Phase Angle as a Comprehensive Tool for Nutritional Monitoring and Management in Patients with Crohn’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Design
2.2. Clinical Measurements
2.3. BIA, BMI, FFMI, and PhA
2.4. Malnutrition Diagnosis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.L.; Loftus, E.V.; Kappelman, M.D. Effects of Race and Ethnicity on Diagnosis and Management of Inflammatory Bowel Diseases. Gastroenterology 2021, 160, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, C.F.; Yann, L.H.; Lal, S. Nutritional Management of Crohn’s Disease. Ther. Adv. Gastroenterol. 2013, 6, 231–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balestrieri, P.; Ribolsi, M.; Guarino, M.P.L.; Emerenziani, S.; Altomare, A.; Cicala, M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattiello, R.; Amaral, M.A.; Mundstock, E.; Ziegelmann, P.K. Reference Values for the Phase Angle of the Electrical Bioimpedance: Systematic Review and Meta-Analysis Involving More than 250,000 Subjects. Clin. Nutr. 2020, 39, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Munsell, M.; Harris, M.L. Nationwide Prevalence and Prognostic Significance of Clinically Diagnosable Protein-Calorie Malnutrition in Hospitalized Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2008, 14, 1105–1111. [Google Scholar] [CrossRef]
- Koroušić Seljak, B.; Mlakar Mastnak, D.; Mrevlje, Ž.; Veninšek, G.; Rotovnik Kozjek, N. A Multi-Center Survey on Hospital Malnutrition and Cachexia in Slovenia. Eur. J. Clin. Nutr. 2020, 74, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical Phase Angle and Impedance Vector Analysis—Clinical Relevance and Applicability of Impedance Parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of Adult Malnutrition and Prognosis with Bioelectrical Impedance Analysis: Phase Angle and Impedance Ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef]
- Scicchitano, P.; Ciccone, M.M.; Passantino, A.; Valle, R.; De Palo, M.; Sasanelli, P.; Sanasi, M.; Piscopo, A.; Guida, P.; Caldarola, P.; et al. Congestion and Nutrition as Determinants of Bioelectrical Phase Angle in Heart Failure. Heart Lung 2020, 49, 724–728. [Google Scholar] [CrossRef]
- Gerken, A.L.H.; Rohr-Kräutle, K.-K.; Weiss, C.; Seyfried, S.; Reissfelder, C.; Vassilev, G.; Otto, M. Handgrip Strength and Phase Angle Predict Outcome After Bariatric Surgery. Obes. Surg. 2021, 31, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, M.; Kon, S.S.C.; Jones, S.E.; Canavan, J.L.; Nolan, C.M.; Higginson, I.J.; Gao, W.; Polkey, M.I.; Man, W.D.-C. Bioelectrical Impedance Phase Angle Relates to Function, Disease Severity and Prognosis in Stable Chronic Obstructive Pulmonary Disease. Clin. Nutr. 2015, 34, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-K.; Lim, J.-Y. Phase Angle as a Predictor of Functional Outcomes in Patients Undergoing In-Hospital Rehabilitation after Hip Fracture Surgery. Arch. Gerontol. Geriatr. 2020, 89, 104060. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, I.; Marra, M.; Imperatore, N.; Pagano, M.C.; Santarpia, L.; Alfonsi, L.; Testa, A.; Sammarco, R.; Contaldo, F.; Castiglione, F.; et al. Assessment of Bioelectrical Phase Angle as a Predictor of Nutritional Status in Patients with Crohn’s Disease: A Cross Sectional Study. Clin. Nutr. 2020, 39, 1564–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerenziani, S.; Biancone, L.; Guarino, M.P.L.; Balestrieri, P.; Stasi, E.; Ribolsi, M.; Rescio, M.P.; Altomare, A.; Cocca, S.; Pallone, F.; et al. Nutritional Status and Bioelectrical Phase Angle Assessment in Adult Crohn Disease Patients Receiving Anti-TNFα Therapy. Dig. Liver Dis. 2017, 49, 495–499. [Google Scholar] [CrossRef]
- Ashton, J.J.; Marino, L.V.; Johnson, M.J.; Newell, C.; Price, G.; Dewar, H.; Brampton, R.; Ennis, S.; Griffiths, M.; Coelho, T.; et al. Bioelectrical Spectroscopy Impedance Phase Angle Is Not Associated with Nutritional Status in a Stable Cohort of Paediatric Inflammatory Bowel Disease Patients. Clin. Nutr. ESPEN 2021, 44, 276–281. [Google Scholar] [CrossRef]
- Santos, J.C.D.; Malaguti, C.; Lucca, F.D.A.; Cabalzar, A.L.; Ribeiro, T.C.d.R.; Gaburri, P.D.; Chebli, L.A.; Chebli, J.M.F. Impact of Biological Therapy on Body Composition of Patients with Chron’s Disease. Rev. Assoc. Med. Bras. 2017, 63, 407–413. [Google Scholar] [CrossRef]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F. Development of a Crohn’s Disease Activity Index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis--Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Tanaka, S.; Ando, K.; Kobayashi, K.; Hida, T.; Seki, T.; Suzuki, K.; Ito, K.; Tsushima, M.; Morozumi, M.; Machino, M.; et al. Relationship between Locomotive Syndrome and Body Composition among Community-Dwelling Middle-Age and Elderly Individuals in Japan: The Yakumo Study. Mod. Rheumatol. 2019, 29, 491–495. [Google Scholar] [CrossRef]
- Osuna-Padilla, I.A.; Salazar Arenas, M.D.L.A.; Rodríguez-Moguel, N.C.; Aguilar-Vargas, A.; Montano Rivas, J.A.; Ávila-Ríos, S. Phase Angle as Predictor of Malnutrition in People Living with HIV/AIDS. Nutr. Clin. Pract. 2021. [Google Scholar] [CrossRef] [PubMed]
- Więch, P.; Dąbrowski, M.; Bazaliński, D.; Sałacińska, I.; Korczowski, B.; Binkowska-Bury, M. Bioelectrical Impedance Phase Angle as an Indicator of Malnutrition in Hospitalized Children with Diagnosed Inflammatory Bowel Diseases-A Case Control Study. Nutrients 2018, 10, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.-F. The Montreal Classification of Inflammatory Bowel Disease: Controversies, Consensus, and Implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Santarpia, L.; Alfonsi, L.; Castiglione, F.; Pagano, M.C.; Cioffi, I.; Rispo, A.; Sodo, M.; Contaldo, F.; Pasanisi, F. Nutritional Rehabilitation in Patients with Malnutrition Due to Crohn’s Disease. Nutrients 2019, 11, 2947. [Google Scholar] [CrossRef] [Green Version]
- Vasseur, F.; Gower-Rousseau, C.; Vernier-Massouille, G.; Dupas, J.L.; Merle, V.; Merlin, B.; Lerebours, E.; Savoye, G.; Salomez, J.L.; Cortot, A.; et al. Nutritional Status and Growth in Pediatric Crohn’s Disease: A Population-Based Study. Am. J. Gastroenterol. 2010, 105, 1893–1900. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving Role of Diet in the Pathogenesis and Treatment of Inflammatory Bowel Diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Yin, W.; Li, Z.; Zhang, W. Modulation of Bone and Marrow Niche by Cholesterol. Nutrients 2019, 11, 1394. [Google Scholar] [CrossRef] [Green Version]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s Disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [Green Version]
- Kaniewska, M.; Bartnik, W.; Gonciarz, M.; Kłopocka, M.; Linke, K.; Małecka-Panas, E.; Radwan, P.; Reguła, J.; Rydzewska, G. Iron Deficiency Anaemia in Patients with Inflammatory Bowel Disease: National Consultant for Gastroenterology Working Group Recommendations. Prz. Gastroenterol. 2014, 9, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voegtlin, M.; Vavricka, S.R.; Schoepfer, A.M.; Straumann, A.; Voegtlin, J.; Rogler, G.; Ballabeni, P.; Pittet, V.; Buser, A.; Fried, M.; et al. Prevalence of Anaemia in Inflammatory Bowel Disease in Switzerland: A Cross-Sectional Study in Patients from Private Practices and University Hospitals. J. Crohn’s Colitis 2010, 4, 642–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filmann, N.; Rey, J.; Schneeweiss, S.; Ardizzone, S.; Bager, P.; Bergamaschi, G.; Koutroubakis, I.; Lindgren, S.; Morena, F.D.L.; Moum, B.; et al. Prevalence of Anemia in Inflammatory Bowel Diseases in European Countries: A Systematic Review and Individual Patient Data Meta-Analysis. Inflamm. Bowel. Dis. 2014, 20, 936–945. [Google Scholar] [CrossRef]
- Thomas, D.W.; Hinchliffe, R.F.; Briggs, C.; Macdougall, I.C.; Littlewood, T.; Cavill, I. British Committee for Standards in Haematology Guideline for the Laboratory Diagnosis of Functional Iron Deficiency. Br. J. Haematol. 2013, 161, 639–648. [Google Scholar] [CrossRef]
- Polito, J.M.; Childs, B.; Mellits, E.D.; Tokayer, A.Z.; Harris, M.L.; Bayless, T.M. Crohn’s Disease: Influence of Age at Diagnosis on Site and Clinical Type of Disease. Gastroenterology 1996, 111, 580–586. [Google Scholar] [CrossRef]
- Freeman, H.J. Age-Dependent Phenotypic Clinical Expression of Crohn’s Disease. J. Clin. Gastroenterol. 2005, 39, 774–777. [Google Scholar] [CrossRef]
- Zook, E.G. Change Over. Hand 2009, 4, 341. [Google Scholar] [CrossRef] [Green Version]
- Cantoro, L.; Lenti, M.V.; Monterubbianesi, R.; Cicala, M.; Giannarelli, D.; Papi, C.; Kohn, A.; Di Sabatino, A. Early-Onset versus Late-Onset Crohn’s Disease: An Italian Cohort Study. United Eur. Gastroenterol. J. 2020, 8, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Mañas, L.; Rodríguez-Sánchez, B.; Carnicero, J.A.; Rueda, R.; García-Garcia, F.J.; Pereira, S.L.; Sulo, S. Impact of Nutritional Status According to GLIM Criteria on the Risk of Incident Frailty and Mortality in Community-Dwelling Older Adults. Clin. Nutr. 2021, 40, 1192–1198. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, D.; Locquet, M.; Reginster, J.-Y.; Cavalier, E.; Bruyère, O.; Beaudart, C. Mortality in Malnourished Older Adults Diagnosed by ESPEN and GLIM Criteria in the SarcoPhAge Study. J. Cachexia Sarcopenia Muscle 2020, 11, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- GLIM Consortium; de van der Schueren, M.A.E.; Keller, H.; Cederholm, T.; Barazzoni, R.; Compher, C.; Correia, M.I.T.D.; Gonzalez, M.C.; Jager-Wittenaar, H.; Pirlich, M.; et al. Global Leadership Initiative on Malnutrition (GLIM): Guidance on Validation of the Operational Criteria for the Diagnosis of Protein-Energy Malnutrition in Adults. Clin. Nutr. 2020, 39, 2872–2880. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Chan, R.S.M.; Kwok, T.; Lee, J.S.W.; Woo, J. Malnutrition According to GLIM Criteria and Adverse Outcomes in Community-Dwelling Chinese Older Adults: A Prospective Analysis. J. Am. Med. Dir. Assoc. 2021, 22, 1953.e4–1959.e4. [Google Scholar] [CrossRef]
- Fiorindi, C.; Luceri, C.; Dragoni, G.; Piemonte, G.; Scaringi, S.; Staderini, F.; Nannoni, A.; Ficari, F.; Giudici, F. GLIM Criteria for Malnutrition in Surgical IBD Patients: A Pilot Study. Nutrients 2020, 12, 2222. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Pichard, C. Low Phase Angle Determined by Bioelectrical Impedance Analysis Is Associated with Malnutrition and Nutritional Risk at Hospital Admission. Clin. Nutr. 2013, 32, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, S.; Nakajima, T.; Nozawa, N.; Katayanagi, S.; Ishizaka, H.; Mizushima, Y.; Matsumoto, K.; Nishikawa, K.; Toyama, Y.; Takahashi, R.; et al. Phase Angle as an Indicator of Sarcopenia, Malnutrition, and Cachexia in Inpatients with Cardiovascular Diseases. J. Clin. Med. 2020, 9, 2554. [Google Scholar] [CrossRef]
- Yasui-Yamada, S.; Oiwa, Y.; Saito, Y.; Aotani, N.; Matsubara, A.; Matsuura, S.; Tanimura, M.; Tani-Suzuki, Y.; Kashihara, H.; Nishi, M.; et al. Impact of Phase Angle on Postoperative Prognosis in Patients with Gastrointestinal and Hepatobiliary-Pancreatic Cancer. Nutrition 2020, 79–80, 110891. [Google Scholar] [CrossRef]
- Irisawa, H.; Mizushima, T. Correlation of Body Composition and Nutritional Status with Functional Recovery in Stroke Rehabilitation Patients. Nutrients 2020, 12, 1923. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD. Nutrients 2019, 11, 2583. [Google Scholar] [CrossRef] [Green Version]
- Jensen, G.L.; Cederholm, T.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report from the Global Clinical Nutrition Community. JPEN J. Parenter Enter. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Sigall Boneh, R.; Sarbagili Shabat, C.; Yanai, H.; Chermesh, I.; Ben Avraham, S.; Boaz, M.; Levine, A. Dietary Therapy with the Crohn’s Disease Exclusion Diet Is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. J. Crohn’s Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portuondo, J.I.; Probstfeld, L.; Massarweh, N.N.; Le, L.; Wei, Q.; Chai, C.Y.; Taylor, J.; Awad, S.S.; Tran Cao, H.S. Malnutrition in Elective Surgery: How Traditional Markers Might Be Failing Surgeons and Patients. Surgery 2020, 168, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhu, C.; Feng, S.; Xu, L.; Hu, S.; Chen, H.; Chen, H.; Yao, S.; Wang, X.; Chen, Y. Economic Burden and Health Care Access for Patients with Inflammatory Bowel Diseases in China: Web-Based Survey Study. J. Med. Internet Res. 2021, 23, e20629. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Vardi, H.; Schwartz, D.; Friger, M.; Kiudelis, G.; Kupčinskas, J.; Fumery, M.; Gower-Rousseau, C.; Lakatos, L.; Lakatos, P.L.; et al. Health-Care Costs of Inflammatory Bowel Disease in a Pan-European, Community-Based, Inception Cohort during 5 Years of Follow-up: A Population-Based Study. Lancet Gastroenterol. Hepatol. 2020, 5, 454–464. [Google Scholar] [CrossRef]
- Park, K.T.; Ehrlich, O.G.; Allen, J.I.; Meadows, P.; Szigethy, E.M.; Henrichsen, K.; Kim, S.C.; Lawton, R.C.; Murphy, S.M.; Regueiro, M.; et al. Corrigendum to The Cost of Inflammatory Bowel Disease: An Initiative from the Crohn’s & Colitis Foundation. Inflamm. Bowel Dis. 2020, 26, 1118. [Google Scholar] [CrossRef]
- Weisshof, R.; Chermesh, I. Micronutrient Deficiencies in Inflammatory Bowel Disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Vermeulen, K.M.; Lopes, M.M.G.D.; Alves, C.X.; Brito, N.J.N.; das Graças Almeida, M.; Leite-Lais, L.; Vale, S.H.L.; Brandão-Neto, J. Bioelectrical Impedance Vector Analysis and Phase Angle on Different Oral Zinc Supplementation in Eutrophic Children: Randomized Triple-Blind Study. Nutrients 2019, 11, 1215. [Google Scholar] [CrossRef] [Green Version]


| Variable | CD Patients (n = 169) | |
|---|---|---|
| Men (n = 127) | Women (n = 42) | |
| Age (year) | 30.87 ± 11.05 | 31.10 ± 12.39 |
| BMI (kg/m2) | 19.93 ± 3.38 | 18.93 ± 2.94 |
| PhA (°) | 6.01 ± 0.75 | 5.28 ± 0.76 |
| WBC (×109/L) | 6.24 ± 2.18 | 5.82 ± 1.68 |
| HGB (g/L) | 129.51 ± 24.83 | 108.98 ± 16.86 |
| PLT (×109/L) | 303.62 ± 104.06 | 318.12 ± 121.63 |
| ALB (g/L) | 41.18 ± 5.54 | 38.93 ± 6.29 |
| FIB (g/L) | 3.79 ± 1.36 | 3.88 ± 1.15 |
| ESR (mm/h) | 40.28 ± 32.11 | 52.14 ± 35.40 |
| CRP (mg/L) | 18.80 ± 24.85 | 19.63 ± 27.30 |
| Disease activity, n (%) | ||
| Activity | 42 (33.1%) | 14 (33.3%) |
| Remission | 85 (66.9%) | 28 (66.7%) |
| Age of diagnosis, n (%) | ||
| A1 | 8 (6.3%) | 5 (11.9%) |
| A2 | 86 (67.7%) | 30 (71.4%) |
| A3 | 33 (26.0%) | 7 (16.7%) |
| Location, n (%) | ||
| L1 | 26 (20.5%) | 5 (11.9%) |
| L2 | 6 (4.7%) | 2 (4.8%) |
| L3 | 92 (72.4%) | 34 (81.0%) |
| L4 | 3 (2.4%) | 1 (2.4%) |
| Behavior, n (%) | ||
| B1 | 64 (50.4%) | 19 (45.2%) |
| B2 | 38 (29.9%) | 22 (52.4%) |
| B3 | 8 (6.3%) | 0 (0.0%) |
| B2+B3 | 17 (13.4%) | 1 (2.4%) |
| Combined perianal fistulas, n (%) | ||
| Yes | 89 (70.1%) | 19 (45.2%) |
| No | 38 (29.9%) | 23 (54.8%) |
| History of gastrointestinal surgery, n (%) | ||
| Yes | 62 (48.8%) | 18 (42.9%) |
| No | 65 (51.2%) | 24 (57.1%) |
| Men (n = 127) | p-Value | Women (n = 42) | p-Value | |||
|---|---|---|---|---|---|---|
| Normal (n = 53) | Malnutrition (n = 74) | Normal (n = 10) | Malnutrition (n = 32) | |||
| Age (year) | 32.91 ± 10.59 | 29.44 ± 11.51 | 0.025 * | 37.50 ± 14.74 | 28.91 ± 11.05 | 0.085 |
| BMI (kg/m2) | 22.74 ± 3.28 | 17.99 ± 2.02 | 0.000 * | 22.93 ± 1.40 | 17.68 ± 2.01 | 0.000 * |
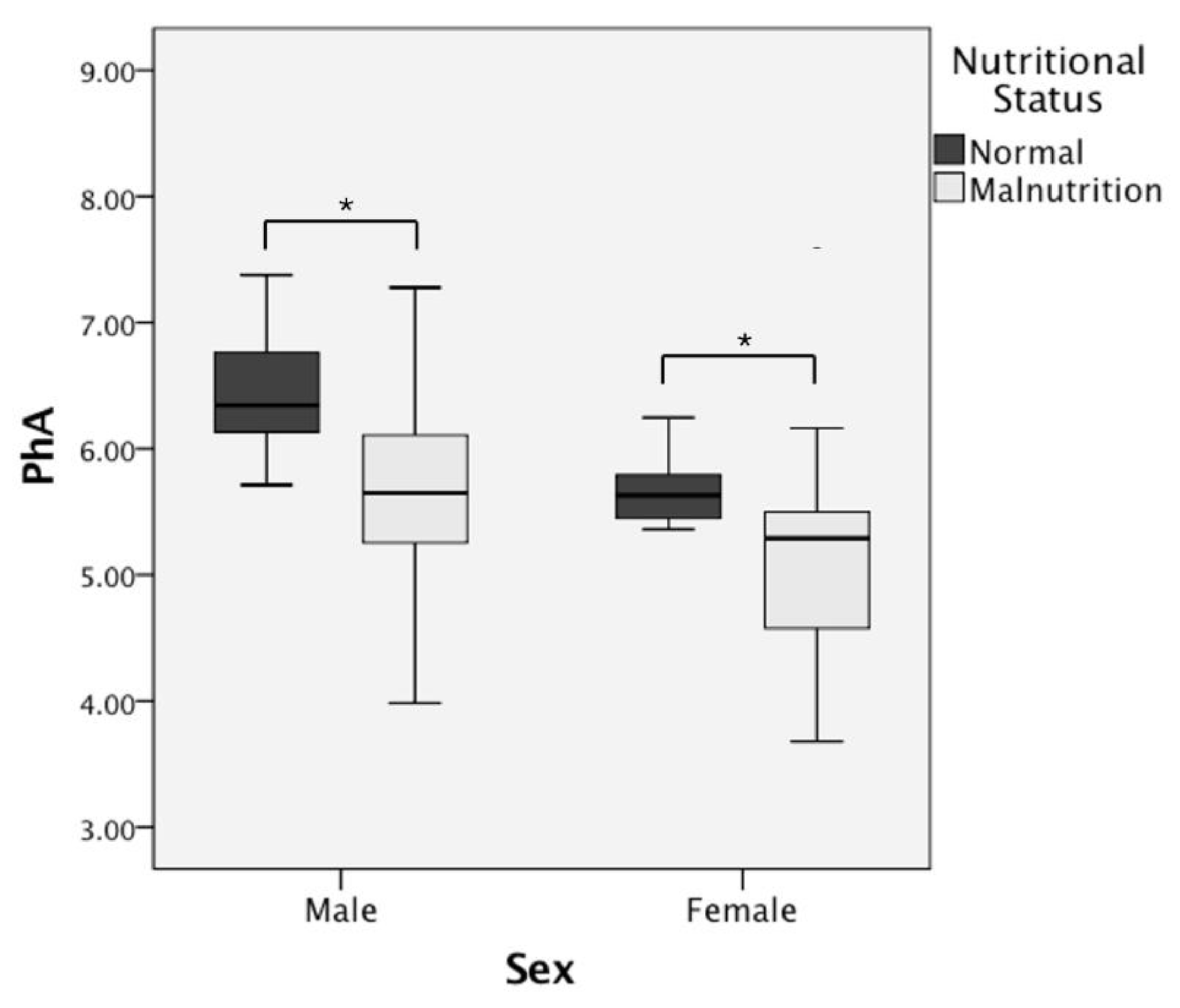
| PhA (°) | 6.45 ± 0.51 | 5.65 ± 0.75 | 0.000 * | 5.63 ± 0.36 | 5.17 ± 0.82 | 0.010 * |
| WBC (×109/L) | 6.27 ± 2.09 | 6.41 ± 2.35 | 0.584 | 5.95 ± 0.71 | 5.78 ± 1.90 | 0.478 |
| HGB (g/L) | 134.82 ± 26.74 | 124.72 ± 22.47 | 0.009 * | 113.60 ± 13.04 | 107.53 ± 17.82 | 0.367 |
| PLT (×109/L) | 291.07 ± 105.92 | 316.59 ± 100.58 | 0.102 | 294.50 ± 66.32 | 325.50 ± 134.36 | 0.555 |
| ALB (g/L) | 42.44 ± 4.50 | 39.88 ± 6.36 | 0.027 * | 40.13 ± 4.50 | 38.55 ± 6.77 | 0.525 |
| FIB (g/L) | 3.49 ± 1.15 | 4.03 ± 1.52 | 0.021 * | 3.96 ± 0.92 | 3.86 ± 1.23 | 0.595 |
| ESR (mm/h) | 33.61 ± 30.00 | 44.94 ± 32.97 | 0.014 * | 46.60 ± 30.41 | 53.88 ± 37.10 | 0.565 |
| CRP (mg/L) | 13.48 ± 22.06 | 25.61 ± 31.81 | 0.005 * | 7.94 ± 5.42 | 23.28 ± 30.33 | 0.626 |
| Disease activity, n (%) | 0.004 * | 0.306 | ||||
| Activity | 10 (18.9%) | 32 (43.2%) | 2 (20.0%) | 12 (37.5%) | ||
| Remission | 43 (81.1%) | 42 (56.8%) | 8 (80.0%) | 20 (62.5%) | ||
| Age of diagnosis, n (%) | 0.064 | 0.075 | ||||
| A1 | 1 (1.9%) | 8 (10.8%) | 1 (10.0%) | 4 (12.5%) | ||
| A2 | 40 (75.5%) | 45 (60.8%) | 5 (50.0%) | 25 (78.1%) | ||
| A3 | 12 (22.6%) | 21 (28.4%) | 4 (40.0%) | 3 (9.4%) | ||
| Location, n (%) | 0.908 | 0.192 | ||||
| L1 | 12 (22.6%) | 14 (18.9%) | 3 (30.0%) | 2 (6.3%) | ||
| L2 | 3 (5.7%) | 3 (4.1%) | 0 (0.0%) | 2 (6.3%) | ||
| L3 | 37 (69.8%) | 55 (74.3%) | 7 (70.0%) | 27 (84.4%) | ||
| L4 | 1 (1.9%) | 2 (2.8%) | 0 (0.0%) | 1 (3.1%) | ||
| Behavior, n (%) | 0.968 | 0.822 | ||||
| B1 | 26 (49.1%) | 38 (51.4%) | 5 (50.0%) | 14 (43.8%) | ||
| B2 | 16 (30.2%) | 22 (29.7%) | 5 (50.0%) | 17 (53.1%) | ||
| B3 | 4 (7.5%) | 4 (5.4%) | 0 (0.0%) | 0 (0.0%) | ||
| B2+B3 | 7 (13.2%) | 10 (13.5%) | 0 (0.0%) | 1 (3.1%) | ||
| Combined perianal fistulas, n (%) | 0.217 | 0.477 | ||||
| Yes | 34 (64.2%) | 55 (74.3%) | 6 (60.0%) | 13 (40.6%) | ||
| No | 19 (35.8%) | 34 (64.2%) | 4 (40.0%) | 19 (59.4%) | ||
| History of gastrointestinal surgery, n (%) | 0.753 | 0.875 | ||||
| Yes | 25 (47.2%) | 37 (50.0%) | 5 (50.0%) | 13 (40.6%) | ||
| No | 28 (52.8%) | 44 (50.0%) | 5 (50.0%) | 19 (59.4%) | ||
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Age (years) | 0.953 | 0.907–1.002 | 0.058 |
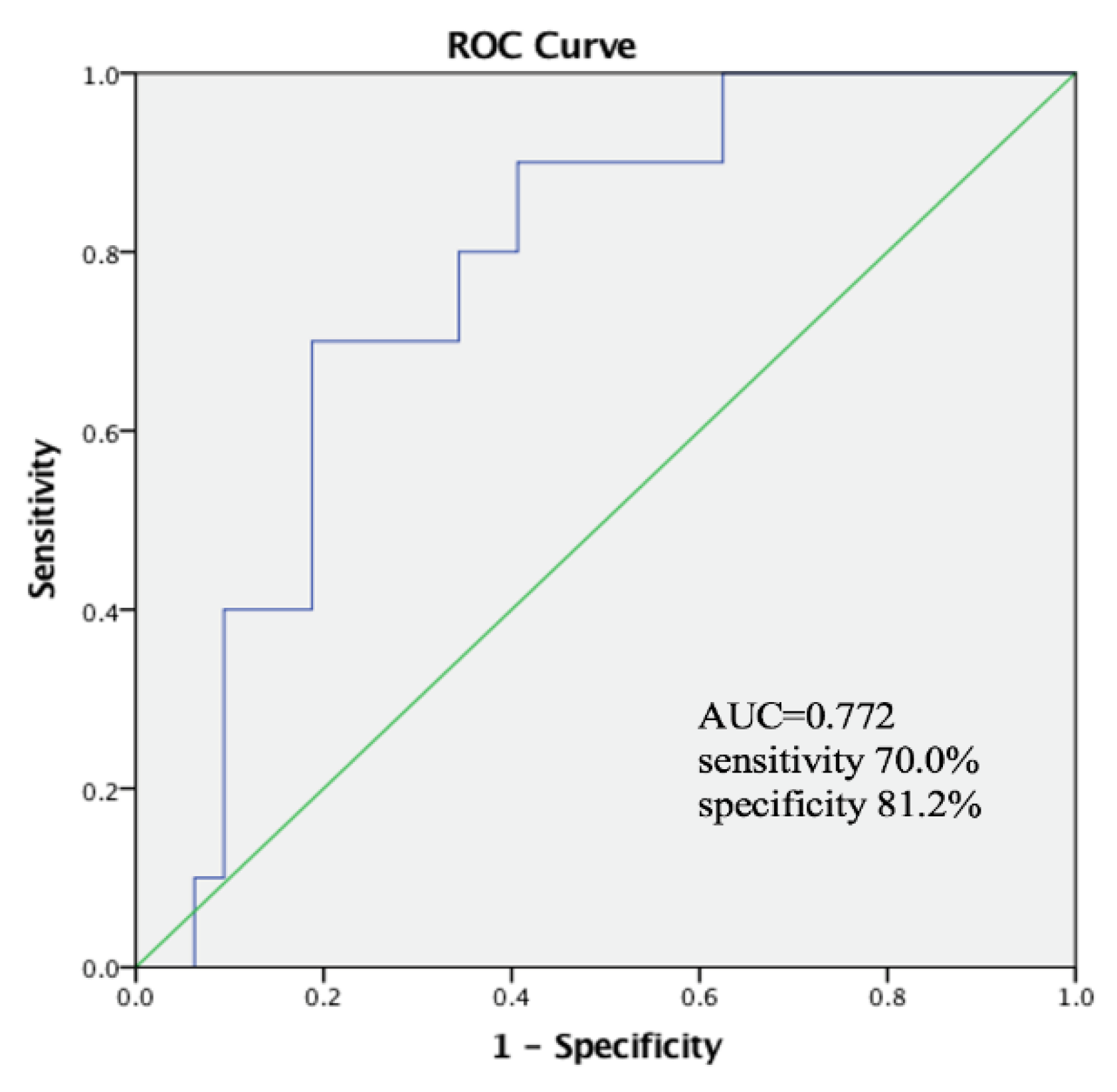
| PhA (°) | 0.150 | 0.062–0.362 | 0.000 * |
| HGB (g/L) | 0.985 | 0.963–1.007 | 0.186 |
| ALB (g/L) | 0.977 | 0.869–1.099 | 0.700 |
| FIB (g/L) | 1.236 | 0.611–2.499 | 0.556 |
| ESR (mm/h) | 0.997 | 0.972–1.023 | 0.824 |
| CRP (mg/L) | 1.001 | 0.970–1.032 | 0.970 |
| Disease activity, n (%) | |||
| Remission | - | - | - |
| Activity | 2.178 | 0.698–6.800 | 0.180 |
| Male Malnutrition (n = 74) | p-Value | Female Malnutrition (n = 32) | p-Value | |||
|---|---|---|---|---|---|---|
| Moderate Malnutrition (n = 40) | Severe Malnutrition (n = 34) | Moderate Malnutrition (n = 10) | Severe Malnutrition (n = 22) | |||
| Age (year) | 29.22 ± 9.90 | 29.15 ± 12.38 | 0.398 | 30.70 ± 15.88 | 28.36 ± 8.45 | 0.682 |
| BMI (kg/m2) | 19.23 ± 1.69 | 16.88 ± 2.33 | 0.000 * | 19.28 ± 1.30 | 16.95 ± 1.87 | 0.001 * |
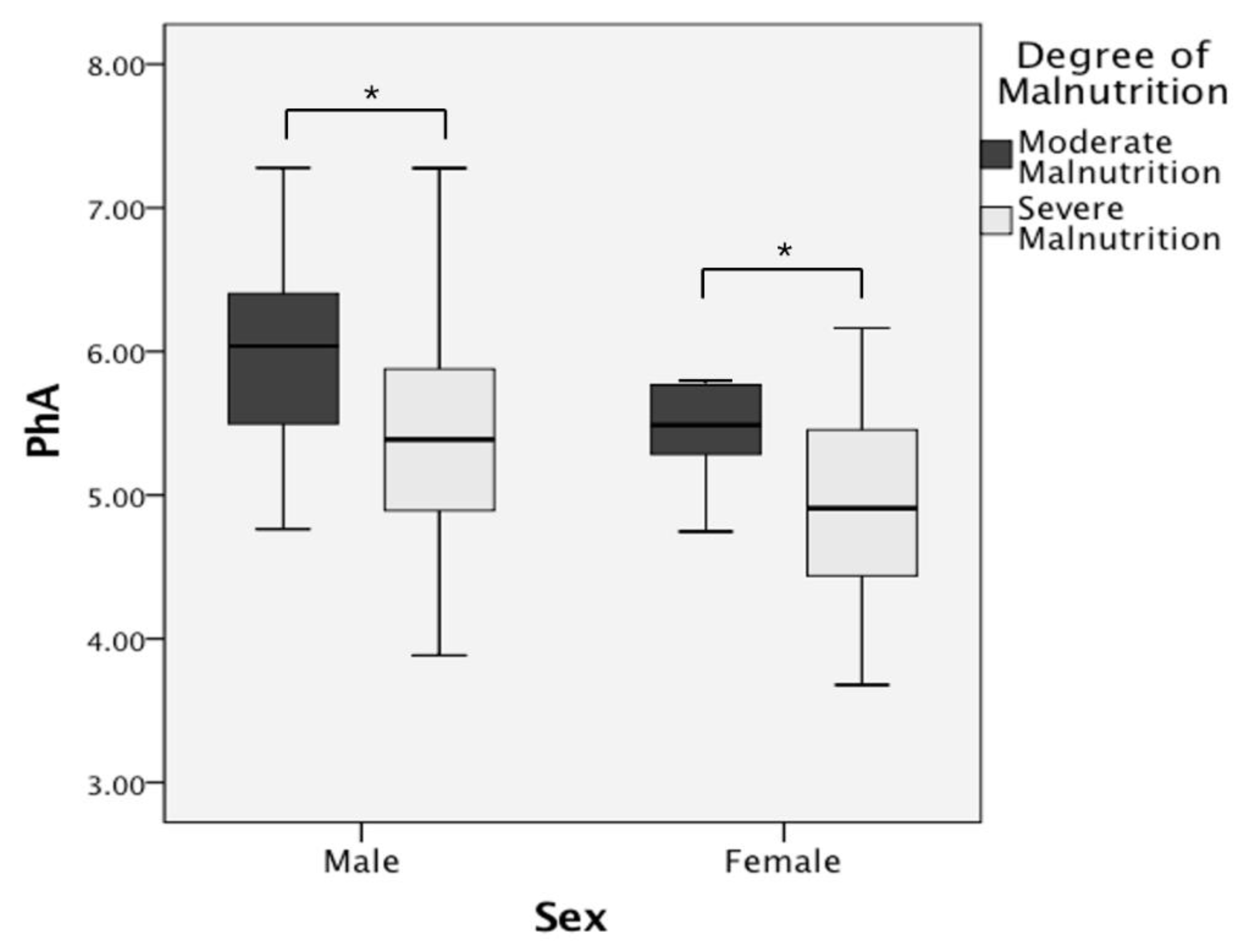
| PhA (°) | 5.96 ± 0.59 | 5.39 ± 0.76 | 0.001 * | 5.76 ± 0.90 | 4.90 ± 0.64 | 0.016 * |
| WBC (×109/L) | 5.94 ± 2.17 | 6.59 ± 2.45 | 0.278 | 6.20 ± 1.50 | 5.60 ± 2.05 | 0.222 |
| HGB (g/L) | 131.28 ± 21.99 | 118.44 ± 20.11 | 0.002 * | 118.20 ± 10.65 | 102.68 ± 18.47 | 0.015 * |
| PLT (×109/L) | 295.75 ± 101.98 | 338.88 ± 107.55 | 0.045 * | 259.90 ± 93.49 | 364.41 ± 133.35 | 0.034 * |
| ALB (g/L) | 42.59 ± 4.89 | 37.34 ± 5.88 | 0.000 * | 42.00 ± 4.35 | 36.98 ± 7.17 | 0.024 * |
| FIB (g/L) | 3.51 ± 1.37 | 4.64 ± 1.35 | 0.002 * | 3.32 ± 0.88 | 4.10 ± 1.31 | 0.074 |
| ESR (mm/h) | 32.97 ± 27.66 | 60.62 ± 32.35 | 0.000 * | 44.80 ± 35.98 | 58.00 ± 37.68 | 0.299 |
| CRP (mg/L) | 17.84 ± 22.73 | 31.01 ± 28.05 | 0.011 * | 7.22 ± 7.67 | 30.59 ± 33.97 | 0.074 |
| Disease activity, n (%) | 0.003 * | 0.325 | ||||
| Activity | 11 (27.5%) | 21 (61.8%) | 2 (20.0%) | 10 (45.5%) | ||
| Remission | 29 (72.5%) | 13 (38.2%) | 8 (80.0%) | 12 (54.5%) | ||
| Age of diagnosis, n (%) | 0.205 | 0.041 * | ||||
| A1 | 3 (7.5%) | 5 (14.7%) | 3 (30.0%) | 1 (4.5%) | ||
| A2 | 28 (70.0%) | 17 (50.0%) | 5 (50.0%) | 20 (90.9%) | ||
| A3 | 9 (22.5%) | 12 (35.3%) | 2 (20.0%) | 1 (4.5%) | ||
| Location, n (%) | 0.759 | 0.457 | ||||
| L1 | 9 (22.5%) | 5 (14.7%) | 0 (0.0%) | 2 (9.1%) | ||
| L2 | 1 (2.5%) | 2 (5.9%) | 1 (10.0%) | 1 (4.5%) | ||
| L3 | 29 (72.5%) | 26 (76.5%) | 9 (90.0%) | 18 (81.8%) | ||
| L4 | 1 (2.5%) | 1 (2.9%) | 0 (0.0%) | 1 (4.5%) | ||
| Behavior, n (%) | 0.338 | 0.106 | ||||
| B1 | 19 (47.5%) | 19 (55.9%) | 7 (70.0%) | 7 (31.8%) | ||
| B2 | 11 (27.5%) | 11 (32.4%) | 3 (30.0%) | 14 (63.6%) | ||
| B3 | 2 (5.0%) | 2 (5.9%) | 0 (0.0%) | 0 (0.0%) | ||
| B2+B3 | 8 (20.0%) | 2 (5.9%) | 0 (0.0%) | 1 (4.5%) | ||
| Combined perianal fistulas, n (%) | 0.145 | 0.662 | ||||
| Yes | 27 (67.5%) | 28 (82.4%) | 3 (30.0%) | 10 (45.5%) | ||
| No | 13 (32.5%) | 6 (17.6%) | 7 (70.0%) | 12 (54.5%) | ||
| History of gastrointestinal surgery, n (%) | 0.641 | 1.000 | ||||
| Yes | 21 (52.5%) | 16 (47.1%) | 4 (40.0%) | 9 (40.9%) | ||
| No | 19 (47.5%) | 18 (52.9%) | 6 (60.0%) | 13 (59.1%) | ||
| OR | 95%CI | p-Value | |
|---|---|---|---|
| PhA (°) | 0.246 | 0.079–0.767 | 0.016 * |
| HGB (g/L) | 0.965 | 0.918–1.016 | 0.173 |
| PLT (×109/L) | 0.994 | 0.985–1.004 | 0.245 |
| ALB (g/L) | 0.951 | 0.809–1.117 | 0.540 |
| FIB (g/L) | 2.362 | 0.952–5.861 | 0.064 |
| ESR (mm/h) | 1.019 | 0.981–1.058 | 0.329 |
| CRP (mg/L) | 0.965 | 0.921–1.012 | 0.142 |
| Disease activity | |||
| Remission | - | - | - |
| Activity | 0.602 | 0.123–2.954 | 0.532 |
| OR | 95%CI | p-Value | |
|---|---|---|---|
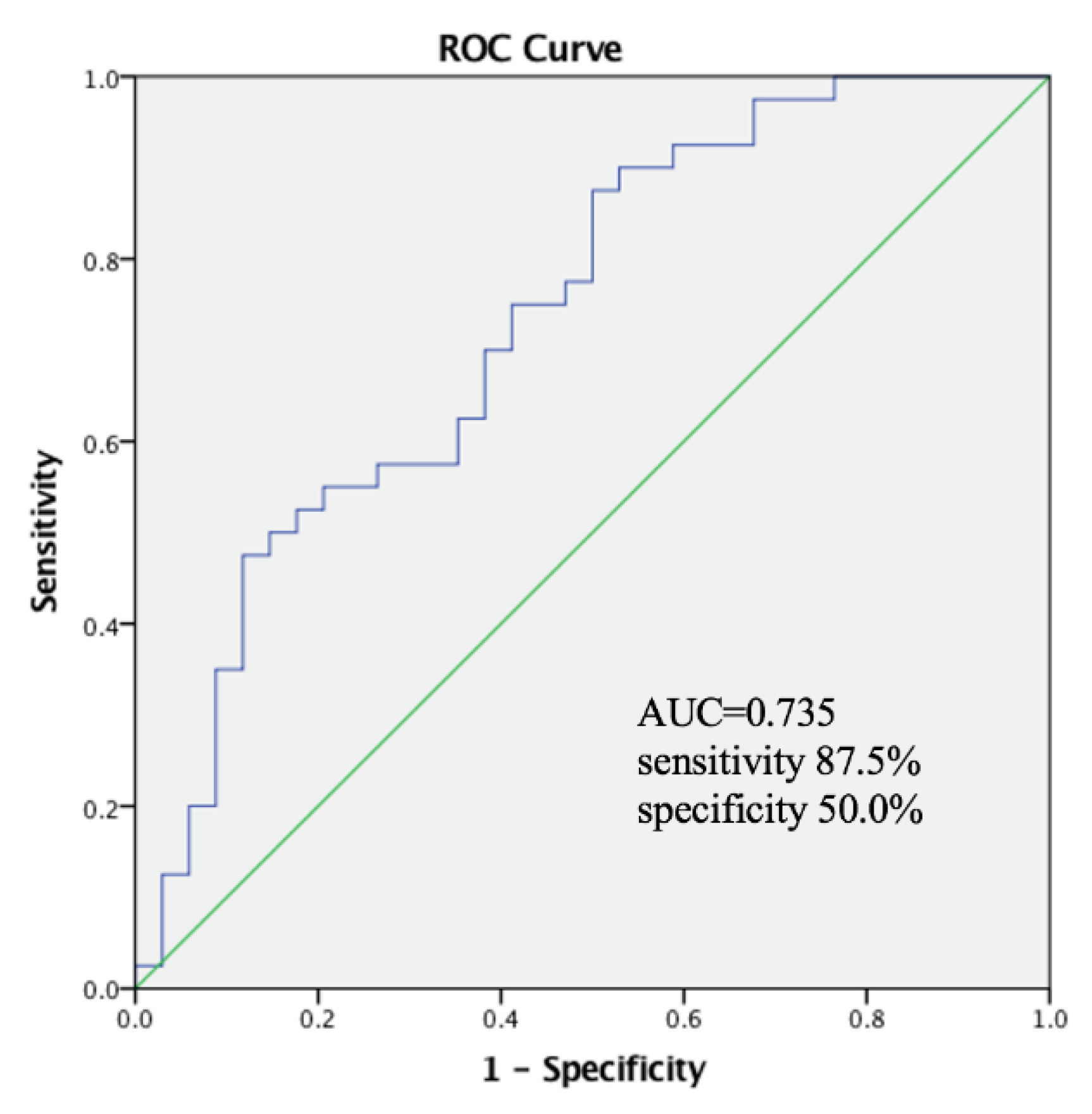
| PhA (°) | 0.000 | 0.000–0.627 | 0.037 * |
| HGB (g/L) | 0.855 | 0.702–1.042 | 0.855 |
| PLT (×109/L) | 1.026 | 0.992–1.060 | 0.133 |
| ALB (g/L) | 0.771 | 0.482–1.232 | 0.771 |
| Age of diagnosis | |||
| A1 | - | - | - |
| A2 | 728.109 | 0.008–62604377.3 | 0.172 |
| A3 | 1.026 | 0.992–1.060 | 0.133 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Xu, D.; Li, Y.; Peng, Y.; Liu, X. Phase Angle as a Comprehensive Tool for Nutritional Monitoring and Management in Patients with Crohn’s Disease. Nutrients 2022, 14, 2260. https://doi.org/10.3390/nu14112260
Peng Z, Xu D, Li Y, Peng Y, Liu X. Phase Angle as a Comprehensive Tool for Nutritional Monitoring and Management in Patients with Crohn’s Disease. Nutrients. 2022; 14(11):2260. https://doi.org/10.3390/nu14112260
Chicago/Turabian StylePeng, Ziheng, Duo Xu, Yong Li, Yu Peng, and Xiaowei Liu. 2022. "Phase Angle as a Comprehensive Tool for Nutritional Monitoring and Management in Patients with Crohn’s Disease" Nutrients 14, no. 11: 2260. https://doi.org/10.3390/nu14112260
APA StylePeng, Z., Xu, D., Li, Y., Peng, Y., & Liu, X. (2022). Phase Angle as a Comprehensive Tool for Nutritional Monitoring and Management in Patients with Crohn’s Disease. Nutrients, 14(11), 2260. https://doi.org/10.3390/nu14112260






