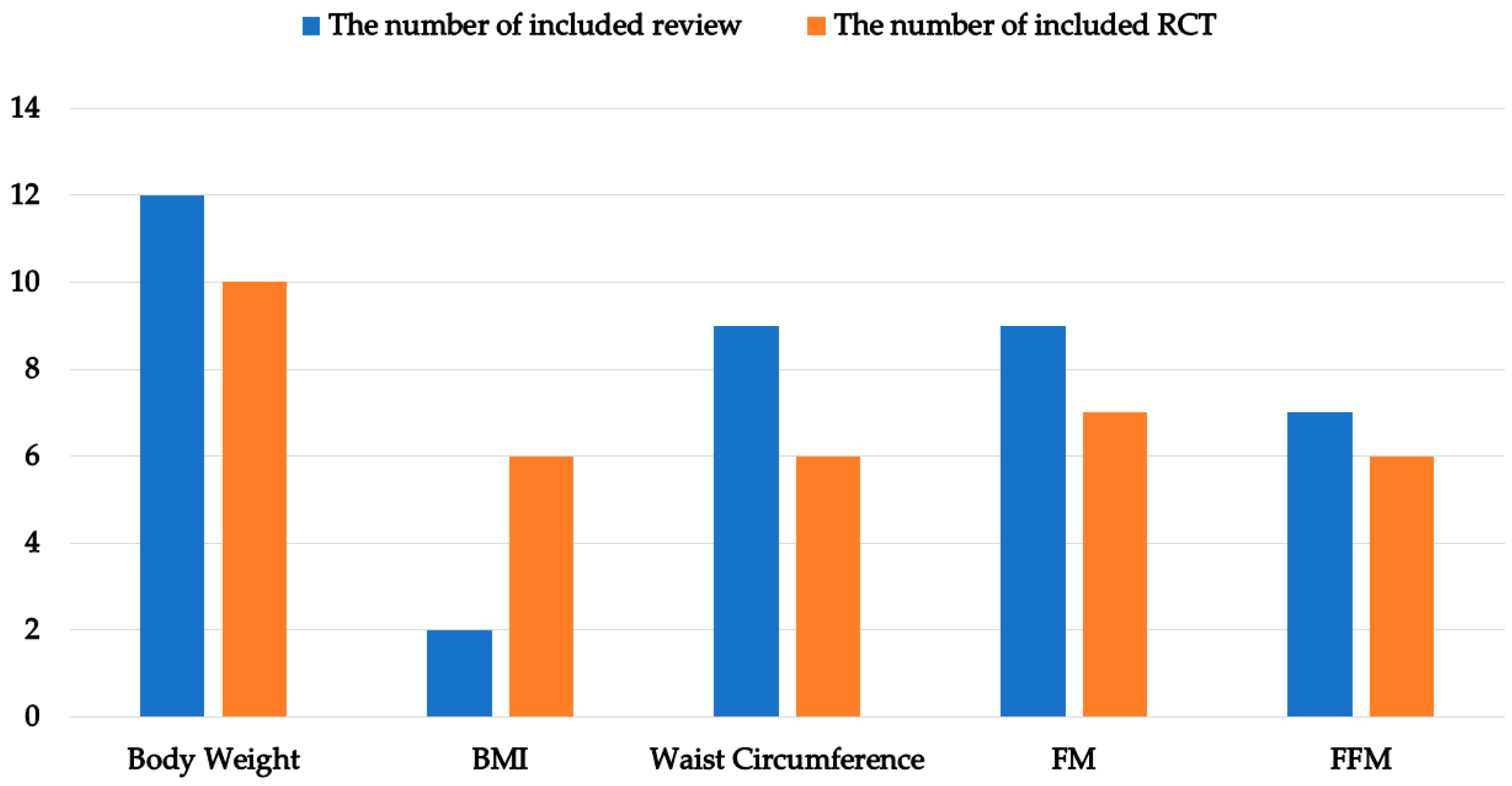
3.2. Characteristics of Included Reviews and RCTs
3.3. Outcomes for Reported Data of Included Reviews and Included RCTs
3.4. Summary of Findings from the Meta-Analyses of the Included Reviews and Results of Our Meta-Analysis
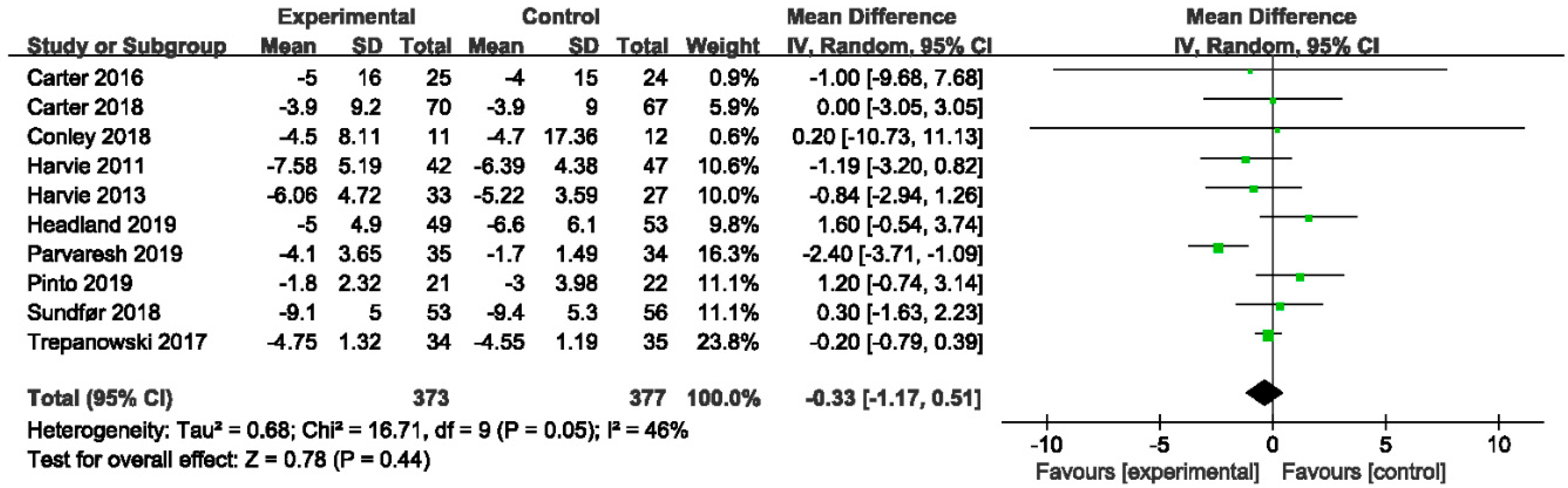
3.4.1. The Effect of IER vs. CER on Body Weight
3.4.2. The Effect of IER vs. CER on BMI
3.4.3. The Effect of IER vs. CER on Waist Circumference
3.4.4. The Effects of IER vs. CER on Body Composition
3.5. The Methodological Quality of the Included Reviews According to AMSTAR 2
3.6. Assessment of the Included RCTs According to ROB2
3.6.1. Allocation
3.6.2. Deviations from Intended Interventions
3.6.3. Missing Outcome Data
3.6.4. Measurement of the Outcome
3.6.5. Selection of the Reported Result
4. Discussion
4.1. Main Findings and Possible Explanations
4.2. Limitations of the Present Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | Alternate Day Fasting |
| AMSTAR | A Measurement Tool to Assess Systematic Reviews |
| BMI | Body Mass Index |
| CER | Continuous Energy Restriction |
| CR | Calorie Restriction |
| CVD | Cardiovascular Disease |
| CG | Control Group |
| CI | Confidence Interval |
| ER | Energy Restriction |
| EG | Experimental Group |
| FM | Fat Mass |
| FFM | Fat-Free Mass |
| GRADE | The Grading of Recommendations Assessment, Development and Evaluation |
| IER | Intermittent Energy Restriction |
| MD | Mean Difference |
| PRISMA | The Statement of Preferred Reporting Items for Systematic Review and Meta-analyses |
| PF | Periodic Fasting |
| RCT | Randomized Control Trial |
| ROB | The Cochrane Risk-of-Bias Tool |
| TRF | Time-Restricted Feeding |
| T2DM | Diabetes Mellitus Type 2 |
| VLED | Very Low Energy Diet |
| WHO | World Health Organization |
References
- Rapando, C.; Nyagero, J.; Wakhu, F. Feeding Habits associated with overweight and obesity amongst secondary School students in Private and Public schools in Langata Nairobi Kenya. IJSRP 2017, 7, 498–505. [Google Scholar]
- Hajek, A.; Brettschneider, C.; van der Leeden, C.; Lühmann, D.; Oey, A.; Wiese, B.; Weyerer, S.; Werle, J.; Fuchs, A.; Pentzek, M.; et al. Prevalence and factors associated with obesity among the oldest old. Arch. Gerontol. Geriatr. 2020, 89, 104069. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, W.M.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Lemamsha, H.; Randhawa, G.; Papadopoulos, C. Prevalence of Overweight and Obesity among Libyan Men and Women. BioMed Res. Int. 2019, 2019, 8531360. [Google Scholar] [CrossRef] [PubMed]
- Luhar, S.; Timæus, I.M.; Jones, R.; Cunningham, S.; Patel, S.A.; Kinra, S.; Clarke, L.; Houben, R. Forecasting the prevalence of overweight and obesity in India to 2040. PLoS ONE 2020, 15, e0229438. [Google Scholar]
- Koliaki, C.; Spinos, T.; Spinou, Μ.; Brinia, Μ.E.; Mitsopoulou, D.; Katsilambros, N. Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthcare 2018, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Norris, T.; Cole, T.J.; Bann, D.; Hamer, M.; Hardy, R.; Li, L.; Viner, R.; Johnson, W. Duration of obesity exposure between ages 10 and 40 years and its relationship with cardiometabolic disease risk factors: A cohort study. PLoS Med. 2020, 17, e1003387. [Google Scholar] [CrossRef]
- Bragg, F.; Li, L.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Chen, J.; Collins, R.; Peto, R.; Wang, C.; et al. Risks and Population Burden of Cardiovascular Diseases Associated with Diabetes in China: A Prospective Study of 0.5 Million Adults. PLoS Med. 2016, 13, e1002026. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, Y.; Zeng, X.; Wang, H.; Yin, P.; Wang, L.; Liu, Y.; Liu, J.; Qi, J.; Ran, S.; et al. Burden of Cardiovascular Diseases in China, 1990–2016: Findings From the 2016 Global Burden of Disease Study. JAMA Cardiol. 2019, 4, 342–352. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Global Burden of Disease. Obesity. 2017. Available online: https://ghdx.healthdata.org/gbd-results-tool (accessed on 16 December 2021).
- Halpern, B.; Mendes, T.B. Intermittent fasting for obesity and related disorders: Unveiling myths, facts, and presumptions. Arch. Endocrinol. Metab. 2021, 65, 14–23. [Google Scholar] [CrossRef]
- Pannen, S.T.; Maldonado, S.G.; Nonnenmacher, T.; Sowah, S.A.; Gruner, L.F.; Watzinger, C.; Nischwitz, K.; Ulrich, C.M.; Kaaks, R.; Schübel, R.; et al. Adherence and Dietary Composition during Intermittent vs. Continuous Calorie Restriction: Follow-Up Data from a Randomized Controlled Trial in Adults with Overweight or Obesity. Nutrients 2021, 13, 1195. [Google Scholar] [CrossRef]
- Maroofi, M.; Nasrollahzadeh, J. Effect of intermittent versus continuous calorie restriction on body weight and cardiometabolic risk markers in subjects with overweight or obesity and mild-to-moderate hypertriglyceridemia: A randomized trial. Lipids Health Dis. 2020, 19, 216. [Google Scholar] [CrossRef]
- Schubel, R.; Nattenmuller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Okechukwu, C.E. Intermittent energy restriction as a health promotion strategy to improve visceral adiposity and cardiometabolic health in obese older adults. J. Educ Health Promot. 2020, 9, 79. [Google Scholar] [CrossRef]
- Tinsley, G.M.; La Bounty, P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef]
- Cioffi, I.; Evangelista, A.; Ponzo, V.; Ciccone, G.; Soldati, L.; Santarpia, L.; Contaldo, F.; Pasanisi, F.; Ghigo, E.; Bo, S. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: A systematic review and meta-analysis of randomized controlled trials. J. Transl. Med. 2018, 16, 371. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.M.; Bordoli, C.; Buckner, L.P.; Kim, C.; Kaplan, P.C.; Del Arenal, I.M.; Jeffcock, E.J.; Hall, W.L. Intermittent energy restriction is comparable to continuous energy restriction for cardiometabolic health in adults with central obesity: A randomized controlled trial; the Met-IER study. Clin. Nutr. 2020, 39, 1753–1763. [Google Scholar] [CrossRef]
- Steger, F.L.; Donnelly, J.E.; Hull, H.R.; Li, X.; Hu, J.; Sullivan, D.K. Intermittent and continuous energy restriction result in similar weight loss, weight loss maintenance, and body composition changes in a 6 month randomized pilot study. Clin. Obes. 2021, 11, e12430. [Google Scholar] [CrossRef]
- Donna Ryan Heaner, M. Guidelines (2013) for managing overweight and obesity in adults. Obesity 2014, 22, S1–S410. [Google Scholar]
- Harvie, M.; Howell, A. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting amongst Obese, Overweight and Normal Weight Subjects-A Narrative Review of Human and Animal Evidence. Behav. Sci. 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enriquez Guerrero, A.; San Mauro Martin, I.; Garicano Vilar, E.; Camina Martin, M.A. Effectiveness of an intermittent fasting diet versus continuous energy restriction on anthropometric measurements, body composition and lipid profile in overweight and obese adults: A meta-analysis. Eur. J. Clin. Nutr. 2021, 75, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Seimon, R.V.; Roekenes, J.A.; Zibellini, J.; Zhu, B.; Gibson, A.A.; Hills, A.P.; Wood, R.E.; King, N.A.; Byrne, N.M.; Sainsbury, A. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol. Cell Endocrinol. 2015, 418, 153–172. [Google Scholar] [CrossRef] [Green Version]
- Roman, Y.M.; Dominguez, M.C.; Easow, T.M.; Pasupuleti, V.; White, C.M.; Hernandez, A.V. Effects of intermittent versus continuous dieting on weight and body composition in obese and overweight people: A systematic review and meta-analysis of randomized controlled trials. Int. J. Obes. 2019, 43, 2017–2027. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Jane, L.; Atkinson, G.; Jaime, V.; Hamilton, S.; Waller, G.; Harrison, S. Intermittent fasting interventions for the treatment of overweight and obesity in adults aged 18 years and over: A systematic review protocol. JBI Database Syst. Rev Implement Rep. 2015, 13, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Grade Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [Green Version]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Minozzi, S.; Dwan, K.; Borrelli, F.; Filippini, G. Reliability of the revised Cochrane risk-of-bias tool for randomised trials (RoB2) improved with the use of implementation instruction. J. Clin. Epidemiol. 2022, 141, 99–105. [Google Scholar] [CrossRef]
- Allaf, M.; Elghazaly, H.; Mohamed, O.G.; Fareen MF, K.; Zaman, S.; Salmasi, A.M.; Tsilidis, K.; Dehghan, A. Intermittent fasting for the prevention of cardiovascular disease. Cochrane. Database Syst. Rev. 2021, 1, CD013496. [Google Scholar] [CrossRef]
- Harris, L.; Hamilton, S.; Azevedo, L.B.; Olajide, J.; De Brun, C.; Waller, G.; Vicki, W.; Tracey, S.; Mike, L.; Catherine, H.; et al. Intermittent fasting interventions for the treatment of overweight and obesity in adults aged 18 years and over: A systematic review and meta-analysis. JBI Database Syst. Rev. Implement Rep. 2018, 16, 507–547. [Google Scholar] [CrossRef] [Green Version]
- Headland, M.; Clifton, P.M.; Carter, S.; Keogh, J.B. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Intermittent Energy Restriction Trials Lasting a Minimum of 6 Months. Nutrients 2016, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Zahringer, J.; Nitschke, K.; Torbahn, G.; Lohner, S.; Kuhn, T.; Fontanae, L.; Veronese, N.; Schmucker, C.; Meerpohl, J.J. Impact of intermittent energy restriction on anthropometric outcomes and intermediate disease markers in patients with overweight and obesity: Systematic review and meta-analyses. Crit. Rev. Food Sci. Nutr. 2021, 61, 1293–1304. [Google Scholar] [CrossRef]
- Vitale, R.; Kim, Y. The Effects of Intermittent Fasting on Glycemic Control and Body Composition in Adults with Obesity and Type 2 Diabetes: A Systematic Review. Metab. Syndr. Relat. Disord. 2020, 18, 450–461. [Google Scholar] [CrossRef]
- Davis, C.S.; Clarke, R.E.; Coulter, S.N.; Rounsefell, K.N.; Walker, R.E.; Rauch, C.E.; Huggins, C.E.; Ryan, L. Intermittent energy restriction and weight loss: A systematic review. Eur. J. Clin. Nutr. 2016, 70, 292–299. [Google Scholar] [CrossRef]
- Harris, L.; McGarty, A.; Hutchison, L.; Ells, L.; Hankey, C. Short-term intermittent energy restriction interventions for weight management: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Wang, J.; Zhang, J.; Xu, J. Intermittent Versus Continuous Energy Restriction for Weight Loss and Metabolic Improvement: A Meta-Analysis and Systematic Review. Obesity 2021, 29, 108–115. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Gabel, K.; Kroeger, C.M.; Trepanowski, J.F.; Hoddy, K.K.; Cienfuegos, S.; Kalam, F.; Varady, K.A. Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obesity 2019, 27, 1443–1450. [Google Scholar] [CrossRef]
- Parvaresh, A.; Razavi, R.; Abbasi, B.; Yaghoobloo, K.; Hassanzadeh, A.; Mohammadifard, N.; Safavi, S.M.; Hadi, A.; Clark, C.C.T. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: A randomized clinical trial. Complement Ther. Med. 2019, 47, 102187. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 122, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Res. Clin. Pract. 2019, 151, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conley, M.; Le Fevre, L.; Haywood, C.; Proietto, J. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutr. Diet. 2018, 75, 65–72. [Google Scholar] [CrossRef]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef] [Green Version]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef] [Green Version]
- Headland, M.L.; Clifton, P.M.; Keogh, J.B. Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. Int. J. Obes. 2019, 43, 2028–2036. [Google Scholar] [CrossRef]
- Sundfor, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.O.; Schlundt, D.G.; Sbrocco, T.; Sharp, T.; Popecordle, J.; Stetson, B.; Kaler, M.; Heim, C. Evaluation of an Alternating-Calorie Diet with and without Exercise in the Treatment of Obesity. Am. J. Clin. Nutr. 1989, 50, 248–254. [Google Scholar] [CrossRef]
- Panizza, C.E.; Lim, U.; Yonemori, K.M.; Cassel, K.D.; Wilkens, L.R.; Harvie, M.N.; Maskarinec, G.; Delp, E.; Lampe, J.W.; Shepherd, J.; et al. Effects of Intermittent Energy Restriction Combined with a Mediterranean Diet on Reducing Visceral Adiposity: A Randomized Active Comparator Pilot Study. Nutrients 2019, 11, 1386. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, S.R.; Halset, E.H.; Gasbakk, S.; Rehfeld, J.F.; Kulseng, B.; Truby, H.; Martins, C. Compensatory mechanisms activated with intermittent energy restriction: A randomized control trial. Clin. Nutr. 2018, 37, 815–823. [Google Scholar] [CrossRef]
- Byrne, N.M.; Sainsbury, A.; King, N.A.; Hills, A.P.; Wood, R.E. Intermittent energy restriction improves weight loss efficiency in obese men: The MATADOR study. Int. J. Obes. 2018, 42, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Ash, S.; Reeves, M.M.; Yeo, S.; Morrison, G.; Carey, D.; Capra, S. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with Type II diabetes: A randomised trial. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Arguin, H.; Dionne, I.J.; Senechal, M.; Bouchard, D.R.; Carpentier, A.C.; Ardilouze, J.L.; Tremblay, A.; Leblanc, C.; Brochu, M. Short-and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: A pilot study. Menopause 2012, 19, 870–876. [Google Scholar] [CrossRef]
- Huffman, K.M.; Redman, L.M.; Landerman, L.R.; Pieper, C.F.; Stevens, R.D.; Muehlbauer, M.J.; Wenner, B.R.; Bain, J.R.; Kraus, V.B.; Newgard, C.B.; et al. Caloric restriction alters the metabolic response to a mixed-meal: Results from a randomized, controlled trial. PLoS ONE 2012, 7, e28190. [Google Scholar] [CrossRef] [Green Version]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef]
- Wilhelmi de Toledo, F.; Grundler, F.; Sirtori, C.R.; Ruscica, M. Unravelling the health effects of fasting: A long road from obesity treatment to healthy life span increase and improved cognition. Ann. Med. 2020, 52, 147–161. [Google Scholar] [CrossRef]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Cardiometabolic Benefits of Intermittent Fasting. Annu. Rev. Nutr. 2021, 41, 333–361. [Google Scholar] [CrossRef]

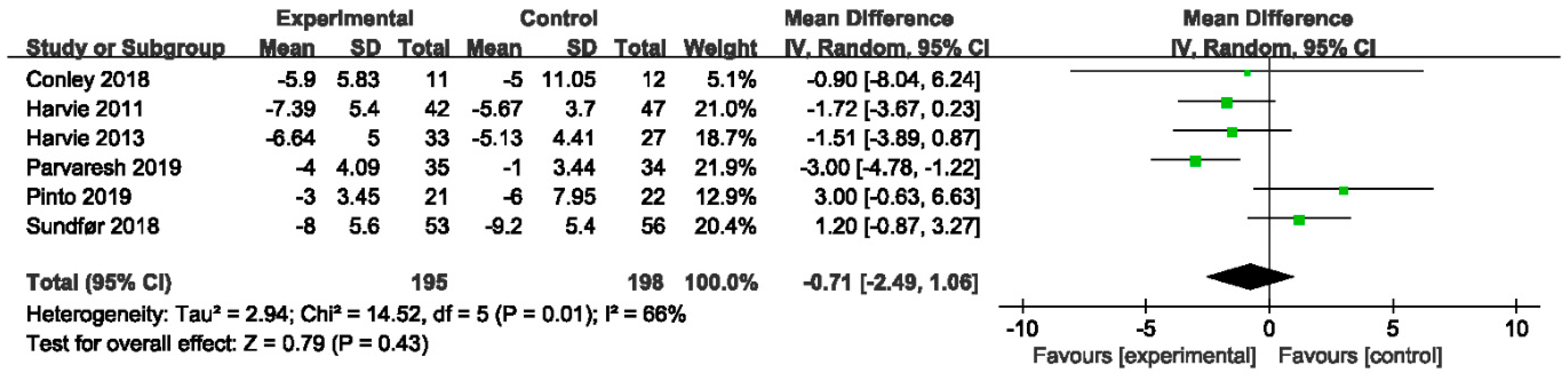

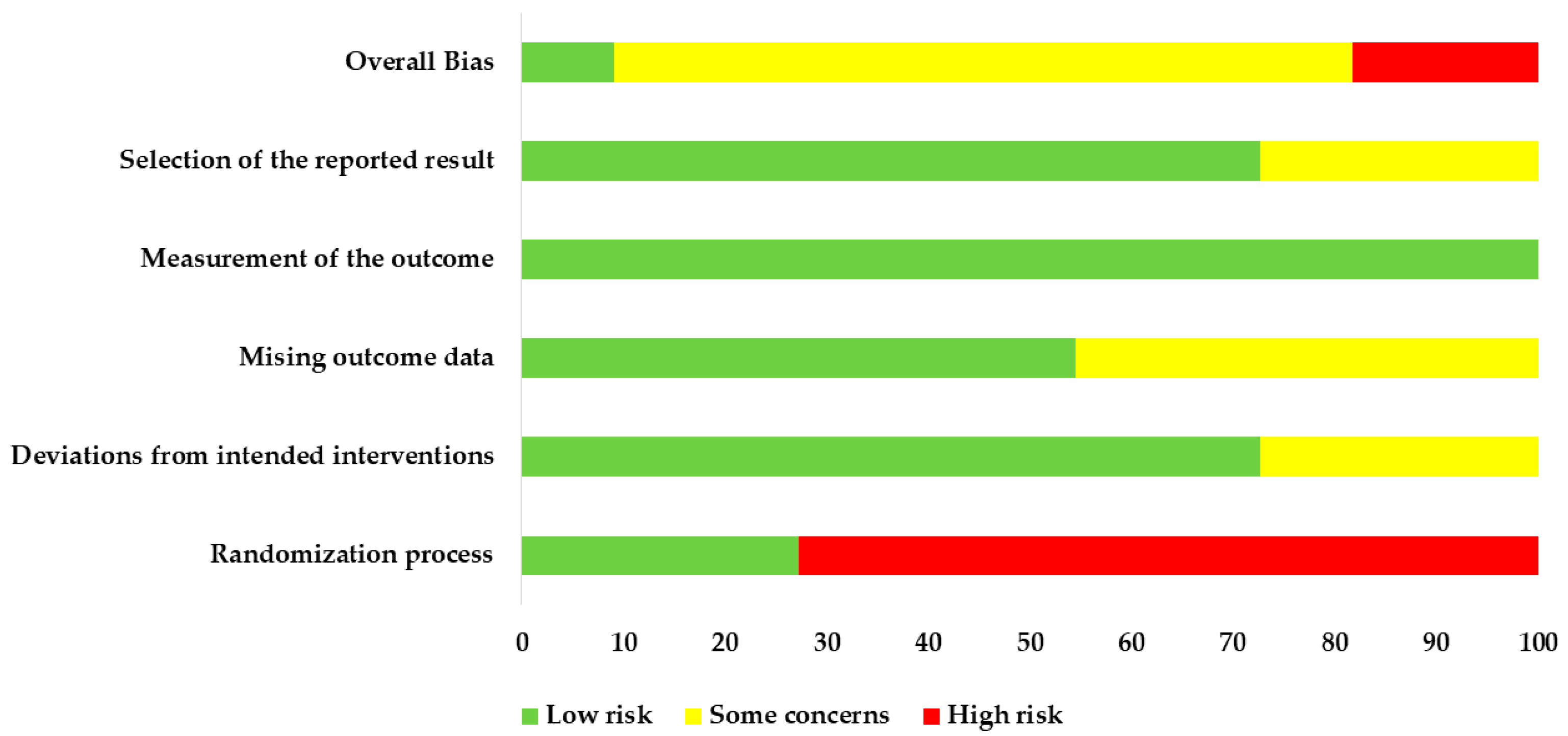
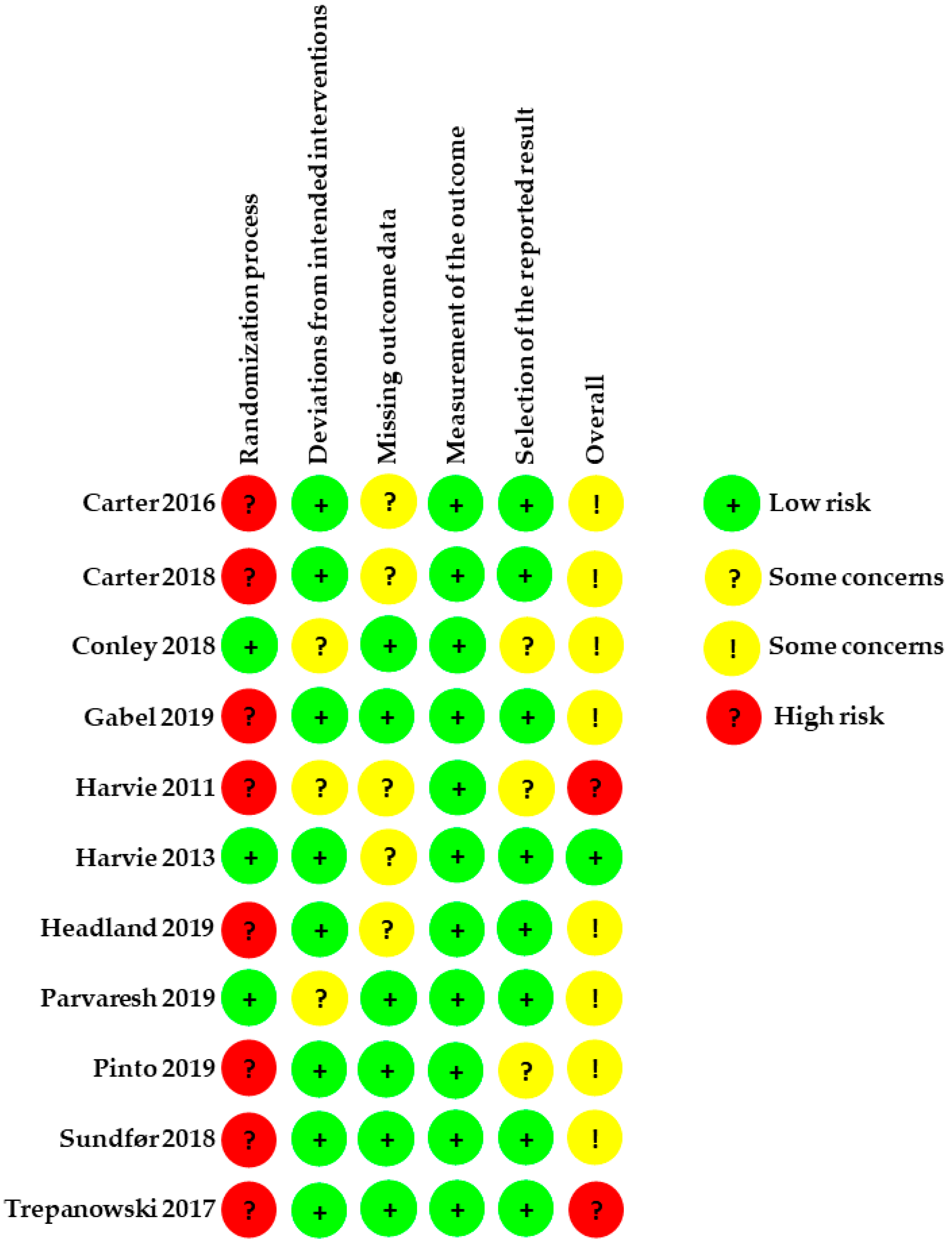
| Author, Year | Date of Search | No. Studies Included | Population | Intervention | Comparison Intervention | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Sample Size | Age (Year) | BMI (kg/m2) | Diet Form | Total Duration (Week) | Diet Form | Total Duration (Week) | |||
| Cochrane systematic review | ||||||||||
| Allaf, 2021 [32] | 2019.12 | 26 | 1. Male 2. Menopausal women with metabolic syndrome 3. Adults with T2DM 4. Professional cyclists 5. Adults with overweight or obesity | 1125 | 18–70 | 20–45 | IER: 5:2 diet/ADF/TRF/Other* | 16–96 | CER/Regular diet | 4–96 |
| Non-Cochrane systematic review | ||||||||||
| Seimon, 2015 [25] | 2014.11 | 12 | 1. Male with T2DM overweight/obesity 2. Female with overweight or obesity 3. Male with obesity and female 4. Male with obesity and female with T2DM | 1440 | 17–79 | 20–45 | IER: ADF/Other* | 5–50 | CER/Regular diet/No control | 5–50 |
| Davis, 2016 [37] | 2013.09 | 8 | 1. Adults with T2DM 2. Postmenopausal women 3. Premenopausal women | 390 | 34.3–61.8 | 28.6–37.3 | IER: 5:2 diet/Other* | 5–48 | CER/No control | 5–48 |
| Headland, 2016 [34] | 2016.04 | 9 | 1. All Female 2. All Male 3. All female and male 4. Female with T2DM | 981 | 18–70 | 24–40 | IER: 5:2 diet/Other* | 10–96 | CER/Regular diet/VLED/No control | 10–96 |
| Cioffi, 2018 [19] | 2018.05 | 11 | All adults with overweight/obesity (1) Men and women (2) All men (3) Adults with T2DM (4) Dysmetabolic conditions (5) All women | 630 | 30–71 | 24–46 | IER: 5:2 diet/ADF/Other* | 8–24 | CER/Regular diet/No control | 8–24 |
| Harris and Hamilton, 2018 [33] | 2015.11 | 6 | Adults with overweight or obesity, except adults with diabetes | 400 | 37–49 | 26–35.6 | IER: 5:2 diet/ADF/Other* | 12–48 | CER/Regular diet | 12–48 |
| Harris and McGarty, 2018 [38] | 2015.09 | 5 | All adults with overweight/obesity (1) Men and women (2) Adults with T2DM (3) All women | 376 | 42.6–61.0 | 33.1–44.6 | IER: Other* | 14–48 | CER/Regular diet | 14–48 |
| Roman, 2019 [26] | 2018.02 | 9 | 1. All adults with overweight/obesity 2. Only adults with diabetes 3. Excluded adults with diabetes | 782 | 39.6–61.5 | 24–45 | IER: 5:2 diet/Other* | 12–52 | CER/Regular diet | 12–52 |
| Vitale, 2020 [36] | 2020.01 | 5 | Adults with T2DM and obesity (had T2DM between 1 and 25 years in duration) | 351 | 46–71 | 27.6–41.8 | IER: 5:2 diet/Other* | 12–24 | CER/Mediterranean diet/ | 12–24 |
| Guerrero, 2021 [24] | 2019.01 | 18 | Adults with overweight/obesity | 1219 | 18–70 | ≥25 | IER: 5:2 diet/ADF/Other* | 6–48 | CER/Regular diet | 6–48 |
| He, 2021 [39] | 2019.12 | 11 | All adults with overweight/obesity (1) Men and women (2) Adults with T2DM (3) All women (4) All men | 850 | 28–71 | 26–43 | IER: 5:2 diet/ADF/Other* | 8–48 | CER/Regular diet | 8–48 |
| Schwingshackl, 2021 [35] | 2019.03 | 17 | 1. Adults with overweight/obesity 2. Adults with T2DM | 1328 | 31.7–67.6 | 26–35.3 | IER: 5:2 diet/ADF/Other* | 12–52 | CER/Regular diet | 12–52 |
| Author, Year | Population | Intervention | Comparison | Duration (Week) | ||||
|---|---|---|---|---|---|---|---|---|
| Type | Sample Size | BMI (kg/m2) | Age (Year) | IER Form | IER Protocol | CER Protocol | ||
| Carter, 2016 [43] | T2DM | EG:25 CG:24 | ≥27 | >18 | 5:2 Diet | 400–600 kcal/day on 2 fast days and regular diet on 5 feed days | 1200–1500 kcal/day | 12 |
| Carter, 2018 [44] | T2DM | EG:70 CG:67 | ≥27 | >18 | 5:2 Diet | 500–600 kcal/d for 2 days and regular diet for 5 days | 1200–1500 kcal/day | 96 |
| Conley, 2018 [45] | Male with no T2DM | EG:11 CG:12 | ≥30 | 55–75 | 5:2 Diet | 600 kcal/day on 2 fast days and regular diet on 5 feed days | 25% energy restriction every day | 24 |
| Gabel, 2019 [41] | Adults with no T2DM | EG:11 CG:17 | 25.0–39.9 | 18–65 | ADF | 25% of the energy need on fast days and 125% of energy needs on feed days | 75% energy needs every day | 48 |
| Harvie, 2011 [47] | Premenopausal women with no T2DM | EG:45 CG:47 | 24–40 | 30–45 | 5:2 Diet | 25% of the energy need on 2 fast days and regular diet for 5 days | 25% energy restriction every day | 24 |
| Harvie, 2013 [46] | Women with no T2DM | EG:33 CG:33 | 24–45 | 20–69 | 5:2 Diet | 25% of the energy need on 2 fast days and regular diet for 5 days | 25% energy restriction every day | 16 |
| Headland, 2019 [48] | Adults with no T2DM | EG:49 CG:53 | >25 | 18–72 | 5:2 Diet | 500/600 kcal (F/M) on 2 fast days and regular diet for 5 days | 1000–1200 kcal/day (F/M) | 48 |
| Parvaresh, 2019 [42] | Adults with no T2DM | EG:35 CG:34 | 25–40 | 25–60 | ADF | 25% energy needs on fast days; 100% needs on alternating feast days | 25% energy restriction every day | 8 |
| Pinto, 2019 [20] | Adults with no T2DM | EG:21 CG:22 | >25 | 35–75 | 5:2 Diet | 600 kcal on 2 fast days and regular diet for 5 days | 25% energy restriction every day | 4 |
| Sundfør, 2018 [49] | Adults with no T2DM | EG:54 CG:58 | 30–45 | 21–70 | 5:2 Diet | 400/600 kcal (F/M) on 2 fast days and regular diet for 5 days | 26–28% energy restriction every day | 48 |
| Trepanowski, 2017 [40] | Adults with no T2DM | EG:34 CG:35 | 25–39.9 | 18–64 | ADF | 25% of the energy need on fast days and 125% of energy needs on feed days | 25% energy restriction every day | 24 |
| Author, Year | Body Weight | BMI | Waist Circumference | FM | FFM |
|---|---|---|---|---|---|
| IER vs. CER (Analyzed separately from IER vs. regular diet) | |||||
| Harris and Hamilton, 2018 | 0.156 b | / | 0.002 b | 0.014 c | 0.958 b |
| Allaf, 2021 (≤12 weeks) | 0.05 b | 0.01 a | 0.20 a | / | / |
| Allaf, 2021 (>12 weeks) | 0.33 b | 0.51 b | 0.49 c | / | / |
| Schwingshackl, 2021 | 0.02 a | / | 0.25 a | 0.007 a | / |
| IER vs. CER (Not analyzed separately from IER vs. regular diet) | |||||
| Headland, 2016 | 0.458 c | / | / | / | / |
| Cioffi, 2018 | 0.27 a | / | 0.83 c | 0.66 a | 0.58 a |
| Harris and McGarty, 2018 | 0.15 c | / | / | / | / |
| Roman, 2019 | 0.29 a | / | 0.71 b | 0.56 b | 0.03 b |
| Guerrero, 2021 | N a | N a | N b | N c | N c |
| He, 2021 | 0.006 a | / | 0.61 b | 0.08 a | 0.09 a |
| Our meta-analysis | 0.44 | 0.14 | 0.43 | 0.98 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, F.; Chen, H.; Liu, L.; Zhang, S.; Luo, W.; Wang, G.; Hu, X. Comparison of the Effects of Intermittent Energy Restriction and Continuous Energy Restriction among Adults with Overweight or Obesity: An Overview of Systematic Reviews and Meta-Analyses. Nutrients 2022, 14, 2315. https://doi.org/10.3390/nu14112315
Wang J, Wang F, Chen H, Liu L, Zhang S, Luo W, Wang G, Hu X. Comparison of the Effects of Intermittent Energy Restriction and Continuous Energy Restriction among Adults with Overweight or Obesity: An Overview of Systematic Reviews and Meta-Analyses. Nutrients. 2022; 14(11):2315. https://doi.org/10.3390/nu14112315
Chicago/Turabian StyleWang, Jun, Fang Wang, Hongxiu Chen, Li Liu, Shuai Zhang, Wenjing Luo, Guan Wang, and Xiuying Hu. 2022. "Comparison of the Effects of Intermittent Energy Restriction and Continuous Energy Restriction among Adults with Overweight or Obesity: An Overview of Systematic Reviews and Meta-Analyses" Nutrients 14, no. 11: 2315. https://doi.org/10.3390/nu14112315
APA StyleWang, J., Wang, F., Chen, H., Liu, L., Zhang, S., Luo, W., Wang, G., & Hu, X. (2022). Comparison of the Effects of Intermittent Energy Restriction and Continuous Energy Restriction among Adults with Overweight or Obesity: An Overview of Systematic Reviews and Meta-Analyses. Nutrients, 14(11), 2315. https://doi.org/10.3390/nu14112315