The Association between Daily Dietary Intake of Riboflavin and Lung Function Impairment Related with Dibutyl Phthalate Exposure and the Possible Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Extraction
2.3. Exposure of Phthalate
2.4. Lung Function and Fractional Exhaled Nitrous Oxide
2.5. Daily Dietary Intake of Riboflavin
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Included Population
3.2. Subjects with Different Level of Urinary MBP
3.3. Effects of DBP Exposure on Lung Function Impairment and Blood Cells
3.4. Subgroup Analyses
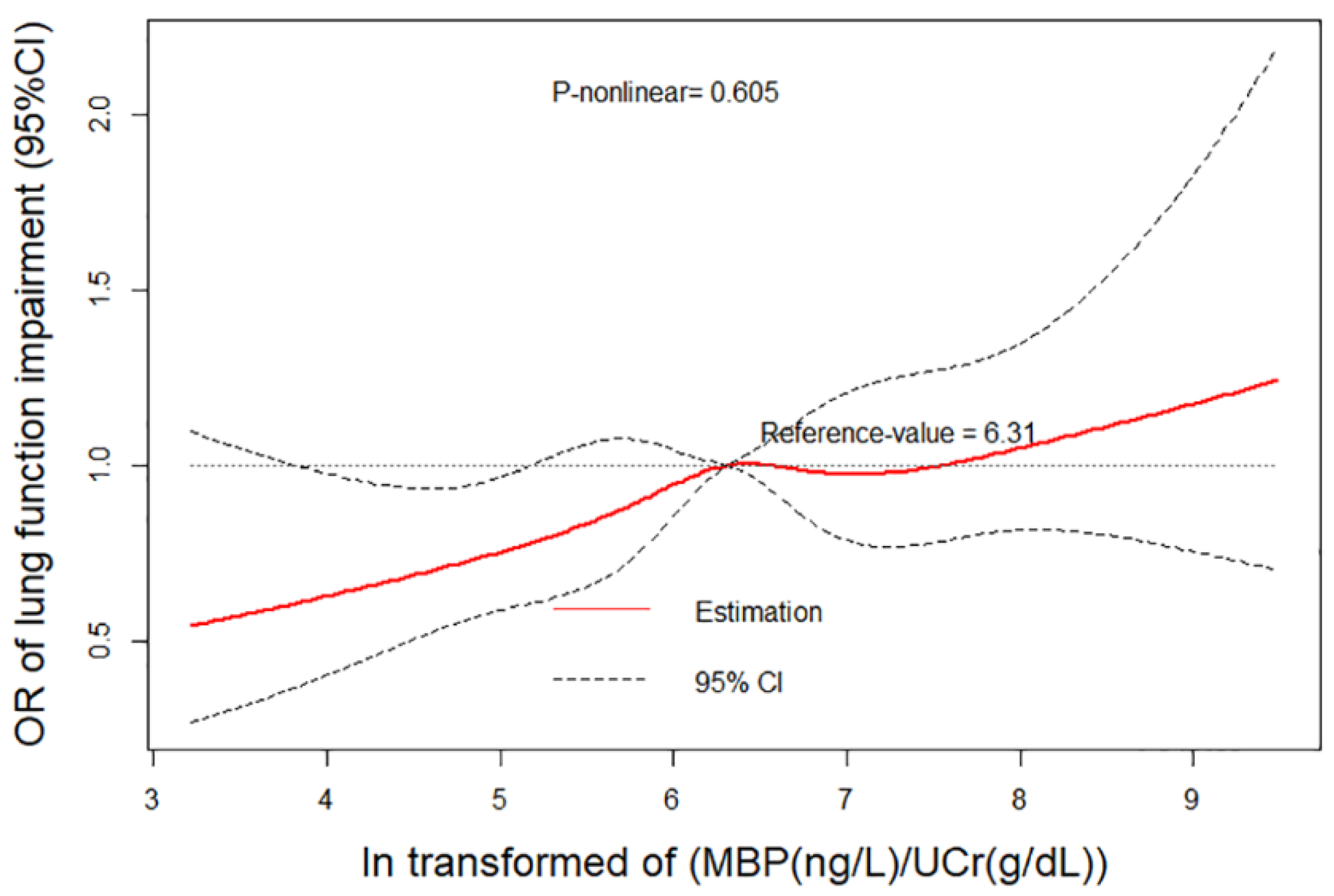
3.5. Relationship between DDIR and Lung Function Impairment Related with DBP Exposure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eales, J.; Bethel, A.; Galloway, T.; Hopkinson, P.; Morrissey, K.; Short, R.; Garside, R. Human health impacts of exposure to phthalate plasticizers: An overview of reviews. Environ Int. 2022, 158, 106903. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Reidy, J.A.; Preau, J.L.; Samandar, E.; Needham, L.L.; Calafat, A.M. Measurement of eight urinary metabolites of di(2-ethylhexyl) phthalate as biomarkers for human exposure assessment. Biomarkers 2006, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; Chevrier, C.; Hond, E.D.; Fernandez, M.F.; Pierik, F.; Philippat, C.; Slama, R.; Toft, G.; Vandentorren, S.; Wilhelm, M.; et al. Exposure to brominated flame retardants, perfluorinated compounds, phthalates and phenols in European birth cohorts: ENRIECO evaluation, first human biomonitoring results, and recommendations. Int. J. Hyg. Environ. Health 2013, 216, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kupsco, A.; Just, A.; Calafat, A.M.; Oken, E.; Braun, J.M.; Sanders, A.P.; Mercado-Garcia, A.; Cantoral, A.; Pantic, I.; et al. Maternal Phthalates Exposure and Blood Pressure during and after Pregnancy in the PROGRESS Study. Environ. Health Perspect. 2021, 129, 127007. [Google Scholar] [CrossRef]
- Gao, D.; Zou, Z.; Li, Y.; Chen, M.; Ma, Y.; Chen, L.; Wang, X.; Yang, Z.; Dong, Y.; Ma, J. Association between urinary phthalate metabolites and dyslipidemia in children: Results from a Chinese cohort study. Environ. Pollut. 2021, 295, 118632. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, Q.; Wang, X.; Wu, K.H. Urinary levels of Phthalate metabolite mixtures and pulmonary function in adolescents. Environ. Pollut. 2022, 293, 118595. [Google Scholar] [CrossRef]
- Schulz, C.O.; Rubin, R.J. Distribution, metabolism, and excretion of di-2-ethylhexyl phthalate in the rat. Environ. Health Perspect. 1973, 3, 123–129. [Google Scholar] [CrossRef]
- Abdi, S.; Sobhanardakani, S.; Lorestani, B.; Cheraghi, M.; Panahi, H.A. Analysis and health risk assessment of phthalate esters (PAEs) in indoor dust of preschool and elementary school centers in city of Tehran, Iran. Environ. Sci. Pollut. Res. Int. 2021, 28, 61151–61162. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hsu, Y.L.; Huang, M.S.; Tsai, M.J.; Ko, Y.C. Ginger suppresses phthalate ester-induced airway remodeling. J. Agric. Food Chem. 2011, 59, 3429–3438. [Google Scholar] [CrossRef]
- Preece, A.S.; Knutz, M.; Lindh, C.H.; Bornehag, C.G.; Shu, H. Prenatal phthalate exposure and early childhood wheeze in the SELMA study. J. Expo. Sci. Environ. Epidemiol. 2021, 32, 303–311. [Google Scholar] [CrossRef]
- Wang, X.; Han, B.; Wu, P.; Li, S.; Lv, Y.; Lu, J.; Yang, Q.; Li, J.; Zhu, Y.; Zhang, Z. Dibutyl phthalate induces allergic airway inflammation in rats via inhibition of the Nrf2/TSLP/JAK1 pathway. Environ. Pollut. 2020, 267, 115564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Han, M.; Ren, Y.; Yang, X.; Duan, L.; Zeng, Y.; Li, J. Dibutyl phthalate aggravated asthma-like symptoms through oxidative stress and increasing calcitonin gene-related peptide release. Ecotoxicol. Environ. Saf. 2020, 199, 110740. [Google Scholar] [CrossRef] [PubMed]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B₂) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Choi, H.; Kim, J. Association between Dietary Nutrient Intake and Chronic Obstructive Pulmonary Disease Severity: A Nationwide Population-Based Representative Sample. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 49–58. [Google Scholar] [CrossRef]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef]
- Osorio Ovarian Tumor Tissue Analysis (OTTA) Consortium; Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Toloczko, A.; Hein, A.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef] [Green Version]
- Sicińska, P. Di-n-butyl phthalate, butylbenzyl phthalate, and their metabolites exhibit different apoptotic potential in human peripheral blood mononuclear cells. Food Chem. Toxicol. 2019, 133, 110750. [Google Scholar] [CrossRef]
- Maestre-Batlle, D.; Huff, R.D.; Schwartz, C.; Alexis, N.E.; Tebbutt, S.J.; Turvey, S.; Bølling, A.K.; Carlsten, C. Dibutyl Phthalate Augments Allergen-induced Lung Function Decline and Alters Human Airway Immunology. A Randomized Crossover Study. Am. J. Respir. Crit. Care Med. 2020, 202, 672–680. [Google Scholar] [CrossRef]
- Grytting, V.S.; Olderbø, B.P.; Holme, J.A.; Samuelsen, J.T.; Solhaug, A.; Becher, R.; Bølling, A.K. Di-n-butyl phthalate modifies PMA-induced macrophage differentiation of THP-1 monocytes via PPARγ. Toxicol. Vitr. 2019, 54, 168–177. [Google Scholar] [CrossRef]
- Rajaram, M.V.; Brooks, M.N.; Morris, J.D.; Torrelles, J.B.; Azad, A.K.; Schlesinger, L.S. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J. Immunol. 2010, 185, 929–942. [Google Scholar] [CrossRef] [Green Version]
- Evren, E.; Ringqvist, E.; Tripathi, K.P.; Sleiers, N.; Rives, I.C.; Alisjahbana, A.; Gao, Y.; Sarhan, D.; Halle, T.; Sorini, C.; et al. Distinct developmental pathways from blood monocytes generate human lung macrophage diversity. Immunity 2021, 54, 259–275.e7. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.T.; Hansen, J.S.; Hansen, E.W.; Clausen, P.A.; Nielsen, G.D. Airway inflammation and adjuvant effect after repeated airborne exposures to di-(2-ethylhexyl)phthalate and ovalbumin in BALB/c mice. Toxicology 2007, 235, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Sapey, E.; Stockley, J.A.; Greenwood, H.; Ahmad, A.; Bayley, D.; Lord, J.M.; Insall, R.H.; Stockley, R.A. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 183, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Ayadhi, L.Y.; Alanazi, M.M.; Alfardan, A.S.; Attia, S.M.; Algahtani, M.; Bakheet, S. Dysregulated Nrf2 signaling in response to di(2-ethylhexyl) phthalate in neutrophils of children with autism. Int. Immunopharmacol. 2022, 106, 108619. [Google Scholar] [CrossRef] [PubMed]
- Karakoyun, B.; Ertaş, B.; Yüksel, M.; Akakın, D.; Çevik, Ö.; Şener, G. Ameliorative effects of riboflavin on acetic acid-induced colonic injury in rats. Clin. Exp. Pharmacol. Physiol. 2018, 45, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Seekamp, A.; Hultquist, D.E.; Till, G.O. Protection by vitamin B2 against oxidant-mediated acute lung injury. Inflammation 1999, 23, 449–460. [Google Scholar] [CrossRef]
- Sanches, S.C.; Ramalho, L.N.; Mendes-Braz, M.; Terra, V.A.; Cecchini, R.; Augusto, M.J.; Ramalho, F.S. Riboflavin (vitamin B-2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food Chem. Toxicol. 2014, 67, 65–71. [Google Scholar] [CrossRef]
| Total | Lung Function Impairment | p | ||
|---|---|---|---|---|
| No | Yes | |||
| Sample size | 4631 | 3889 | 742 | |
| Sex (men, %) | 2375 (51.28%) | 1943 (49.96%) | 432 (58.22%) | <0.001 |
| Age (years old) | <0.001 | |||
| 18–45 | 2344 (50.62%) | 2100 (54.00%) | 244 (32.88%) | |
| 45–60 | 1135 (24.51%) | 957 (24.61%) | 178 (23.99%) | |
| ≥60 | 1152 (24.88%) | 832 (21.39%) | 320 (43.13%) | |
| BMI (kg/m2) | <0.001 | |||
| <18.5 | 83 (1.79%) | 69 (1.78%) | 14 (1.90%) | |
| 18.5–25 | 1341 (28.96%) | 1081 (27.96%) | 260 (35.28%) | |
| 25–30 | 1534 (33.12%) | 1295 (33.50%) | 239 (32.43%) | |
| ≥30 | 1645 (35.52%) | 1421 (36.76%) | 224 (30.39%) | |
| Race | <0.001 | |||
| Mexican American | 734 (15.85%) | 667 (17.15%) | 67 (9.03%) | |
| Other Hispanic | 483 (10.43%) | 424 (10.90%) | 59 (7.95%) | |
| Non-Hispanic White | 1955 (42.22%) | 1535 (39.47%) | 420 (56.60%) | |
| Non-Hispanic Black | 1031 (22.26%) | 873 (22.45%) | 158 (21.29%) | |
| Other Race | 428 (9.24%) | 390 (10.03%) | 38 (5.12%) | |
| Education | 0.014 | |||
| Below high school | 1187 (25.65%) | 985 (25.35%) | 202 (27.26%) | |
| High school | 1078 (23.30%) | 882 (22.70%) | 196 (26.45%) | |
| Above high school | 2362 (51.05%) | 2019 (51.96%) | 343 (46.29%) | |
| Smoking status (yes, %) | <0.001 | |||
| Never | 2383 (54.79%) | 2140 (58.86%) | 243 (34.08%) | |
| Former | 977 (22.46%) | 758 (20.85%) | 219 (30.72%) | |
| Current | 989 (22.74%) | 738 (20.30%) | 251 (35.20%) | |
| Respiratory disease (yes, %) | 565 (12.20%) | 396 (10.18%) | 169 (22.78%) | <0.001 |
| History of diabetes (yes, %) | 466 (10.07%) | 373 (9.60%) | 93 (12.53%) | 0.015 |
| History of hypertension (yes, %) | 1376 (29.74%) | 1109 (28.54%) | 267 (36.03%) | <0.001 |
| Mono-benzyl phthalate | 6.32 ± 0.98 | 6.30 ± 0.98 | 6.40 ± 0.96 | 0.007 |
| Lung function impairment | 742 (16.02%) | 0 (0%) | 742 (100%) | |
| FeNO | 16.91 ± 15.05 | 16.67 ± 14.31 | 18.18 ± 18.39 | 0.039 |
| Blood cells | ||||
| Monocyte number (cells/uL) | 529.34 ± 183.65 | 524.46 ± 180.27 | 554.93 ± 198.65 | <0.001 |
| Neutrophils number (cells/uL) | 4259.81 ± 2071.89 | 4227.04 ± 2127.74 | 4431.69 ± 1741.19 | 0.006 |
| Daily dietary intake of riboflavin (mg) | 2.10 ± 1.35 | 2.09 ± 1.33 | 2.19 ± 1.44 | 0.077 |
| Characteristics | Quartile of ln(MBP/UCr), Range (Median) | Ptrend | |||
|---|---|---|---|---|---|
| 2.15~5.69 (5.23) | 5.69~6.31 (6.03) | 6.31~6.93 (6.59) | 6.93~10.20 (7.40) | ||
| Sample size | 1164 | 1155 | 1151 | 1161 | |
| Sex (men, %) | 689 (59.19%) | 615 (53.25%) | 572 (49.70%) | 499 (42.98%) | <0.001 |
| Age (years old) | <0.001 | ||||
| 18–45 | 564 (48.45%) | 526 (45.54%) | 589 (51.17%) | 665 (57.28%) | |
| 45–60 | 272 (23.37%) | 316 (27.36%) | 284 (24.67%) | 263 (22.65%) | |
| ≥60 | 328 (28.18%) | 313 (27.10%) | 278 (24.15%) | 233 (20.07%) | |
| BMI (kg/m2) | 0.715 | ||||
| <18.5 | 11 (0.90%) | 21 (1.87%) | 18 (1.72%) | 33 (2.99%) | |
| 18.5–25 | 339 (27.79%) | 310 (27.60%) | 351 (33.59%) | 341 (30.89%) | |
| 25–30 | 435 (35.66%) | 396 (35.26%) | 338 (32.34%) | 365 (33.06%) | |
| ≥30 | 373 (30.57%) | 426 (37.93%) | 434 (41.53%) | 412 (37.32%) | |
| Race | <0.001 | ||||
| Mexican American | 198 (17.01%) | 158 (13.68%) | 192 (16.68%) | 186 (16.02%) | |
| Other Hispanic | 126 (10.82%) | 131 (11.34%) | 109 (9.47%) | 117 (10.08%) | |
| Non-Hispanic White | 421 (36.17%) | 466 (40.35%) | 508 (44.14%) | 560 (48.23%) | |
| Non-Hispanic Black | 271 (23.28%) | 282 (24.42%) | 255 (22.15%) | 223 (19.21%) | |
| Other Race | 148 (12.71%) | 118 (10.22%) | 87 (7.56%) | 75 (6.46%) | |
| Education | <0.001 | ||||
| Below high school | 258 (22.18%) | 271 (23.46%) | 319 (27.72%) | 339 (29.27%) | |
| High school | 236 (20.29%) | 255 (22.08%) | 301 (26.15%) | 286 (24.70%) | |
| Above high school | 669 (57.52%) | 629 (54.46%) | 531 (46.13%) | 533 (46.03%) | |
| Smoking status (yes, %) | <0.001 | ||||
| Never | 640 (57.92%) | 608 (55.37%) | 583 (54.38%) | 552 (51.40%) | |
| Former | 276 (24.98%) | 261 (23.77%) | 245 (22.85%) | 195 (18.16%) | |
| Current | 189 (17.10%) | 229 (20.86%) | 244 (22.76%) | 327 (30.45%) | |
| Respiratory disease (yes, %) | 128 (11.00%) | 131 (11.34%) | 142 (12.34%) | 164 (14.13%) | 0.016 |
| History of diabetes (yes, %) | 114 (9.80%) | 127 (11.01%) | 120 (10.43%) | 105 (9.04%) | 0.470 |
| History of hypertension (yes, %) | 362 (31.10%) | 364 (31.57%) | 334 (29.07%) | 316 (27.22%) | 0.018 |
| Lung function impairment | 155 (13.32%) | 187 (16.19%) | 200 (17.38%) | 200 (17.23%) | 0.007 |
| FeNO | 197 (17.78%) | 183 (16.73%) | 170 (15.47%) | 168 (15.40%) | 0.092 |
| Monocyte number (cells/uL) | 510.29 ± 172.93 | 519.58 ± 178.28 | 540.40 ± 186.68 | 547.01 ± 193.75 | <0.001 |
| Neutrophils number (cells/uL) | 4028.43 ± 1513.40 | 4172.29 ± 1756.88 | 4298.82 ± 1759.86 | 4537.27 ± 2918.24 | <0.001 |
| Outcomes | Model | Quartiles of ln(MBP/UCr), Range (Median) | Ptrend | |||
|---|---|---|---|---|---|---|
| 2.15~5.69 (5.23) | 5.69~6.31 (6.03) | 6.31~6.93 (6.59) | 6.93~10.20 (7.40) | |||
| Lung function impairment | Model 1 | 1.00 (Reference) | 1.26 (1.00, 1.58) | 1.37 (1.09, 1.72) | 1.35 (1.08, 1.70) | 0.007 |
| Model 2 | 1.00 (Reference) | 1.23 (0.97, 1.56) | 1.36 (1.08, 1.73) | 1.44 (1.14, 1.83) | 0.002 | |
| Model 3 | 1.00 (Reference) | 1.25 (0.98, 1.61) | 1.35 (1.05, 1.73) | 1.36 (1.06, 1.75) | 0.015 | |
| Model 4 | 1.00 (Reference) | 1.24 (0.97, 1.59) | 1.34 (1.05, 1.72) | 1.35 (1.05, 1.73) | 0.017 | |
| High monocyte | Model 1 | 1.00 (Reference) | 1.20 (0.97, 1.48) | 1.47 (1.20, 1.81) | 1.43 (1.16, 1.76) | <0.001 |
| Model 2 | 1.00 (Reference) | 1.20 (0.96, 1.49) | 1.42 (1.15, 1.76) | 1.40 (1.13, 1.74) | 0.001 | |
| Model 3 | 1.00 (Reference) | 1.25 (0.99, 1.56) | 1.37 (1.10, 1.72) | 1.29 (1.02, 1.62) | 0.023 | |
| Model 4 | 1.00 (Reference) | 1.27 (1.02, 1.60) | 1.41 (1.13, 1.77) | 1.32 (1.05, 1.66) | 0.011 | |
| High neutrophil | Model 1 | 1.00 (Reference) | 1.16 (0.95, 1.41) | 1.32 (1.08, 1.60) | 1.66 (1.37, 2.01) | <0.001 |
| Model 2 | 1.00 (Reference) | 1.14 (0.93, 1.39) | 1.21 (0.99, 1.48) | 1.46 (1.20, 1.78) | <0.001 | |
| Model 3 | 1.00 (Reference) | 1.13 (0.92, 1.40) | 1.16 (0.94, 1.44) | 1.29 (1.04, 1.59) | 0.022 | |
| Model 4 | 1.00 (Reference) | 1.16 (0.94, 1.43) | 1.20 (0.98, 1.49) | 1.33 (1.08, 1.64) | 0.008 | |
| Subgroup Variable | OR quartile 4 vs. 1 (95% CI) | p | Pinteraction |
|---|---|---|---|
| Sex | |||
| woman | 1.33 (0.90, 2.00) | 0.160 | 0.752 |
| man | 1.35 (0.94, 1.93) | 0.102 | |
| Age (years old) | |||
| 18–45 | 1.20 (0.76, 1.90) | 0.435 | |
| 45–60 | 1.62 (0.94, 2.82) | 0.082 | 0.451 |
| ≥60 | 1.36 (0.88, 2.09) | 0.166 | 0.975 |
| Race | |||
| Mexican American | 1.07 (0.51, 2.26) | 0.855 | |
| Other Hispanic | 4.25 (1.40, 14.53) | 0.142 | 0.309 |
| Non-Hispanic White | 1.57 (1.08, 2.29) | 0.020 | 0.406 |
| Non-Hispanic Black | 1.51 (0.85, 2.70) | 0.161 | 0.950 |
| Other Race | 0.35 (0.06, 1.40) | 0.172 | 0.164 |
| Education | |||
| Below high school | 1.45 (0.84, 2.54) | 0.181 | |
| High school | 1.45 (0.84, 2.55) | 0.183 | 0.834 |
| Above high school | 1.40 (0.97, 2.04) | 0.075 | 0.625 |
| History of diabetes | |||
| No | 1.46 (1.10, 1.94) | 0.009 | |
| Yes | 0.84 (0.38, 1.85) | 0.670 | 0.126 |
| Respiratory disease | |||
| No | 1.47 (1.09, 1.98) | 0.012 | |
| Yes | 1.05 (0.58, 1.90) | 0.933 | 0.409 |
| Smoke status | |||
| Never | 1.70 (1.10, 2.63) | 0.017 | |
| Former | 1.46 (0.87, 2.44) | 0.154 | 0.474 |
| Current | 1.08 (0.68, 1.73) | 0.748 | 0.232 |
| History of hypertension | |||
| No | 1.64 (1.17, 2.31) | 0.006 | |
| Yes | 1.05 (0.68, 1.62) | 0.999 | 0.031 |
| Daily dietary intake of riboflavin (mg) | |||
| Low/normal (≤1.8) | 1.85 (1.25, 2.75) | 0.004 | |
| Higher (>1.8) | 1.07 (0.74, 1.54) | 0.717 | 0.026 |
| Outcomes | Model | Quartile of ln(MBP/UCr), Range (Median) | Ptrend | ||||
|---|---|---|---|---|---|---|---|
| 2.15~5.69 (5.23) | 5.69~6.31 (6.03) | 6.31~6.93 (6.59) | 6.93~10.20 (7.40) | ||||
| Low/normal DDIR (n = 2178) | Lung function impairment | Model 1 | 1.00 (Reference) | 1.59 (1.12, 2.26) | 1.63 (1.15, 2.31) | 1.80 (1.28, 2.54) | 0.002 |
| Model 2 | 1.00 (Reference) | 1.43 (1.00, 2.05) | 1.54 (1.08, 2.21) | 1.94 (1.36, 2.79) | <0.001 | ||
| Model 3 | 1.00 (Reference) | 1.41 (0.97, 2.06) | 1.40 (0.96, 2.05) | 1.85 (1.27, 2.70) | 0.003 | ||
| Model 4 | 1.00 (Reference) | 1.41 (0.97, 2.06) | 1.44 (0.99, 2.11) | 1.85 (1.27, 2.71) | 0.002 | ||
| High neutrophils | Model 1 | 1.00 (Reference) | 1.22 (0.91, 1.65) | 1.47 (1.10, 1.97) | 1.73 (1.30, 2.30) | <0.001 | |
| Model 2 | 1.00 (Reference) | 1.27 (0.94, 1.72) | 1.39 (1.04, 1.87) | 1.56 (1.17, 2.09) | 0.003 | ||
| Model 3 | 1.00 (Reference) | 1.28 (0.93, 1.76) | 1.33 (0.97, 1.82) | 1.42 (1.04, 1.95) | 0.032 | ||
| Model 4 | 1.00 (Reference) | 1.26 (0.92, 1.73) | 1.37 (1.00, 1.87) | 1.45 (1.06, 1.97) | 0.018 | ||
| High monocytes | Model 1 | 1.00 (Reference) | 1.11 (0.80, 1.54) | 1.37 (1.00, 1.88) | 1.42 (1.04, 1.94) | 0.013 | |
| Model 2 | 1.00 (Reference) | 1.12 (0.80, 1.56) | 1.30 (0.94, 1.80) | 1.42 (1.03, 1.97) | 0.021 | ||
| Model 3 | 1.00 (Reference) | 1.13 (0.80, 1.61) | 1.23 (0.87, 1.74) | 1.22 (0.87, 1.73) | 0.225 | ||
| Model 4 | 1.00 (Reference) | 1.14 (0.81, 1.62) | 1.26 (0.89, 1.77) | 1.24 (0.88, 1.75) | 0.193 | ||
| High DDIR (n = 2251) | Lung function impairment | Model 1 | 1.00 (Reference) | 1.12 (0.80, 1.55) | 1.18 (0.86, 1.63) | 1.09 (0.78, 1.51) | 0.570 |
| Model 2 | 1.00 (Reference) | 1.14 (0.81, 1.61) | 1.26 (0.90, 1.77) | 1.15 (0.82, 1.63) | 0.350 | ||
| Model 3 | 1.00 (Reference) | 1.20 (0.84, 1.72) | 1.32 (0.93, 1.89) | 1.10 (0.76, 1.58) | 0.531 | ||
| Model 4 | 1.00 (Reference) | 1.17 (0.82, 1.66) | 1.29 (0.91, 1.83) | 1.07 (0.75, 1.53) | 0.616 | ||
| High neutrophils | Model 1 | 1.00 (Reference) | 1.15 (0.87, 1.52) | 1.22 (0.92, 1.61) | 1.61 (1.23, 2.11) | 0.001 | |
| Model 2 | 1.00 (Reference) | 1.07 (0.80, 1.42) | 1.08 (0.82, 1.44) | 1.39 (1.05, 1.83) | 0.024 | ||
| Model 3 | 1.00 (Reference) | 1.10 (0.81, 1.49) | 1.06 (0.78, 1.44) | 1.25 (0.92, 1.68) | 0.195 | ||
| Model 4 | 1.00 (Reference) | 1.16 (0.86, 1.56) | 1.11 (0.82, 1.49) | 1.30 (0.97, 1.75) | 0.119 | ||
| High monocytes | Model 1 | 1.00 (Reference) | 1.24 (0.93, 1.66) | 1.50 (1.13, 1.99) | 1.38 (1.04, 1.85) | 0.012 | |
| Model 2 | 1.00 (Reference) | 1.21 (0.90, 1.63) | 1.44 (1.08, 1.93) | 1.34 (0.99, 1.80) | 0.031 | ||
| Model 3 | 1.00 (Reference) | 1.28 (0.94, 1.75) | 1.40 (1.03, 1.92) | 1.34 (0.98, 1.83) | 0.065 | ||
| Model 4 | 1.00 (Reference) | 1.34 (0.99, 1.83) | 1.45 (1.07, 1.97) | 1.37 (1.00, 1.87) | 0.049 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Cheng, S.; Zhang, J.; Yuan, S.; Zhang, L.; Wu, J.; Chen, J.; Tang, M.; Hu, Y.; Tong, S.; et al. The Association between Daily Dietary Intake of Riboflavin and Lung Function Impairment Related with Dibutyl Phthalate Exposure and the Possible Mechanism. Nutrients 2022, 14, 2282. https://doi.org/10.3390/nu14112282
Lin J, Cheng S, Zhang J, Yuan S, Zhang L, Wu J, Chen J, Tang M, Hu Y, Tong S, et al. The Association between Daily Dietary Intake of Riboflavin and Lung Function Impairment Related with Dibutyl Phthalate Exposure and the Possible Mechanism. Nutrients. 2022; 14(11):2282. https://doi.org/10.3390/nu14112282
Chicago/Turabian StyleLin, Jilei, Siying Cheng, Jing Zhang, Shuhua Yuan, Lei Zhang, Jinhong Wu, Jiande Chen, Mingyu Tang, Yabin Hu, Shilu Tong, and et al. 2022. "The Association between Daily Dietary Intake of Riboflavin and Lung Function Impairment Related with Dibutyl Phthalate Exposure and the Possible Mechanism" Nutrients 14, no. 11: 2282. https://doi.org/10.3390/nu14112282
APA StyleLin, J., Cheng, S., Zhang, J., Yuan, S., Zhang, L., Wu, J., Chen, J., Tang, M., Hu, Y., Tong, S., Zhao, L., & Yin, Y. (2022). The Association between Daily Dietary Intake of Riboflavin and Lung Function Impairment Related with Dibutyl Phthalate Exposure and the Possible Mechanism. Nutrients, 14(11), 2282. https://doi.org/10.3390/nu14112282






