Increased Breastfeeding Proportion Is Associated with Improved Gross Motor Skills at 3–5 Years of Age: A Pilot Study
Abstract
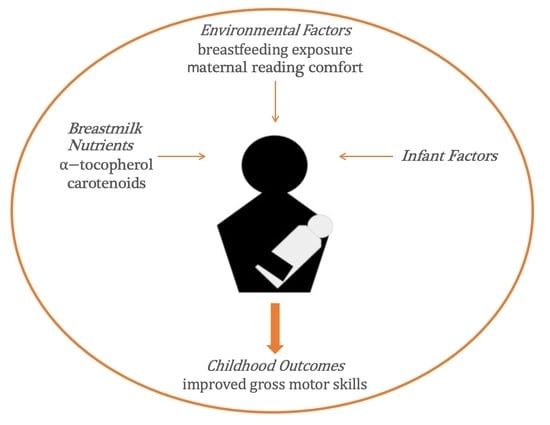
:1. Introduction
2. Materials and Methods
2.1. Enrollment
2.2. Breastfeeding Category Determination
2.3. Breastmilk Nutrient Analysis
2.4. Neurodevelopmental Questionnaires
2.5. Statistical Approaches
3. Results
3.1. Patient Characteristics
3.2. Breastfeeding Category Associated with Problem Solving and Motor Skills
3.3. Breastmilk Nutrients Correlate with Gross Motor and Problem-Solving Skills
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Questionnaire | Mean ± SD or Number Yes (%) |
|---|---|
| ASQ | |
| Communication | 54.1 ± 11.0 |
| Gross motor | 54.2 ± 7.4 |
| Fine motor | 45.2 ± 15.2 |
| Problem solving | 54.5 ± 15.6 |
| Personal/social | 50.5 ± 13.3 |
| Hears well | 30 (90.1) |
| Speaks like other toddlers | 27 (81.2) |
| Easily understood | 32 (96.9) |
| Easily understood by others | 30 (90.1) |
| Walks, runs, and climbs like others | 32 (96.9) |
| ASQ concerns: | |
| Family history of childhood deafness | 2 (6.1) |
| Concerns about vision | 2 (6.1) |
| Any medical problems | 5 (15.1) |
| Concerns about behavior | 4 (12.1) |
| Other concerns | 10 (30.3) |
| PEDS (all concerns) | |
| Global cognition | 9 (27.3) |
| Expressive language | 6 (18.2) |
| Receptive language | 3 (9.1) |
| Fine motor | 1 (3.0) |
| Gross motor | 0 (0) |
| Behavior | 8 (24.2) |
| Social/emotional | 3 (9.1) |
| Self help | 1 (3.0) |
| School | 3 (9.1) |
| Other | 1 (3.0) |
| Total predictive | 0.64 ± 1.14 |
| Total non-predictive | 0.45 ± 0.94 |
| CDI | |
| Age of child | 3.9 ± 0.6 |
| Social | 36.3 ± 4.6 |
| Self help | 30.8 ± 5.8 |
| Gross motor | 25.9 ± 2.2 |
| Fine motor | 25.1 ± 3.9 |
| Expressive language | 46.7 ± 6.3 |
| Language comprehension | 46.2 ± 7.4 |
| Letters | 6.9 ± 4.2 |
| Numbers | 10.2 ± 2.5 |
| General development | 59.0 ± 8.2 |
| CDI concerns: | |
| Number < 25 | 1.3 ± 1.8 |
| Number of areas < 25 | 0.8 ± 1.8 |
| Number of problems | 2.4 ± 3.4 |
| Questionnaire Overall Score | Unadjusted Spearman Coefficient (p-Value) | Partially Adjusted Model Spearman Coefficient (p-Value) | Fully Adjusted Model Spearman Coefficient (p-Value) |
|---|---|---|---|
| ASQ | −0.05 (0.77) | −0.21 (0.29) | −0.23 (0.26) |
| PEDS | −0.05 (0.64) | 0.04 (0.69) | 0.02 (0.84) |
| CDI | −0.16 (0.31) | −0.04 (0.81) | −0.12 (0.46) |
| Questionnaire (n = 33) | Spearman Coefficient (p-Value) |
|---|---|
| ASQ | |
| Communication | 0.13 (0.45) |
| Gross motor | 0.13 (0.46) |
| Fine motor | 0.05 (0.79) |
| Problem solving | 0.35 (0.05) |
| Personal/social | 0.14 (0.43) |
| Hears well | 0.21 (0.25) |
| Speaks like other toddlers | 0.18 (0.31) |
| Easily understood | 0.14 (0.43) |
| Easily understood by others | 0.15 (0.39) |
| Walks, runs, and climbs like others | 0.14 (0.43) |
| Family history of childhood deafness | 0.19 (0.30) |
| Concerns about vision | −0.007 (0.97) |
| Any medical problems | 0.13 (0.47) |
| Concerns about behavior | −0.07 (0.71) |
| Other concerns | −0.10 (0.58) |
| PEDS | |
| Global cognition | −0.24 (0.19) |
| Expressive language | 0.058 (0.75) |
| Receptive language | −0.09 (0.62) |
| Fine motor | 0.13 (0.47) |
| Gross motor | NA |
| Behavior | 0.05 (0.79) |
| Social/emotional | −0.09 (0.62) |
| Self help | 0.13 (0.47) |
| School | 0.07 (0.69) |
| Other | 0.13 (0.47) |
| Total predictive | −0.11 (0.54) |
| Total non-predictive | 0.057 (0.75) |
| CDI | |
| Social | 0.26 (0.15) |
| Self help | −0.08 (0.65) |
| Gross motor | 0.34 (0.06) |
| Fine motor | −0.04 (0.84) |
| Expressive language | 0.03 (0.87) |
| Language comprehension | −0.004 (0.98) |
| Letters | −0.28 (0.12) |
| Numbers | 0.03 (0.89) |
| General development | −0.13 (0.46) |
| Number <25 | −0.16 (0.37) |
| Number of areas <25 | −0.20 (0.27) |
| Number of problems | −0.32 (0.07) |
| Questionnaire (n = 33) | Spearman Coefficient (p-Value) |
|---|---|
| ASQ | |
| Communication | −0.01 (0.95) |
| Gross motor | 0.28 (0.15) |
| Fine motor | 0.10 (0.62) |
| Problem solving | 0.39 (0.04) |
| Personal/social | 0.16 (0.42) |
| Hears well | 0.20 (0.30) |
| Speaks like other toddlers | 0.14 (0.47) |
| Easily understood | −0.02 (0.90) |
| Easily understood by others | 0.03 (0.87) |
| Walks, runs, and climbs like others | 0.04 (0.85) |
| Family history of childhood deafness | 0.14 (0.49) |
| Concerns about vision | 0.15 (0.43) |
| Any medical problems | 0.18 (0.37) |
| Concerns about behavior | −0.02 (0.90) |
| Other concerns | 0.05 (0.77) |
| PEDS | |
| Global cognition | −0.21 (0.29) |
| Expressive language | 0.21 (0.29) |
| Receptive language | 0.07 (0.73) |
| Fine motor | 0.04 (0.83) |
| Gross motor | NA |
| Behavior | 0.16 (0.40) |
| Social/emotional | 0.07 (0.74) |
| Self help | 0.04 (0.83) |
| School | 0.14 (0.48) |
| Other | 0.09 (0.64) |
| Total predictive | −0.03 (0.89) |
| Total non-predictive | 0.17 (0.38) |
| CDI | |
| Social | 0.10 (0.61) |
| Self help | −0.02 (0.89) |
| Gross motor | 0.47 (0.01) |
| Fine motor | 0.04 (0.86) |
| Expressive language | 0.03 (0.86) |
| Language comprehension | −0.11 (0.58) |
| Letters | −0.17 (0.38) |
| Numbers | 0.22 (0.25) |
| General development | −0.02 (0.91) |
| Number <25 | −0.13 (0.51) |
| Number of areas <25 | −0.08 (0.67) |
| Number of problems | −0.19 (0.34) |
| Questionnaire (n = 33) | Spearman Coefficient (p-Value) |
|---|---|
| ASQ | |
| Communication | −0.02 (0.91) |
| Gross motor | 0.41 (0.04) |
| Fine motor | 0.10 (0.63) |
| Problem solving | 0.39 (0.05) |
| Personal/social | 0.15 (0.48) |
| Hears well | 0.20 (0.33) |
| Speaks like other toddlers | 0.20 (0.33) |
| Easily understood | −0.02 (0.93) |
| Easily understood by others | 0.03 (0.88) |
| Walks, runs, and climbs like others | 0.07 (0.74) |
| Family history of childhood deafness | 0.19 (0.37) |
| Concerns about vision | 0.15 (0.46) |
| Any medical problems | 0.21 (0.30) |
| Concerns about behavior | −0.08 (0.70) |
| Other concerns | 0.06 (0.78) |
| PEDS | |
| Global cognition | −0.23 (0.26) |
| Expressive language | 0.20 (0.34) |
| Receptive language | 0.05 (0.82) |
| Fine motor | 0.03 (0.90) |
| Gross motor | NA |
| Behavior | 0.13 (0.54) |
| Social/emotional | 0.01 (0.95) |
| Self help | 0.03 (0.90) |
| School | 0.10 (0.65) |
| Other | 0.15 (0.47) |
| Total predictive | −0.05 (0.81) |
| Total non-predictive | 0.13 (0.52) |
| CDI | |
| Social | 0.12 (0.57) |
| Self help | −0.04 (0.85) |
| Gross motor | 0.52 (0.0075) |
| Fine motor | 0.05 (0.83) |
| Expressive language | 0.04 (0.86) |
| Language comprehension | −0.11 (0.61) |
| Letters | −0.19 (0.37) |
| Numbers | 0.19 (0.36) |
| General development | −0.02 (0.91) |
| Number <25 | −0.16 (0.45) |
| Number of areas <25 | −0.12 (0.58) |
| Number of problems | −0.24 (0.24) |
| Breastmilk Nutrient | Mean ± SD |
|---|---|
| Total α-tocopherol | 4.13 ± 2.24 |
| S-α-tocopherol (% of total) | 0.02 ± 0.02 |
| RSS-α-tocopherol (% of total) | 0.07 ± 0.05 |
| RRS-α-tocopherol (% of total) | 0.06 ± 0.03 |
| RRR-α-tocopherol (% of total) | 0.79 ± 0.12 |
| RSR-α-tocopherol (% of total) | 0.05 ± 0.03 |
| 13 cis-lutein | 8.35 ± 3.87 |
| 13′ cis-lutein | 2.11 ± 1.86 |
| Translutein | 15.34 ± 10.62 |
| Total lutein | 25.81 ± 15.37 |
| % Translutein | 0.56 ± 0.10 |
| Zeaxanthin | 8.96 ± 4.38 |
| 13 cis-beta carotene | 10.78 ± 6.72 |
| Transbeta carotene | 23.22 ± 10.19 |
| Total beta carotene | 33.99 ± 14.06 |
| % Transbeta carotene | 0.67 ± 0.10 |
| Trans lycopene | 1.62 ± 0.94 |
| Tetrahydrofolate (THF) | 19.70 ± 13.13 |
| FA | 27.78 ± 18.81 |
| MTHF | 22.24 ± 8.06 |

References
- Schwarzenberg, S.J.; Georgieff, M.K. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innis, S.M. Impact of maternal diet on human milk composition and neurological development of infants. Am. J. Clin. Nutr. 2014, 99, 734S–741S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.K.; Singhal, A.; Vaidya, U.; Banerjee, S.; Anwar, F.; Rao, S. Optimizing Nutrition in Preterm Low Birth Weight Infants—Consensus Summary. Front. Nutr. 2017, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.J. Nutrient requirements for preterm infant formulas. J. Nutr. 2002, 132, 1395S–1577S. [Google Scholar] [CrossRef] [PubMed]
- Luby, J.L.; Belden, A.C.; Whalen, D.; Harms, M.P.; Barch, D.M. Breastfeeding and Childhood IQ: The Mediating Role of Gray Matter Volume. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Ou, X.; Andres, A.; Cleves, M.A.; Pivik, R.; Snow, J.H.; Ding, Z.; Badger, T.M. Sex-specific association between infant diet and white matter integrity in 8-y-old children. Pediatr. Res. 2014, 76, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Stelmach, I.; Kwarta, P.E.; Jerzyńska, J.; Stelmach, W.; Krakowiak, J.; Karbownik, M.; Podlecka, D.; Hanke, W.; Polańska, K. Duration of breastfeeding and psychomotor development in 1-year-old children—Polish mother and Child cohort study. Int. J. Occup. Med. Environ. Health 2019, 32, 175–184. [Google Scholar] [CrossRef]
- Khan, A.A.; Mohiuddin, O.; Wahid, I.; Khan, B.S.; Khan, S.H. Predicting the Relationship Between Breastfeeding and Gross Motor Milestones Development: The Practice and Prevalence of Breastfeeding in Metropolitan Areas of Sindh, Pakistan. Cureus 2019, 11, e4039. [Google Scholar] [CrossRef] [Green Version]
- Morton, S.U.; Vyas, R.; Gagoski, B.; Vu, C.; Litt, J.; Larsen, R.J.; Kuchan, M.J.; Lasekan, J.B.; Sutton, B.P.; Grant, P.E.; et al. Maternal Dietary Intake of Omega-3 Fatty Acids Correlates Positively with Regional Brain Volumes in 1-Month-Old Term Infants. Cereb. Cortex 2020, 30, 2057–2069. [Google Scholar] [CrossRef]
- Kuchan, M.J.; Moulton, C.J.; Dyer, R.A.; Jensen, S.K.; Schimpf, K.J.; Innis, S.M. RRR-α-tocopherol is the predominant stereoisomer of α-tocopherol in human milk. Curr. Dev. Nutr. 2018, 2, nzy055. [Google Scholar] [CrossRef]
- Jensen, S.K.; Nørgaard, J.V.; Lauridsen, C. Bioavailability of α-tocopherol stereoisomers in rats depends on dietary doses of all-rac-or RRR-α-tocopheryl acetate. Br. J. Nutr. 2006, 95, 477–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golay, P.A.; Moulin, J. Determination of labeled fatty acids content in milk products, infant formula, and adult/pediatric nutritional formula by capillary gas chromatography: Collaborative study, final action 2012.13. J. AOAC Int. 2016, 99, 210–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squires, J.; Bricker, D. Ages & Stages Questionnaires®, Third Edition (ASQ®-3): A Parent-Completed Child Monitoring System; Paul H. Brookes Publishing Co., Inc.: Baltimore, MD, USA, 2009. [Google Scholar]
- Glascoe, F.P. Collaborating with Parents; PEDStest.com, LLC: Nolensville, TN, USA, 2013. [Google Scholar]
- Ireton, H.; Glascoe, F.P. Assessin Children’s Development Using Parents’ Reports: The Child Development Inventory. Clin. Pediatr. 1995, 34, 248–255. [Google Scholar] [CrossRef]
- Libertus, K.; Violi, D.A. Sit to talk: Relation between motor skills and language development in infancy. Front. Psychol. 2016, 7, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, W.W.; Tan, P.T.; Cai, S.; Fok, D.; Chua, M.C.; Lim, S.B.; Shek, L.P.; Chan, S.-Y.; Tan, K.H.; Yap, F.; et al. Nutrients or nursing? Understanding how breast milk feeding affects child cognition. Eur. J. Nutr. 2020, 59, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Oddy, W.H.; Robinson, M.; Kendall, G.E.; Li, J.; Zubrick, S.R.; Stanley, F.J. Breastfeeding and early child development: A prospective cohort study. Acta Paediatr. Int. J. Paediatr. 2011, 100, 992–999. [Google Scholar] [CrossRef]
- McCrory, C.; Murray, A. The effect of breastfeeding on neuro-development in infancy. Matern. Child Health J. 2013, 17, 1680–1688. [Google Scholar] [CrossRef]
- Bell, K. The Relationship Between Home Environment and Reading Achievement. Ph.D. Thesis, State University of New York, Albany, NY, USA, 1996. [Google Scholar]
- Rashid, F.L.; Morris, R.D.; Sevcik, R.A. Relationship between home literacy environment and reading achievement in children with reading disabilities. J. Learn. Disabil. 2005, 38, 2–11. [Google Scholar] [CrossRef]
- Davis-Kean, P.E. The influence of parent education and family income on child achievement: The indirect role of parental expectations and the home environment. J. Fam. Psychol. 2005, 19, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Tucker-Drob, E.M.; Harden, K.P. Early childhood cognitive development and parental cognitive stimulation: Evidence for reciprocal gene-environment transactions. Dev. Sci. 2012, 15, 250–259. [Google Scholar] [CrossRef]
- Kuchan, M.J.; Jensen, S.K.; Johnson, E.J.; Lieblein-Boff, J.C. The naturally occurring α-tocopherol stereoisomer RRR-α-tocopherol is predominant in the human infant brain. Br. J. Nutr. 2016, 116, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferslew, K.E.; Acuff, R.V.; Daigneault, E.A.; Woolley, T.W.; Stanton, P.E. Pharmacokinetics and Bioavailability of the RRR and All Racemic Stereoisomers of Alpha-Tocopherol in Humans After Single Oral Administration. J. Clin. Pharmacol. 1993, 33, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Ranard, K.M.; Erdman, J.W. Effects of dietary RRR α-tocopherol vs all-racemic α-tocopherol on health outcomes. Nutr. Rev. 2018, 76, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.S.R.; Dimenstein, R.; Ribeiro, K.D.S. Vitamin e concentration in human milk and associated factors: A literature review. J. Pediatr. 2014, 90, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, C.; Lyden, E.; Furtado, J.; Van Ormer, M.; Schumacher, M.; Kamil, A.; McGinn, E.; Rilett, K.; Elliott, E.; Cave, C.; et al. Vitamin E status and associations in maternal-infant Dyads in the Midwestern United States. Clin. Nutr. 2019, 38, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Sokol, R.J.; Guggenheim, M.; Iannaccone, S.T.; Barkhaus, P.E.; Miller, C.; Silverman, A.; Balistreri, W.F.; Heubi, J.E. Improved Neurologic Function after Long-Term Correction of Vitamin E Deficiency in Children with Chronic Cholestasis. N. Engl. J. Med. 1985, 313, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Traber, M.G. A history of vitamin E. Ann. Nutr. Metab. 2012, 61, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Sokol, R.J.; Ringel, S.P.; Neville, H.E.; Thellman, C.A.; Kayden, H.J. Lack of Tocopherol in Peripheral Nerves of Vitamin E-Deficient Patients with Peripheral Neuropathy. N. Engl. J. Med. 1987, 317, 262–265. [Google Scholar] [CrossRef]
- Ulatowski, L.; Parker, R.; Warrier, G.; Sultana, R.; Butterfield, D.; Manor, D. Vitamin E is essential for Purkinje neuron integrity. Neuroscience 2014, 260, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Giampietri, M.; Lorenzoni, F.; Moscuzza, F.; Boldrini, A.; Ghirri, P. Lutein and neurodevelopment in preterm infants. Front. Neurosci. 2016, 10, 4034. [Google Scholar] [CrossRef] [Green Version]
- Vishwanathan, R.; Kuchan, M.J.; Sen, S.; Johnson, E.J. Lutein and preterm infants with decreased concentrations of brain carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Nolan, J.; Kavanagh, H.; O’Donovan, O. Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. Arch. Biochem. Biophys. 2004, 430, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.M.; Khan, N.A.; Walk, A.M.; Raine, L.B.; Moulton, C.; Cohen, N.J.; Kramer, A.F.; Hammond, B.R., Jr.; Renzi-Hammond, L.; Hillman, C.H. Macular pigment optical density is positively associated with academic performance among preadolescent children. Nutr. Neurosci. 2018, 21, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Mahmassani, H.A.; Switkowski, K.M.; Scott, T.M.; Johnson, E.J.; Rifas-Shiman, S.L.; Oken, E.; Jacques, P.F. Maternal Intake of Lutein and Zeaxanthin during Pregnancy Is Positively Associated with Offspring Verbal Intelligence and Behavior Regulation in Mid-Childhood in the Project Viva Cohort. J. Nutr. 2021, 151, 615–627. [Google Scholar] [CrossRef]
- Renzi, L.M.; Bovier, E.R.; Hammond, B.R. A role for the macular carotenoids in visual motor response. Nutr. Neurosci. 2013, 16, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Bovier, E.R.; Hammond, B.R. A randomized placebo-controlled study on the effects of lutein and zeaxanthin on visual processing speed in young healthy subjects. Arch. Biochem. Biophys. 2015, 572, 54–57. [Google Scholar] [CrossRef] [Green Version]
| Participant Characteristics (n = 33) | Mean ± SD or n (%) |
|---|---|
| Female | 11 (33.3) |
| Gestational age (weeks) | 39.6 ± 1.25 |
| Birth weight (kg) | 3.35 ± 0.47 |
| Apgar 5 score | 8.83 ± 0.38 |
| Mother’s age (years) | 33.45 ± 3.26 |
| Mother’s self-reported ethnicity | |
| Hispanic or Latino | 3 (9.1) |
| Non-Hispanic or Latino | 30 (90.9) |
| Mother’s self-reported race 1 | |
| White | 25 (75.8) |
| Black or African American | 4 (12.1) |
| Asian | 2 (6.1) |
| More than one race | 1 (3.0) |
| Mother’s education 2 | |
| High school diploma or equivalency (GED) | 2 (6.1) |
| Associate degree (junior college) | 3 (9.1) |
| Bachelor’s degree | 7 (21.2) |
| Master’s degree | 11 (33.3) |
| Doctorate professional | 7 (21.2) |
| Other | 3 (9.1) |
| Father’s education 2 | |
| High school diploma or equivalency (GED) | 6 (18.2) |
| Associate degree (junior college) | 2 (6.1) |
| Bachelor’s degree | 12 (36.4) |
| Master’s degree | 8 (24.2 |
| Doctorate professional | 4 (12.1) |
| Other | 1 (3.0) |
| Family income | |
| Less than $25,000 | 2 (6.1) |
| $25,000–$49,000 | 5 (15.2) |
| $50,000–$74,999 | 3 (9.1) |
| $75,000–$99,999 | 2 (6.1) |
| $100,000 or greater | 21 (63.5) |
| Participant Characteristics (n = 33) | 0% BF (n = 2) | 1–25% BF (n = 6) | 75–94% BF (n = 4) | >94% BF (n = 21) | Spearman Coefficient (p-Value) |
|---|---|---|---|---|---|
| Female | 0 (0%) | 2 (33.3%) | 2 (50.0%) | 7 (33.3%) | 0.05 (0.79) |
| Gestational age (weeks) | 39.0 ± 0.6 | 39.6 ± 1.4 | 38.2 ± 0.3 | 39.9 ± 1.2 | 0.34 (0.05) |
| Birth weight (kg) | 2.9 ± 0.7 | 3.3 ± 0.4 | 2.9 ± 0.2 | 3.5 ± 0.4 | 0.35 (0.04) |
| Apgar 5 score | 9.0 ± 0 | 8.6 ± 1.7 | 8.7 ± 0.6 | 8.9 ± 0.3 | 0.32 (0.13) |
| Mother’s age (years) | 28.0 ± 1.4 | 34.8 ± 3.1 | 31.3 ± 4.7 | 34.0 ± 2.53 | 0.21 (0.24) |
| Mother’s ethnicity | −0.15 (0.39) | ||||
| Hispanic or Latino | 0 (0%) | 1 (16.7%) | 1 (25.0%) | 1 (4.8%%) | |
| Non-Hispanic or Latino | 2 (100%) | 5 (83.3%) | 3 (75.0%) | 20 (95.2%%) | |
| Mother’s self-reported race | |||||
| White | 0 (0%) | 3 (50.0%) | 2 (50.0%) | 20 (95.2%) | 0.63 (9.17 × 10−5) |
| Black or African American | 1 (50.0%) | 0 (0%) | 2 (50.0%) | 1 (4.8%) | −0.26 (0.15) |
| Asian | 1 (50.0%) | 1 (16.7%) | 0 (0%) | 0 (0%) | −0.42 (0.01) |
| More than one race | 0 (0%) | 1 (16.7%) | 0 (0%) | 0 (0%) | |
| Mother’s education | 0.42 (0.02) | ||||
| High school diploma or equivalency (GED) | 0 (0%) | 1 (16.7%) | 1 (25.0%) | 0 (0%) | |
| Associate degree (junior college) | 1 (50.0%) | 1 (16.7%) | 1 (25.0%) | 0 (0%) | |
| Bachelor’s degree | 0 (0%) | 2 (33.3%) | 0 (0%) | 5 (23.8%) | |
| Master’s degree | 1 (50.0%) | 0 (0%) | 0 (0%) | 10 (47.6%) | |
| Doctorate professional | 0 (0%) | 1 (16.7%) | 1 (25.0%) | 5 (23.8%) | |
| Other | 0 (0%) | 1 (16.7%) | 1 (25.0%) | 1 (4.8%) | |
| Father’s education | 0.30 (0.09) | ||||
| High school diploma or equivalency (GED) | 0 (0%) | 2 (33.3%) | 3 (75.0%) | 2 (9.5%) | |
| Associate degree (junior college) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (9.5%) | |
| Bachelor’s degree | 1 (50.0%) | 4 (66.7%) | 0 (0%) | 7 (33.3%) | |
| Master’s degree | 1 (50.0%) | 0 (0%) | 0 (0%) | 7 (33.3%) | |
| Doctorate professional | 0 (0%) | 0 (0%) | 1 (25.0%) | 3 (14.3%) | |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Family income | 0.15 (0.40) | ||||
| Less than $25,000 | 0 (0%) | 2 (33.3%) | 0 (0%) | 0 (0%) | |
| $25,000–$49,000 | 0 (0%) | 1 (16.7%) | 2 (50.0%) | 2 (9.5%) | |
| $50,000–$74,999 | 0 (0%) | 0 (0%) | 0 (0%) | 3 (14.3%) | |
| $75,000–$99,999 | 0 (0%) | 0 (0%) | 0 (0%) | 2 (9.5%) | |
| $100,000 or greater | 2 (100.0%) | 3 (50.0%) | 2 (50.0%) | 14 (66.7%) | |
| No response | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Question | Unadjusted * | Partially Adjusted * | Fully Adjusted * |
|---|---|---|---|
| ASQ: problem solving | 0.35 (0.05) | 0.39 (0.04) | 0.39 (0.05) |
| ASQ: gross motor | 0.13 (0.46) | 0.29 (0.15) | 0.41 (0.04) |
| ASQ: fine motor | 0.05 (0.79) | 0.10 (0.62) | 0.10 (0.63) |
| CDI: gross motor | 0.34 (0.06) | 0.47 (0.01) | 0.52 (0.008) |
| CDI: fine motor | −0.04 (0.84) | 0.04 (0.86) | 0.05 (0.83) |
| PEDS: fine motor | 0.13 (0.47) | 0.04 (0.83) | 0.03 (0.90) |
| Nutrient | Question | Spearman Coefficient (p-Value) * |
|---|---|---|
| Total α-tocopherol | ASQ: Gross Motor | 0.88 (0.02) |
| RSR-α-tocopherol | ASQ: Problem Solving | −0.86 (0.03) |
| Translutein | CDI: Gross Motor | 0.98 (0.0007) |
| Total lutein | CDI: Gross Motor | 0.92 (0.01) |
| Zeaxanthin | CDI: Gross Motor | 0.93 (0.0068) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Souza, E.E.; Vyas, R.; Sisitsky, M.; Feldman, H.A.; Gagoski, B.; Litt, J.; Larsen, R.J.; Kuchan, M.J.; Lasekan, J.B.; Sutton, B.P.; et al. Increased Breastfeeding Proportion Is Associated with Improved Gross Motor Skills at 3–5 Years of Age: A Pilot Study. Nutrients 2022, 14, 2215. https://doi.org/10.3390/nu14112215
D’Souza EE, Vyas R, Sisitsky M, Feldman HA, Gagoski B, Litt J, Larsen RJ, Kuchan MJ, Lasekan JB, Sutton BP, et al. Increased Breastfeeding Proportion Is Associated with Improved Gross Motor Skills at 3–5 Years of Age: A Pilot Study. Nutrients. 2022; 14(11):2215. https://doi.org/10.3390/nu14112215
Chicago/Turabian StyleD’Souza, Erica E., Rutvi Vyas, Michaela Sisitsky, Henry A. Feldman, Borjan Gagoski, Jonathan Litt, Ryan J. Larsen, Matthew J. Kuchan, John B. Lasekan, Brad P. Sutton, and et al. 2022. "Increased Breastfeeding Proportion Is Associated with Improved Gross Motor Skills at 3–5 Years of Age: A Pilot Study" Nutrients 14, no. 11: 2215. https://doi.org/10.3390/nu14112215
APA StyleD’Souza, E. E., Vyas, R., Sisitsky, M., Feldman, H. A., Gagoski, B., Litt, J., Larsen, R. J., Kuchan, M. J., Lasekan, J. B., Sutton, B. P., Grant, P. E., Ou, Y., & Morton, S. U. (2022). Increased Breastfeeding Proportion Is Associated with Improved Gross Motor Skills at 3–5 Years of Age: A Pilot Study. Nutrients, 14(11), 2215. https://doi.org/10.3390/nu14112215