Adherence to Dietary Guidelines among Women with and without Gestational Diabetes: Evidence from the Growing up in New Zealand Study
Abstract
1. Introduction
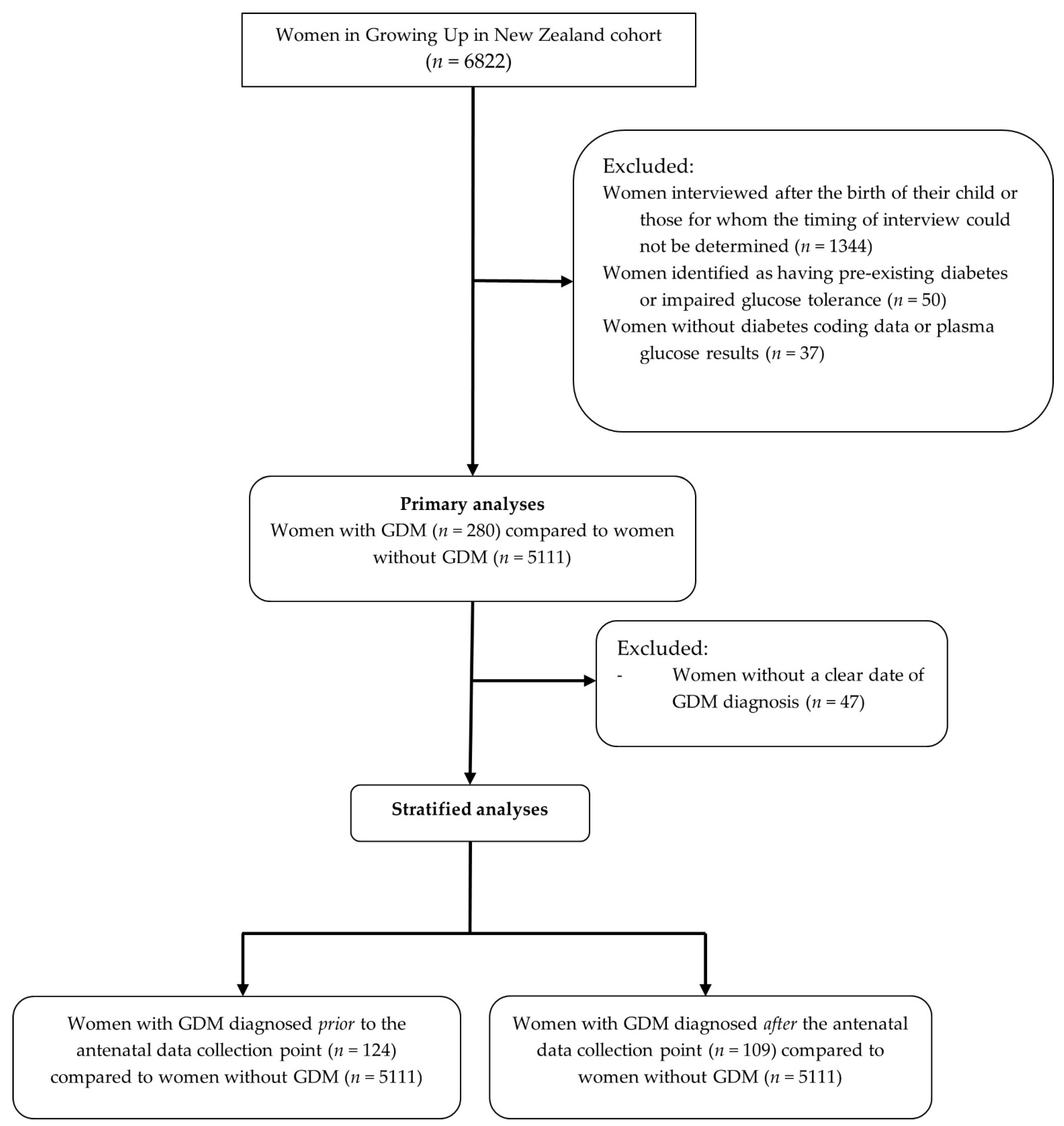
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- National Health and Medical Research Council. Ministry of Health Nutrient Reference Values for Australia and New Zealand. Available online: https://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/n35.pdf (accessed on 4 July 2016).
- Godfrey, K.M.; Barker, D.J. Fetal Nutrition and Adult Disease. Am. J. Clin. Nutr. 2000, 71, 1344S–1352S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; World Health Organization: Geneva, Switzerland, 2013.
- Lawrence, R.L.; Wall, C.R.; Bloomfield, F.H. Prevalence of Gestational Diabetes According to Commonly Used Data Sources: An Observational Study. BMC Pregnancy Childbirth 2019, 19, 349–358. [Google Scholar] [CrossRef] [PubMed]
- di Cianni, G.; Volpe, L.; Lencioni, C.; Miccoli, R.; Cuccuru, I.; Ghio, A.; Chatzianagnostou, K.; Bottone, P.; Teti, G.; del Prato, S.; et al. Prevalence and Risk Factors for Gestational Diabetes Assessed by Universal Screening. Diabetes Res. Clin. Pract. 2003, 62, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.; Ehrlich, S.; Sridhar, S.; Darbinian, J.; Moore, S.; Ferrara, A. Racial/Ethnic Disparities in the Prevalence of Gestational Diabetes Mellitus by BMI. Diabetes Care 2012, 35, 1492–1498. [Google Scholar] [CrossRef]
- Solomon, C.G.; Willett, W.C.; Carey, V.J.; Rich-Edwards, J.; Hunter, D.J.; Colditz, G.A.; Stampfer, M.J.; Speizer, F.E.; Spiegelman, D.; Manson, J.E. A Prospective Study of Pregravid Determinants of Gestational Diabetes Mellitus. J. Am. Med. Assoc. 1997, 278, 1078–1083. [Google Scholar] [CrossRef]
- Tryggvadottir, E.A.; Medek, H.; Birgisdottir, B.E.; Geirsson, R.T.; Gunnarsdottir, I. Association between Healthy Maternal Dietary Pattern and Risk for Gestational Diabetes Mellitus. Eur. J. Clin. Nutr. 2016, 70, 237–242. [Google Scholar] [CrossRef]
- He, J.-R.; Yuan, M.-Y.; Chen, N.-N.; Lu, J.-H.; Hu, C.-Y.; Mai, W.-B.; Zhang, R.-F.; Pan, Y.-H.; Qiu, L.; Wu, Y.-F.; et al. Maternal Dietary Patterns and Gestational Diabetes Mellitus: A Large Prospective Cohort Study in China. Br. J. Nutr. 2015, 113, 1292–1300. [Google Scholar] [CrossRef]
- Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Pre-Pregnancy Dietary Patterns and Risk of Gestational Diabetes Mellitus: Results from an Australian Population-Based Prospective Cohort Study. Diabetologia 2015, 58, 2726–2735. [Google Scholar] [CrossRef]
- Sartorelli, D.S.; Zuccolotto, D.C.C.; Crivellenti, L.C.; Franco, L.J. Dietary Patterns during Pregnancy Derived by Reduced-Rank Regression and Their Association with Gestational Diabetes Mellitus. Nutrition 2019, 60, 191–196. [Google Scholar] [CrossRef]
- Zhang, C.; Schulze, M.B.; Solomon, C.G.; Hu, F.B. A Prospective Study of Dietary Patterns, Meat Intake and the Risk of Gestational Diabetes Mellitus. Diabetologia 2006, 49, 2604–2613. [Google Scholar] [CrossRef]
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The Joint WHO/FAO Expert Consultation on Diet, Nutrition and the Prevention of Chronic Diseases: Process, Product and Policy Implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xu, F.; Zhong, C.; Tong, L.; Li, F.; Li, Q.; Chen, R.; Zhou, X.; Li, X.; Cui, W.; et al. Association between Chinese Dietary Guidelines Compliance Index for Pregnant Women and Risks of Pregnancy Complications in the Tongji Maternal and Child Health Cohort. Nutrients 2021, 13, 829. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Grant, C.C.; Wall, C.R.; Carr, P.E.; Bandara, D.K.; Schmidt, J.M.; Ivory, V.; Inskip, H.M.; Camargo, C.A.J. Adherence to Nutritional Guidelines in Pregnancy: Evidence from the Growing Up in New Zealand Birth Cohort Study. Public Health Nutr. 2014, 17, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Atatoa Carr, P.E.; Grant, C.C.; Robinson, E.M.; Bandara, D.K.; Bird, A.; Ivory, V.C.; Kingi, T.K.; Liang, R.; Marks, E.J.; et al. Cohort Profile: Growing Up in New Zealand. Int. J. Epidemiol. 2013, 42, 65–75. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Prev. Med. 2007, 45, 247–251. [Google Scholar] [CrossRef]
- Statistics New Zealand Statistical Standard for Ethnicity 2005; Statistics New Zealand: Wellington, New Zealand, 2005.
- Salmond, C.E.; Crampton, P.; Atkinson, J. NZDep2006 Index of Deprivation. Available online: http://www.otago.ac.nz/wellington/otago020348.pdf (accessed on 13 February 2019).
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Wall, C.R.; Gammon, C.S.; Bandara, D.K.; Grant, C.C.; Atatoa Carr, P.E.; Morton, S. Dietary Patterns in Pregnancy in New Zealand—Influence of Maternal Socio-Demographic, Health and Lifestyle Factors. Nutrients 2016, 8, 300. [Google Scholar] [CrossRef]
- Ministry of Health Food and Nutrition Guidelines for Healthy Pregnant and Breastfeeding Women: A Background Paper; Ministry of Health: Wellington, New Zealand, 2006; ISBN 9780478317794.
- University of Otago. Ministry of Health Methodology Report for the 2008/09 NZ Adult Nutrition Survey|Ministry of Health NZ; Ministry of Health: Wellington, New Zealand, 2011.
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, Validation and Utilisation of Food-Frequency Questionnaires-a Review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef]
- Morton, S.M.; Atatoa Carr, P.E.; Bandara, D.K.; Grant, C.C.; Ivory, V.C.; Kingi, T.R.; Liang, R.; Perese, L.M.; Peterson, E.; Pryor, J.E.; et al. Growing Up in New Zealand: A Longitudinal Study of New Zealand Children and Their Families. Report 1: Before We Are Born; The University of Auckland: Auckland, New Zealand, 2010. [Google Scholar]
- New Zealand Society for the Study of Diabetes Screening for Diabetes in Asymptomatic Individuals. N. Z. Med. J. 1995, 108, 464–465.
- Simmons, D.; Wolmarans, L.; Cutchie, W.; Johnson, E.; Haslam, A.; Roodt, C.; Rowan, J. Gestational Diabetes Mellitus: Time for Consensus on Screening and Diagnosis. N. Z. Med. J. 2006, 199, U1807. [Google Scholar]
- Ministry of Health. Screening Diagnosis and Management of GDM in NZ: A Clinical Practice Guideline; Ministry of Health: Wellington, New Zealand, 2014.
- Lawrence, R.L.; Wall, C.R.; Bloomfield, F.H.; Crowther, C.A. Dietetic Management of Gestational Diabetes in New Zealand: A Cross-Sectional Survey. Nutr. Diet. 2016, 73, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hassani Zadeh, S.; Boffetta, P.; Hosseinzadeh, M. Dietary Patterns and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Cohort Studies. Clin. Nutr. ESPEN 2020, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gresham, E.; Collins, C.E.; Mishra, G.D.; Byles, J.E.; Hure, A.J. Diet Quality before or during Pregnancy and the Relationship with Pregnancy and Birth Outcomes: The Australian Longitudinal Study on Women’s Health. Public Health Nutr. 2016, 19, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Gicevic, S.; Gaskins, A.J.; Fung, T.T.; Rosner, B.; Tobias, D.K.; Isanaka, S.; Willett, W.C. Evaluating Pre-Pregnancy Dietary Diversity vs. Dietary Quality Scores as Predictors of Gestational Diabetes and Hypertensive Disorders of Pregnancy. PLoS ONE 2018, 13, e0195103. [Google Scholar]
- Lawrence, R.L.; Wall, C.R.; Bloomfield, F.H. Dietary Patterns and Dietary Adaptations in Women with and without Gestational Diabetes: Evidence from the Growing Up in New Zealand Study. Nutrients 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Harding, J.; Wall, C.; Crowther, C. Sociodemographic Factors Associated with Adherence to Dietary Guidelines in Women with Gestational Diabetes: A Cohort Study. Nutrients 2021, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- Olander, E.K.; Atkinson, L.; Edmunds, J.K.; French, D.P. Promoting Healthy Eating in Pregnancy: What Kind of Support Services Do Women Say They Want? Prim. Health Care Res. Dev. 2012, 13, 237–243. [Google Scholar] [CrossRef]
- Hillier, S.E.; Olander, E.K. Women’s Dietary Changes before and during Pregnancy: A Systematic Review. Midwifery 2017, 49, 19–31. [Google Scholar] [CrossRef]
- Wen, L.M.; Flood, V.M.; Simpson, J.M.; Rissel, C.; Baur, L.A. Dietary Behaviours during Pregnancy: Findings from First-Time Mothers in Southwest Sydney, Australia. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 13. [Google Scholar] [CrossRef]
- Malek, L.; Umberger, W.; Makrides, M.; Zhou, S.J. Adherence to the Australian Dietary Guidelines during Pregnancy: Evidence from a National Study. Public Health Nutr. 2016, 19, 1155–1163. [Google Scholar] [CrossRef]
- Bookari, K.; Yeatman, H.; Williamson, M. Exploring Australian Women’s Level of Nutrition Knowledge during Pregnancy: A Cross-Sectional Study. Int. J. Women’s Health 2016, 8, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; von Hurst, P.; Rapson, J.; Conlon, C. Dietary Choices of New Zealand Women during Pregnancy and Lactation. Nutrients 2020, 12, 2692. [Google Scholar] [CrossRef] [PubMed]
- Paterson, H.; Hay-Smith, E.J.C.; Treharne, G.J. Women’s Experiences of Changes in Eating during Pregnancy: A Qualitative Study in Dunedin, New Zealand. N. Z. Coll. Midwives J. 2016, 52, 5–11. [Google Scholar] [CrossRef][Green Version]
- Arrish, J.; Yeatman, H.; Williamson, M. Australian Midwives and Provision of Nutrition Education during Pregnancy: A Cross Sectional Survey of Nutrition Knowledge, Attitudes, and Confidence. Women Birth 2016, 29, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Arrish, J.; Yeatman, H.; Williamson, M. Midwives and Nutrition Education during Pregnancy: A Literature Review. Women Birth J. Aust. Coll. Midwives 2014, 27, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.; Green, T. Nutrition Knowledge and Attitudes of New Zealand Registered Midwives. Nutr. Diet. 2007, 64, 290–294. [Google Scholar] [CrossRef]
- Pan, S.; Dixon, L.; Paterson, H.; Campbell, N.; Sze, Y.; Dixon, L.; Paterson, H.; Campbell, N. New Zealand LMC Midwives’ Approaches to Discussing Nutrition, Activity and Weight Gain during Pregnancy. N. Z. Coll. Midwives J. 2014, 50, 24–29. [Google Scholar]
- Dietitians Board Dietitians Board-Te Mana Mātanga Mātai Kai. Available online: https://www.dietitiansboard.org.nz/public/ (accessed on 1 July 2021).
- Morisset, A.S.; Cote, J.A.; Michaud, A.; Robitaille, J.; Tchernof, A.; Dube, M.C.; Veillette, J.; Weisnagel, S.J. Dietary Intakes in the Nutritional Management of Gestational Diabetes Mellitus. Can. J. Diet. Pract. Res. 2014, 75, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Bowers, K.; Tobias, D.K.; Hu, F.B.; Zhang, C. Prepregnancy Dietary Protein Intake, Major Dietary Protein Sources, and the Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2013, 36, 2001–2008. [Google Scholar] [CrossRef]
- Ministry of Health. Eating and Activity Guidelines for New Zealand Adults; Ministry of Health: Wellington, New Zealand, 2020.
| n (%) | Women without GDM | Women with GDM | p-Value |
|---|---|---|---|
| 5111 (94.8) | 280 (5.2) | ||
| Age group (years) | <0.005 | ||
| <20 | 259 (5.1) | <10 (<10) | |
| 20–24 | 747 (14.6) | 28 (10.0) | |
| 25–29 | 1267 (24.8) | 51 (18.2) | |
| 30–34 | 1662 (31.7) | 95 (33.9) | |
| 35–39 | 1039 (20.3) | 81 (28.9) | |
| 40 and over | 177 (3.5) | 20 (7.1) | |
| Self-prioritised ethnicity | <0.005 | ||
| European | 2915 (57.1) | 103 (36.8) | |
| Māori | 690 (13.5) | 22 (7.9) | |
| Pacific | 631 (12.4) | 55 (19.6) | |
| Asian | 681 (13.3) | 84 (30.0) | |
| Other | 186 (3.6) | 16 (5.7) | |
| Parity | 0.619 | ||
| First child | 2168 (42.4) | 123 (43.9) | |
| Socioeconomic deprivation | 0.021 | ||
| 1 to 2 (least deprived) | 865 (16.9) | 30 (10.7) | |
| 3 to 4 | 978 (19.1) | 45 (16.1) | |
| 5 to 6 | 909 (17.8) | 61 (21.8) | |
| 7 to 8 | 1052 (20.6) | 63 (22.5) | |
| 9 to 10 (most deprived) | 1305 (25.5) | 81 (28.9) | |
| Pre-pregnancy BMI (Kg/m2) | <0.005 | ||
| <18.5 | 192 (4.2) | <10 (<10) | |
| 18.5 to 24.9 | 2559 (56.2) | 103 (41.4) | |
| 25 to 29.9 | 1034 (22.7) | 60 (24.1) | |
| ≥30 | 772 (16.9) | 79 (31.7) | |
| Gestational weight gain | |||
| Gained ≥5 kg | 4460 (88.9) | 229 (83.0) | |
| Gained <5 kg | 377 (7.5) | 32 (11.6) | |
| No change | 43 (0.9) | <10 (<10) | |
| Lost <5 kg | 74 (1.5) | 10 (3.6) | |
| Lost ≥5 kg | 62 (1.2) | <10 (<10) | |
| Physical activity ‡ | |||
| Physically active pre-pregnancy | 2583 (50.5) | 119 (42.5) | 0.009 |
| Physically active during first trimester | 1464 (28.6) | 62 (22.1) | 0.019 |
| Physically active during second & third trimester | 1152 (22.5) | 59 (21.1) | 0.567 |
| Smoking patterns | 0.012 | ||
| Continued smoking | 509 (10.0) | 13 (4.6) | |
| Stopped smoking | 494 (9.7) | 31 (11.1) | |
| Non-smoker | 4094 (80.3) | 236 (84.3) | |
| Alcohol consumption | <0.005 | ||
| Any drinking during pregnancy | 1539 (30.1) | 42 (15.0) | |
| Stopped drinking | 2279 (44.6) | 100 (35.7) | |
| Non-drinker | 1288 (25.2) | 138 (49.3) |
| Adherence to Food Group Recommendations | Primary Analyses | Stratified Analyses | ||
|---|---|---|---|---|
| Women without GDM n = 5109 | Women with GDM n = 280 | GDM Diagnosed Prior to FFQ n = 124 | GDM Diagnosed after FFQ n = 109 | |
| Four food groups | 144 (2.8) | <10 (<10) | <10 (<10) | <10 (<10) |
| Three food groups | 517 (10.1) | 31 (11.1) | <10 (<10) | 13 (11.9) |
| Two food groups | 1295 (25.3) | 68 (24.3) | 32 (25.8) | 27 (24.8) |
| One food group | 1954 (38.2) | 97 (34.6) | 49 (39.5) | 33 (30.3) |
| No food groups | 1199 (23.5) | 75 (26.8) | 32 (25.8) | 31 (28.4) |
| Fruit | 4245 (83.1) | 228 (81.4) | 91 (73.4) * | 95 (87.2) |
| Vegetables | 1382 (27.0) | 87 (31.1) | 37 (29.8) | 35 (32.1) |
| Breads and cereals | 1350 (26.4) | 66 (23.6) | 25 (20.2) | 25 (22.9) |
| Milk and milk products | 2986 (58.4) | 140 (50.0) * | 57 (46.0) * | 60 (55.0) |
| Lean meat, poultry, seafood, eggs, nuts and seeds and legumes | 1073 (21.0) | 81 (28.9) ** | 36 (29.0) * | 30 (27.5) |
| Primary Analyses | Stratified Analyses | |||
|---|---|---|---|---|
| Adherence to Food Group Recommendations | OR (CI) n = 5391 | aOR (CI) n = 4784 | GDM Diagnosed Prior to FFQ and Women without GDM aOR (CI) n = 4647 | GDM Diagnosed After FFQ and Women without GDM aOR (CI) n = 4629 |
| Four vs. at least three food groups | 1.15 (0.58, 2.27) | 0.88 (0.41, 1.89) | 0.69 (0.44, 1.10) | 1.46 (0.78, 2.73) |
| At least three vs. at least two food groups | 1.12 (0.80, 1.58) | 1.12 (0.77, 1.62) | 1.24 (0.81, 1.90) | 1.32 (0.84, 2.06) |
| At least two vs. at least one food groups | 1.01 (0.79, 1.30) | 1.07 (0.81, 1.41) | 0.60 (0.35, 1.00) | 0.80 (0.48, 1.34) |
| At least one vs. no food groups | 0.84 (0.64, 1.10) | 0.95 (0.70, 1.28) | 0.89 (0.60, 1.33) | 0.95 (0.62, 1.46) |
| Four vs. no food groups | 1.00 (0.49, 2.04) | 0.77 (0.34, 1.77) | 1.30 (0.83, 2.04) | 1.06 (0.65, 1.75) |
| Fruit | 0.89 (0.66, 1.22) | 1.00 (0.70, 1.43) | 0.69 (0.44, 1.10) | 1.46 (0.78, 2.73) |
| Vegetables | 1.22 (0.94, 1.58) | 1.24 (0.93, 1.66) | 1.24 (0.81, 1.90) | 1.32 (0.84, 2.06) |
| Breads and cereals | 0.86 (0.65, 1.14) | 0.82 (0.59, 1.14) | 0.60 (0.35, 1.00) | 0.80 (0.48, 1.34) |
| Milk and milk products | 0.71 (0.56, 0.91) * | 0.93 (0.71, 1.22) | 0.89 (0.60, 1.33) | 0.95 (0.62, 1.46) |
| Lean meat, poultry, seafood, eggs, nuts and seeds and legumes | 1.53 (1.17, 2.00) ** | 1.21 (0.88, 1.65) | 1.30 (0.83, 2.04) | 1.06 (0.65, 1.75) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, R.L.; Wall, C.R.; Bloomfield, F.H. Adherence to Dietary Guidelines among Women with and without Gestational Diabetes: Evidence from the Growing up in New Zealand Study. Nutrients 2022, 14, 2145. https://doi.org/10.3390/nu14102145
Lawrence RL, Wall CR, Bloomfield FH. Adherence to Dietary Guidelines among Women with and without Gestational Diabetes: Evidence from the Growing up in New Zealand Study. Nutrients. 2022; 14(10):2145. https://doi.org/10.3390/nu14102145
Chicago/Turabian StyleLawrence, Robyn L., Clare R. Wall, and Frank H. Bloomfield. 2022. "Adherence to Dietary Guidelines among Women with and without Gestational Diabetes: Evidence from the Growing up in New Zealand Study" Nutrients 14, no. 10: 2145. https://doi.org/10.3390/nu14102145
APA StyleLawrence, R. L., Wall, C. R., & Bloomfield, F. H. (2022). Adherence to Dietary Guidelines among Women with and without Gestational Diabetes: Evidence from the Growing up in New Zealand Study. Nutrients, 14(10), 2145. https://doi.org/10.3390/nu14102145







