Caffeic Acid Alleviates Memory and Hippocampal Neurogenesis Deficits in Aging Rats Induced by D-Galactose
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Administrations
2.3. Behavioral Tests
2.4. Sample Preparation
2.5. Immunohistochemistry
2.6. Microscopic Quantification
2.7. Statistical Analysis
3. Results
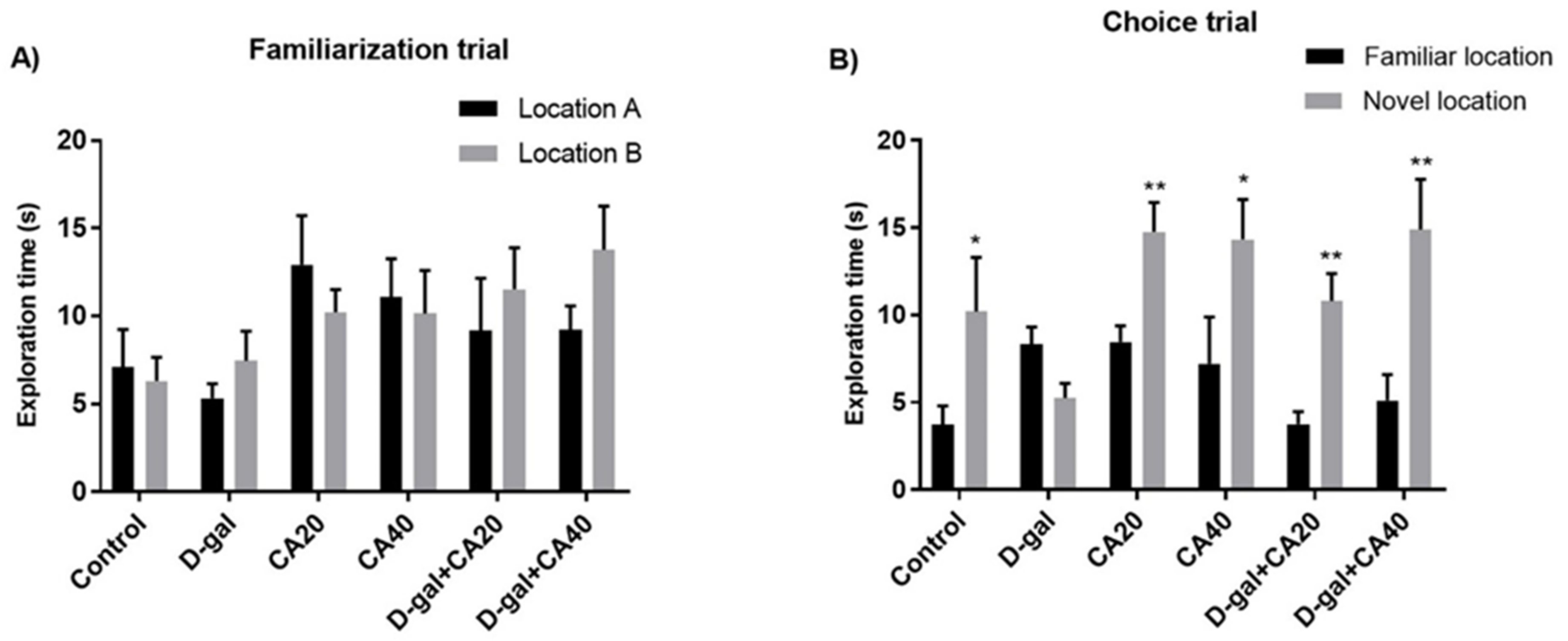
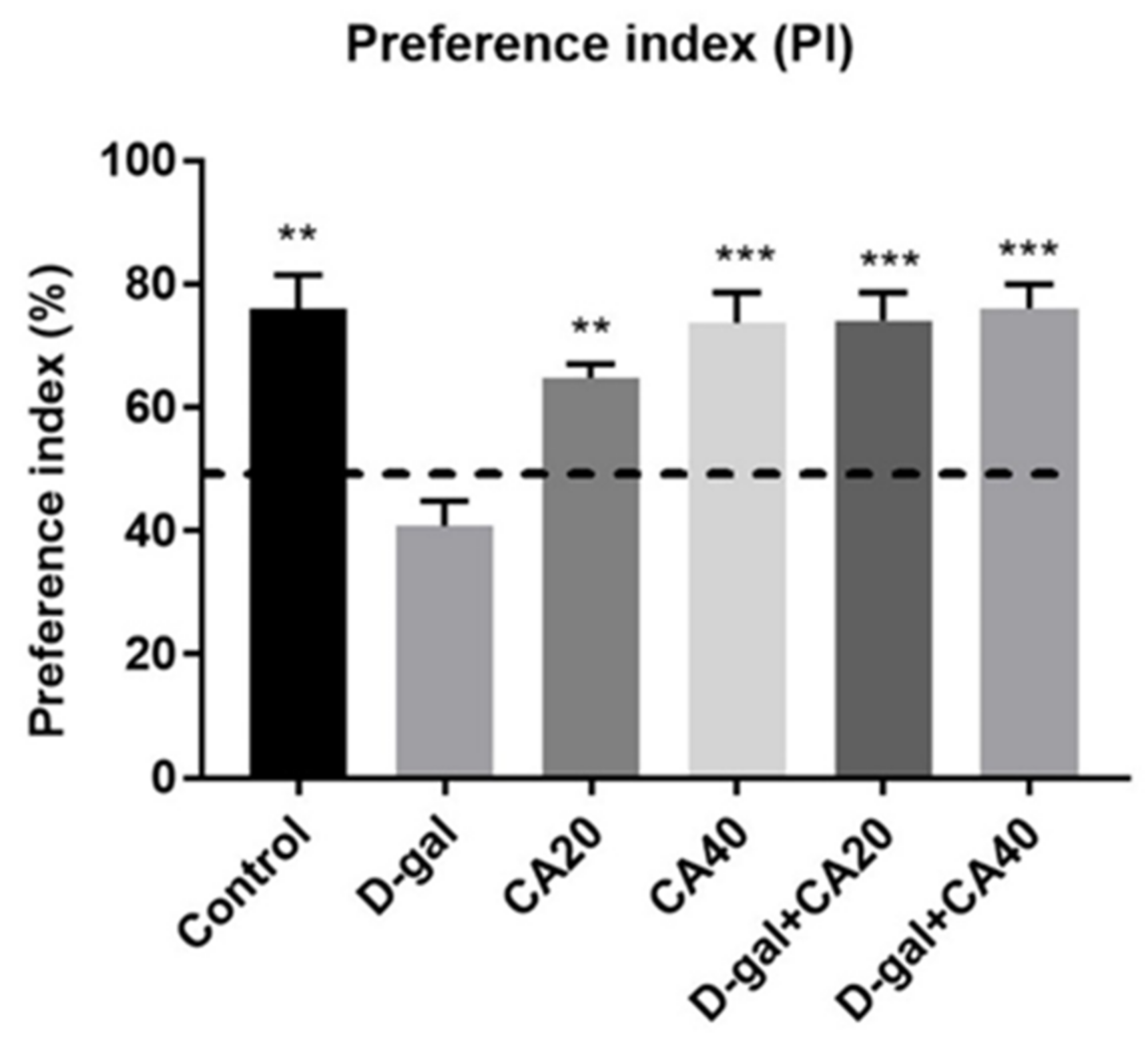
3.1. D-Gal Induced Memory Impairment in NOL Test Is Reversed by CA
3.2. D-Gal Induced Memory Impairment in NOR Test Is Reversed by CA
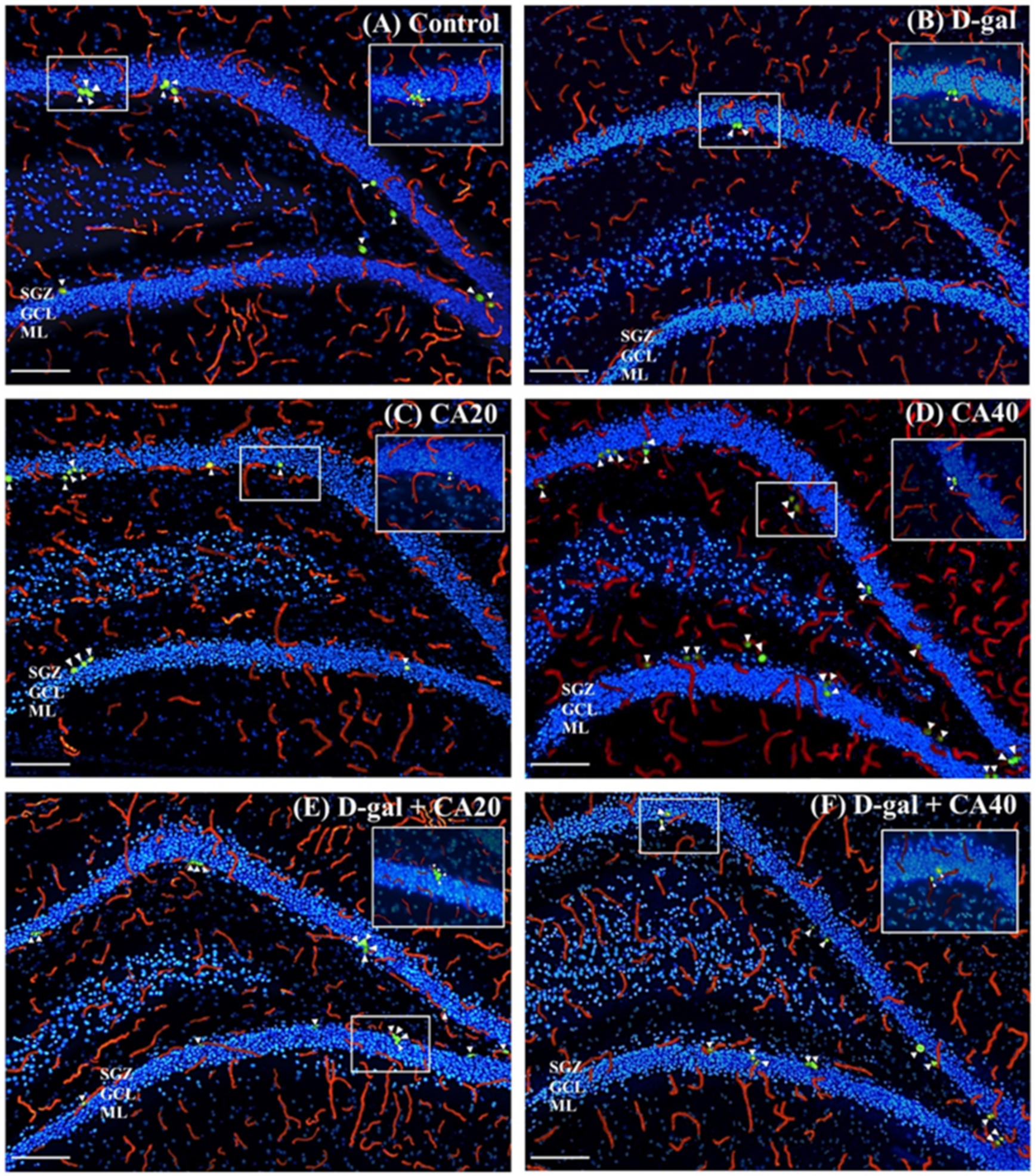
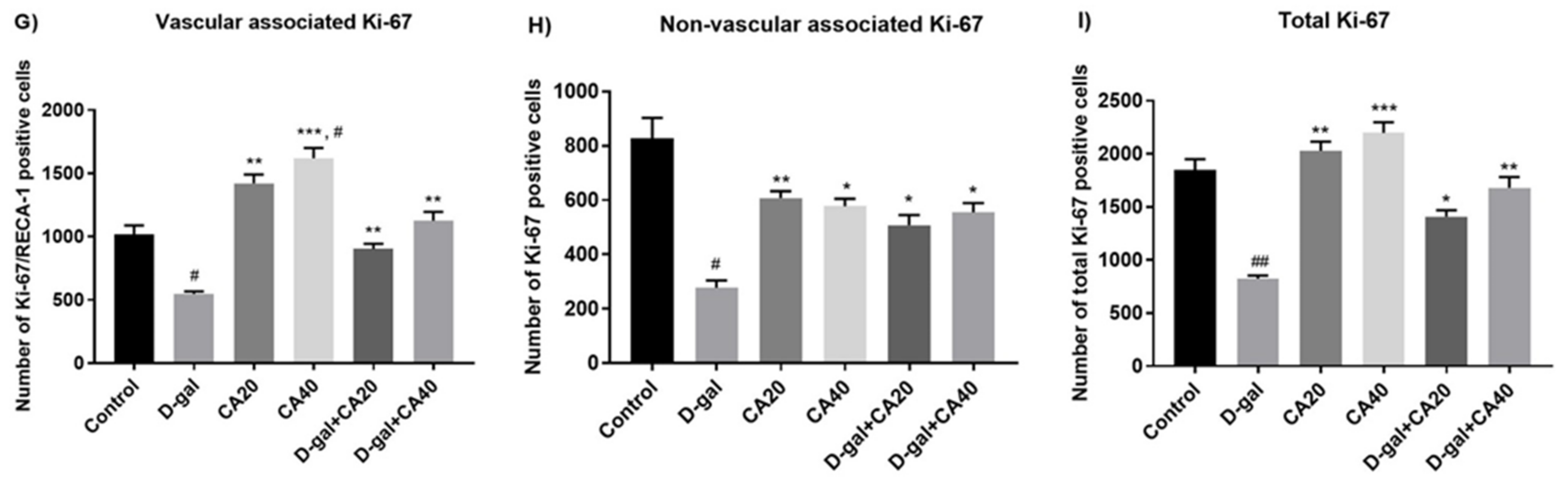
3.3. D-Gal Reduced Cell Proliferation in the Hippocampal Neurogenesis Is Reversed by CA
3.4. D-Gal Reduced Cell Survival Related to Mature Neurons in Hippocampus Is Reversed by CA
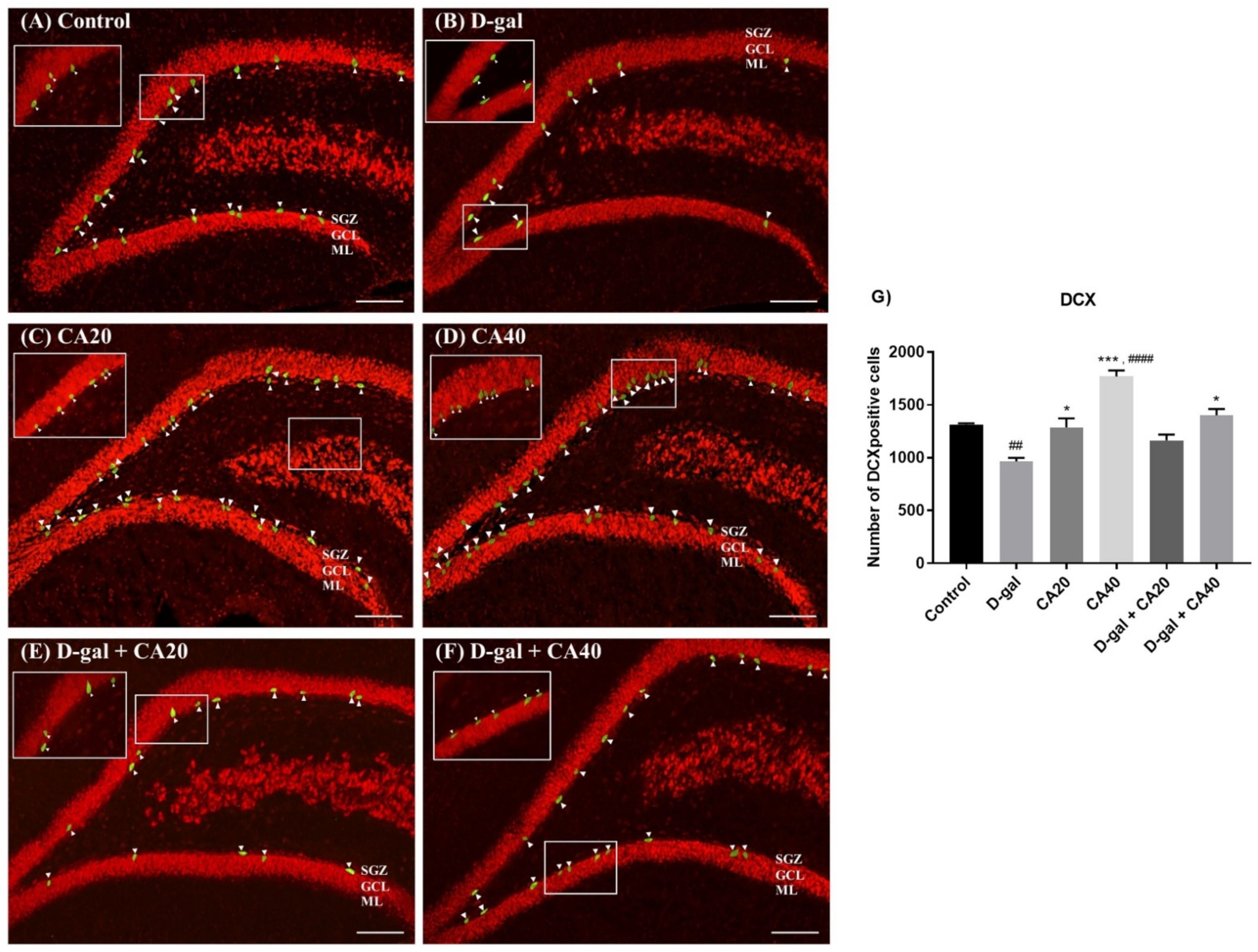
3.5. D-Gal Reduced Immature Neurons in the Hippocampus Is Reversed by CA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, B.; Chen, Y.; Huang, J.; Ning, Y.; Bian, Q.; Shan, Y.; Cai, W.; Zhang, X.; Shen, Z. Icariin improves cognitive deficits and activates quiescent neural stem cells in aging rats. J. Ethnopharmacol. 2012, 142, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, O.; Mattson, M.P.; Peterson, D.A.; Pimplikar, S.W.; van Praag, H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010, 33, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, D.J.; Taylor, C.J.; Bartlett, P.F. Activation of different neural precursor populations in the adult hippocampus: Does this lead to new neurons with discrete functions? Dev. Neurobiol. 2012, 72, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef]
- Floyd, R.A.; Hensley, K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative disease. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Xiang, Q.; Meng, X.; Peng, Y.; Du, N.; Liu, Z.; Sun, Q.; Wang, C.; Liu, X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2014, 5, 158–166. [Google Scholar] [CrossRef]
- Shwe, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp. Gerontol. 2018, 101, 13–36. [Google Scholar] [CrossRef]
- Coelho, A.I.; Berry, G.T.; Rubio-Gozalbo, M.E. Galactose metabolism and health. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 422–427. [Google Scholar] [CrossRef]
- Nam, S.M.; Seo, M.; Seo, J.S.; Rhim, H.; Nahm, S.S.; Cho, I.H.; Chang, B.J.; Kim, H.J.; Choi, S.H.; Nah, S.Y. Ascorbic acid mitigates D-galactose-induced brain aging by increasing hippocampal neurogenesis and improving memory function. Nutrients 2019, 11, 176. [Google Scholar] [CrossRef]
- Ali, T.; Badshah, H.; Kim, T.H.; Kim, M.O. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-KB/JNK signaling pathway in aging mouse model. J. Pineal Res. 2015, 58, 71–85. [Google Scholar] [CrossRef]
- Zhu, J.; Mu, X.; Zeng, J.; Xu, C.; Liu, J.; Zhang, M.; Li, C.; Chen, J.; Li, T.; Wang, Y. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of D-galactose-induced aging. PLoS ONE 2014, 9, e101291. [Google Scholar] [CrossRef]
- Clifford, M. Chlorogenic acids and other cinnamates-nature, occurrence, dietary burden. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Nardini, M.; D’Aquino, M.; Tomassi, G.; Gentili, V.; Di Felice, M.; Scaccini, C. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radic. Biol. Med. 1995, 19, 541–552. [Google Scholar] [CrossRef]
- Chen, Y.F.; Tsai, H.Y.; Wu, T.S. Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. 1995, 61, 2–8. [Google Scholar] [CrossRef]
- Yamada, J.; Tomita, Y. Antimutagenic activity of caffeic acid and related compounds. Biosci. Biotechnol. Biochem. 1996, 60, 328–329. [Google Scholar] [CrossRef]
- Pereira, P.; de Oliveira, P.A.; Ardenghi, P.; Rotta, L.; Henriques, J.A.; Picada, J.N. Neuropharmacological analysis of caffeic acid in rats. Basic Clin. Pharmacol. Toxicol. 2006, 99, 374–378. [Google Scholar] [CrossRef]
- Koga, M.; Nakagawa, S.; Kato, A.; Kusumi, I. Caffeic acid reduces oxidative stress and microglial activation in the mouse hippocampus. Tissue Cell 2019, 60, 14–20. [Google Scholar] [CrossRef]
- Chang, W.; Huang, D.; Lo, Y.M.; Tee, Q.; Kuo, P.; Wu, J.S.; Huang, W.; Shen, S. Protective effect of caffeic acid against Alzheimer’s disease pathogenesis via modulating cerebral insulin signaling, β-amyloid accumulation, and synaptic plasticity in hyperinsulinemic rats. J. Agric. Food Chem. 2019, 67, 7684–7693. [Google Scholar] [CrossRef]
- Ashabi, G.; Alamdary, S.Z.; Ramin, M.; Khodagholi, F. Reduction of hippocampal apoptosis by intracerebroventricular administration of extracellular signal-regulated protein kinase and/or p38 inhibitors in amyloid beta rat model of Alzheimer’s disease: Involvement of nuclear-related factor-2 and nuclear factor-κB. Basic Clin. Pharmacol. Toxicol. 2013, 112, 145–155. [Google Scholar]
- Dix, S.L.; Aggleton, J.P. Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behav. Brain Res. 1999, 99, 191–200. [Google Scholar] [CrossRef]
- Umka Welbat, J.; Sirichoat, A.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Pakdeechote, P.; Sripanidkulchai, B.; Wigmore, P. Asiatic acid prevents the deleterious effects of valproic acid on cognition and hippocampal cell proliferation and survival. Nutrients 2016, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Welbat, J.U.; Chaisawang, P.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Sripanidkulchai, B.; Wigmore, P. Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats. Ann. Anat. 2016, 206, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sirichoat, A.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Leksomboon, R.; Chaichun, A.; Wigmore, P.; Welbat, J.U. Effects of Asiatic acid on spatial working memory and cell proliferation in the adukt rat hippocampus. Nutrients 2015, 7, 8413–8423. [Google Scholar] [CrossRef] [PubMed]
- Chaisawang, P.; Sirichoat, A.; Chaijaroonkhanarak, W.; Pannangrong, W.; Sripanidkulchai, B.; Wigmore, P.; Welbat, J.U. Asiatic acid protects against cognitive deficits and reductions in cell proliferation and survival in the rat hippocampus caused by 5-fluorouracil chemotherapy. PLoS ONE 2017, 12, e0180650. [Google Scholar] [CrossRef]
- Mustafa, S.; Walker, A.; Bennett, G.; Wigmore, P.M. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur. J. Neurosci. 2008, 28, 323–330. [Google Scholar] [CrossRef]
- Kessels, R.P.C.; de Haan, E.H.F.; Kappelle, L.J.; Postma, A. Varieties of human spatial memory: A meta-analysis on the effects of hippocampal lesions. Brain Res. Rev. 2001, 35, 295–303. [Google Scholar] [CrossRef]
- Ortmann, M.R.; Schutte, A.R. The relationship between the perception of axes of symmetry and spatial memory during early childhood. J. Exp. Child. Psychol. 2010, 107, 368–376. [Google Scholar] [CrossRef][Green Version]
- Galati, A.; Michael, C.; Mello, C.; Greenauer, N.M.; Avraamides, M.N. The conversational partner’s perspective affects spatial memory and descriptions. J. Mem. Lang. 2013, 68, 140–159. [Google Scholar] [CrossRef][Green Version]
- Prajit, R.; Sritawan, N.; Suwannakot, K.; Naewla, S.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Chrysin protects against memory and hippocampal neurogenesis depletion in D-galactose-induced aging in rats. Nutrients 2020, 12, 1100. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, B.; Shang, Y.; Yin, Q.; Wang, D.; Xu, S.; Hong, Y.; Hou, X.; Liu, X. Flavonoid-rich ethanol extract from the leaves of Diospyros kaki attenuates D-galactose-induced oxidative stress and neuroinflammation-mediated brain aging in mice. Oxid. Med. Cell Longev. 2018, 2018, 8938207. [Google Scholar] [CrossRef]
- Gao, J.; He, H.; Jiang, W.; Chang, X.; Zhu, L.; Luo, F.; Zhou, R.; Ma, C.; Yan, T. Salidroside ameliorates cognitive impairement in a D-galactose-induced rat model of Alzheimer’s disease. Behav. Brain Res. 2015, 293, 27–33. [Google Scholar] [CrossRef]
- Ullah, F.; Ali, T.; Ullah, N.; Kim, M.O. Caffeine prevents D-galactose-induced cognitive deficits, oxidative stress, neuroinflammation and neurodegeneration in the adult rat brain. Neurochem. Int. 2015, 90, 114–124. [Google Scholar] [CrossRef]
- Winters, B.D.; Saksida, L.M.; Bussey, T.J. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 2008, 32, 1055–1070. [Google Scholar] [CrossRef]
- Clarke, J.R.; Cammarota, M.; Gruart, A.; Izquierdo, I.; Delgado-García, J.M. Plastic modifications induced by object recognition memory processing. Proc. Natl. Acad. Sci. USA 2010, 107, 2652–2657. [Google Scholar] [CrossRef]
- Morici, J.F.; Bekinschtein, P.; Weisstaub, N.V. Medial prefrontal cortex role in recognition memory in rodents. Behav. Brain Res. 2015, 292, 241–251. [Google Scholar] [CrossRef]
- Silvers, J.M.; Harrod, S.B.; Mactutus, C.F.; Brooze, R.M. Automation of the novel object recognition task for use in adolescent rats. J. Neurosci. Methods 2007, 166, 99–103. [Google Scholar] [CrossRef]
- Deshmukh, R.; Kaundal, M.; Bansal, V.; Samardeep. Caffeic acid attenuates oxidative stress, learning and memory deficit in intra-cerebroventricular steptozotocin induced experimental dementia in rats. Biomed. Phamacother. 2016, 81, 56–62. [Google Scholar] [CrossRef]
- Squire, L.R.; Zola, S.M. Episodic memory, semantic memory, and amnesia. Hippocampus 1998, 8, 205–211. [Google Scholar] [CrossRef]
- Palmer, T.D.; Willhoite, A.R.; Gage, F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000, 425, 479–494. [Google Scholar] [CrossRef]
- Leventhal, C.; Rafii, S.; Rafii, D.; Shahar, A.; Goldman, S.A. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol. Cell Neurosci. 1999, 13, 450–464. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Hattiangady, B.; Shetty, A.K. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol. Aging 2008, 29, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Naewla, S.; Sirichoat, A.; Pannangrong, W.; Chaisawang, P.; Wigmore, P.; Welbat, J.U. Hesperidin alleviates methotrexate-induced memory deficits via hippocampal neurogenesis in adult rats. Nutrients 2019, 11, 936. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Sun, Y.; Zhang, W.; Huang, X.; Xue, R.; Zhang, Y.; Wang, Y. Walnut diets up-regulate the decreased hippocampal neurogenesis and age-related cognitive dysfunction in D-galactose induced aged rats. Food Funct. 2018, 9, 4755–4762. [Google Scholar] [CrossRef] [PubMed]
- Massaad, C.A.; Pautler, R.G.; Klann, E. Mitochondrial superoxide: A key player in Alzheimer’s disease. Aging 2009, 1, 758–761. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liao, L.; Pilotte, J.; Xu, T.; Wong, C.C.; Edelman, G.M.; Vanderklish, P.; Yates, J.R. BDNF induces widespread changes in synaptic protein content and up-regulates components of the translation machinery: An analysis using high-throughput proteomics. J. Proteome Res. 2007, 6, 1059–1071. [Google Scholar] [CrossRef]
- Sinclair, L.I.; Tayler, H.M.; Love, S. Synaptic protein levels altered in vascular dementia. Neuropathol. Appl. Neurobiol. 2015, 41, 533–543. [Google Scholar] [CrossRef]



| Groups | Distance Moved (cm) |
|---|---|
| Control | 6655 ± 629.3 |
| D-gal | 6789 ± 419.0 |
| CA20 | 7271 ± 519.5 |
| CA40 | 6792 ± 483.8 |
| D-gal + CA20 | 5733 ± 584.2 |
| D-gal + CA40 | 6640 ± 505.0 |
| Groups | Distance Moved (cm) |
|---|---|
| Control | 7110 ± 303.4 |
| D-gal | 6890 ± 865.6 |
| CA20 | 7482 ± 554.4 |
| CA40 | 6302 ± 569.2 |
| D-gal + CA20 | 5585 ± 360.0 |
| D-gal + CA40 | 6615 ± 660.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saenno, R.; Dornlakorn, O.; Anosri, T.; Kaewngam, S.; Sirichoat, A.; Aranarochana, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Caffeic Acid Alleviates Memory and Hippocampal Neurogenesis Deficits in Aging Rats Induced by D-Galactose. Nutrients 2022, 14, 2169. https://doi.org/10.3390/nu14102169
Saenno R, Dornlakorn O, Anosri T, Kaewngam S, Sirichoat A, Aranarochana A, Pannangrong W, Wigmore P, Welbat JU. Caffeic Acid Alleviates Memory and Hippocampal Neurogenesis Deficits in Aging Rats Induced by D-Galactose. Nutrients. 2022; 14(10):2169. https://doi.org/10.3390/nu14102169
Chicago/Turabian StyleSaenno, Rasa, Oabnithi Dornlakorn, Tanaporn Anosri, Soraya Kaewngam, Apiwat Sirichoat, Anusara Aranarochana, Wanassanun Pannangrong, Peter Wigmore, and Jariya Umka Welbat. 2022. "Caffeic Acid Alleviates Memory and Hippocampal Neurogenesis Deficits in Aging Rats Induced by D-Galactose" Nutrients 14, no. 10: 2169. https://doi.org/10.3390/nu14102169
APA StyleSaenno, R., Dornlakorn, O., Anosri, T., Kaewngam, S., Sirichoat, A., Aranarochana, A., Pannangrong, W., Wigmore, P., & Welbat, J. U. (2022). Caffeic Acid Alleviates Memory and Hippocampal Neurogenesis Deficits in Aging Rats Induced by D-Galactose. Nutrients, 14(10), 2169. https://doi.org/10.3390/nu14102169







