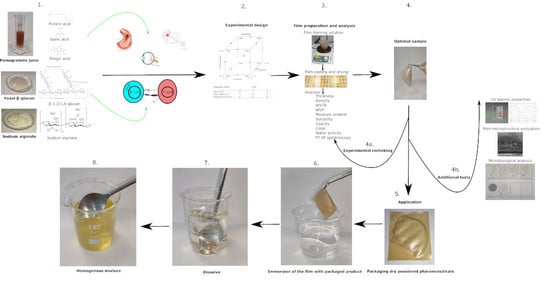
Formulation, Characterization and Optimization of β–Glucan and Pomegranate Juice Based Films for Its Potential in Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Yeast β–Glucan
2.1.2. Preparation of Film-Forming Solution (FFS) and Casting
2.2. Methods
2.2.1. Thickness and Density Measurements
2.2.2. Water Vapor Transmission Rate (WVTR)
2.2.3. Water Vapor Permeability (WVP)
2.2.4. Moisture Content (MC) and Solubility
2.2.5. Film Opacity and Color Measurement
2.2.6. Water Activity
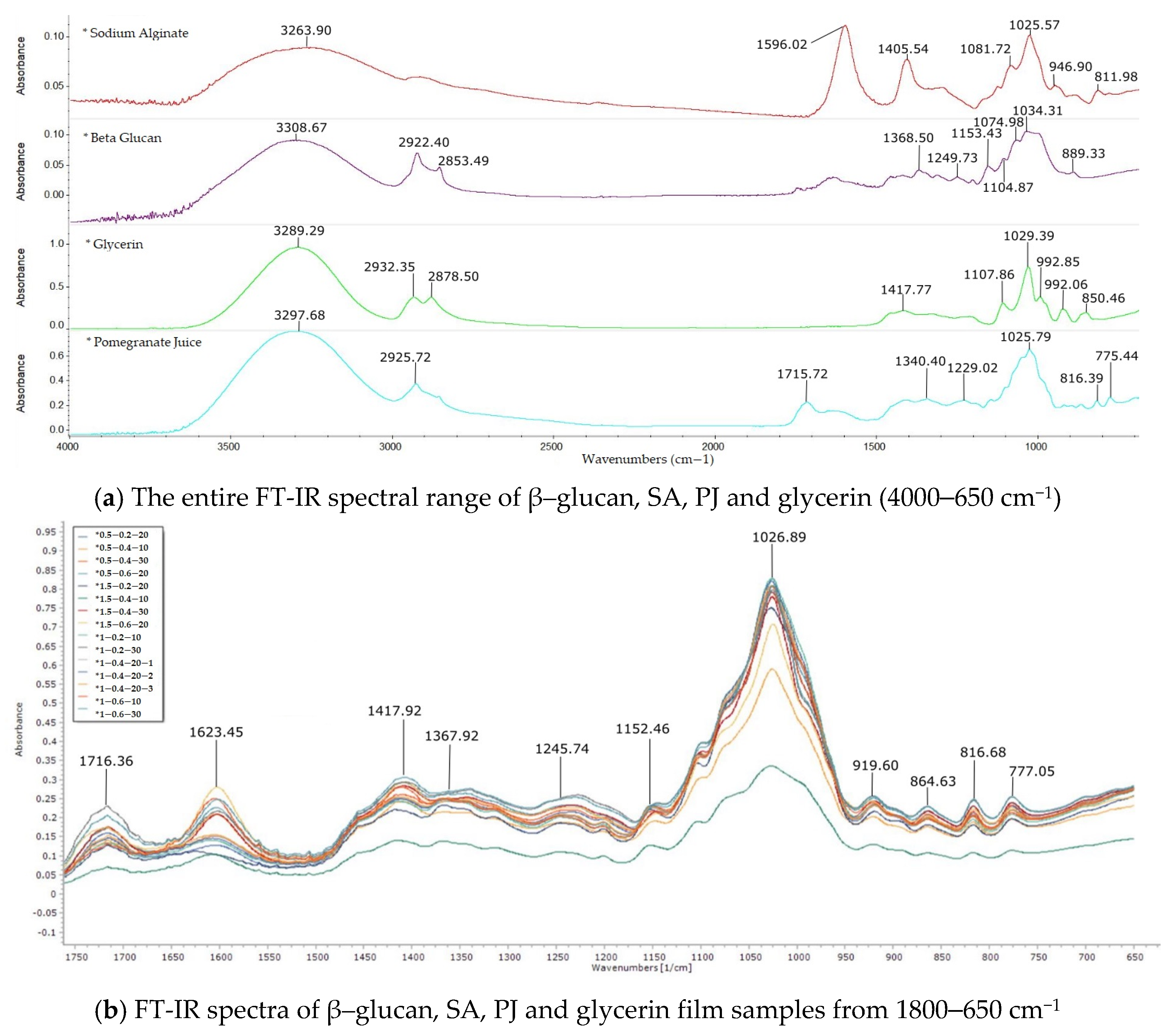
2.2.7. FT-IR Spectroscopy
2.2.8. Scanning Electron Microscopy (SEM)
2.2.9. Model Fitting
3. Results and Discussion
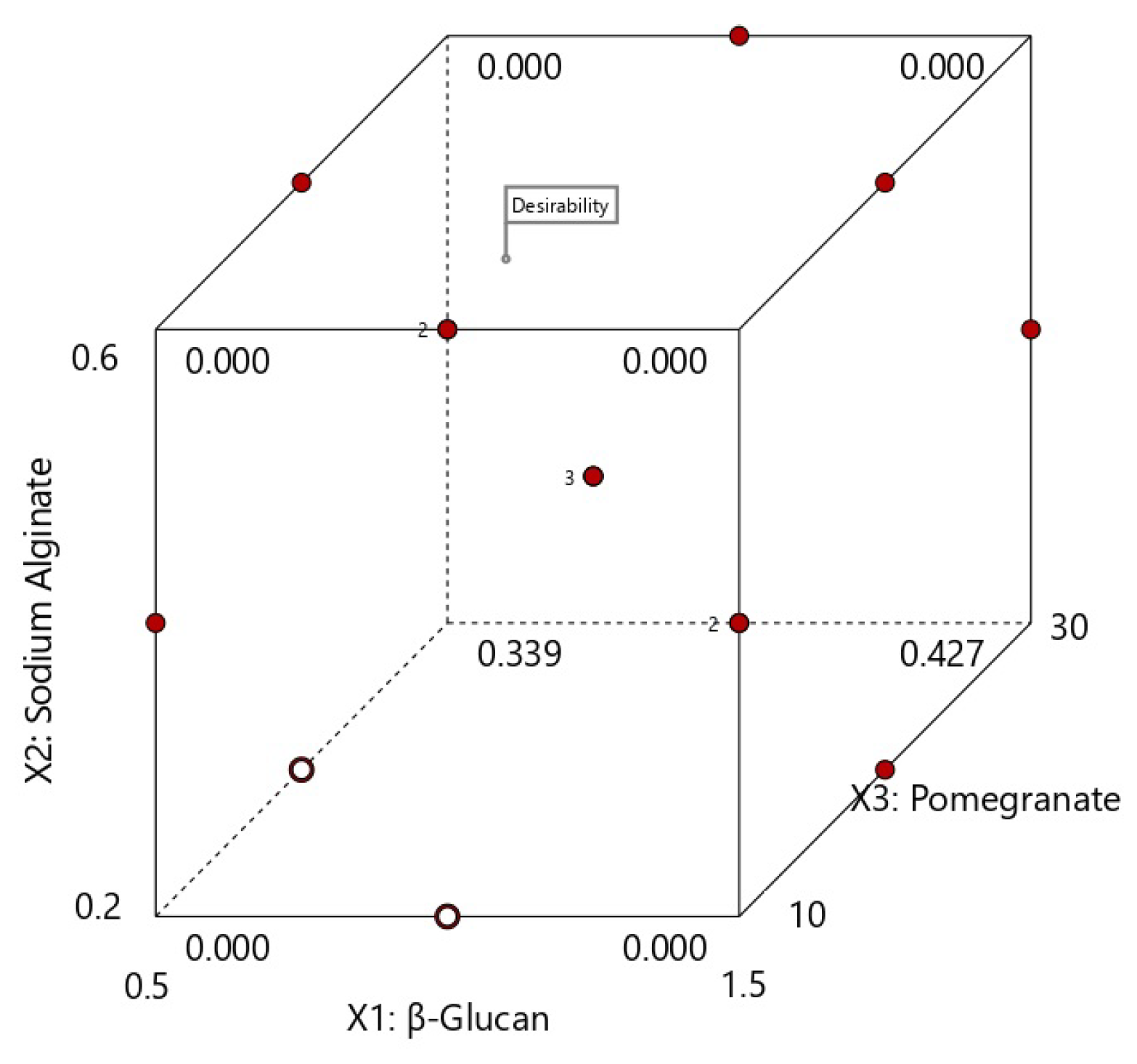
3.1. Optimization of Film Forming Solution by Response Surface Methodology
3.1.1. Statistical Design
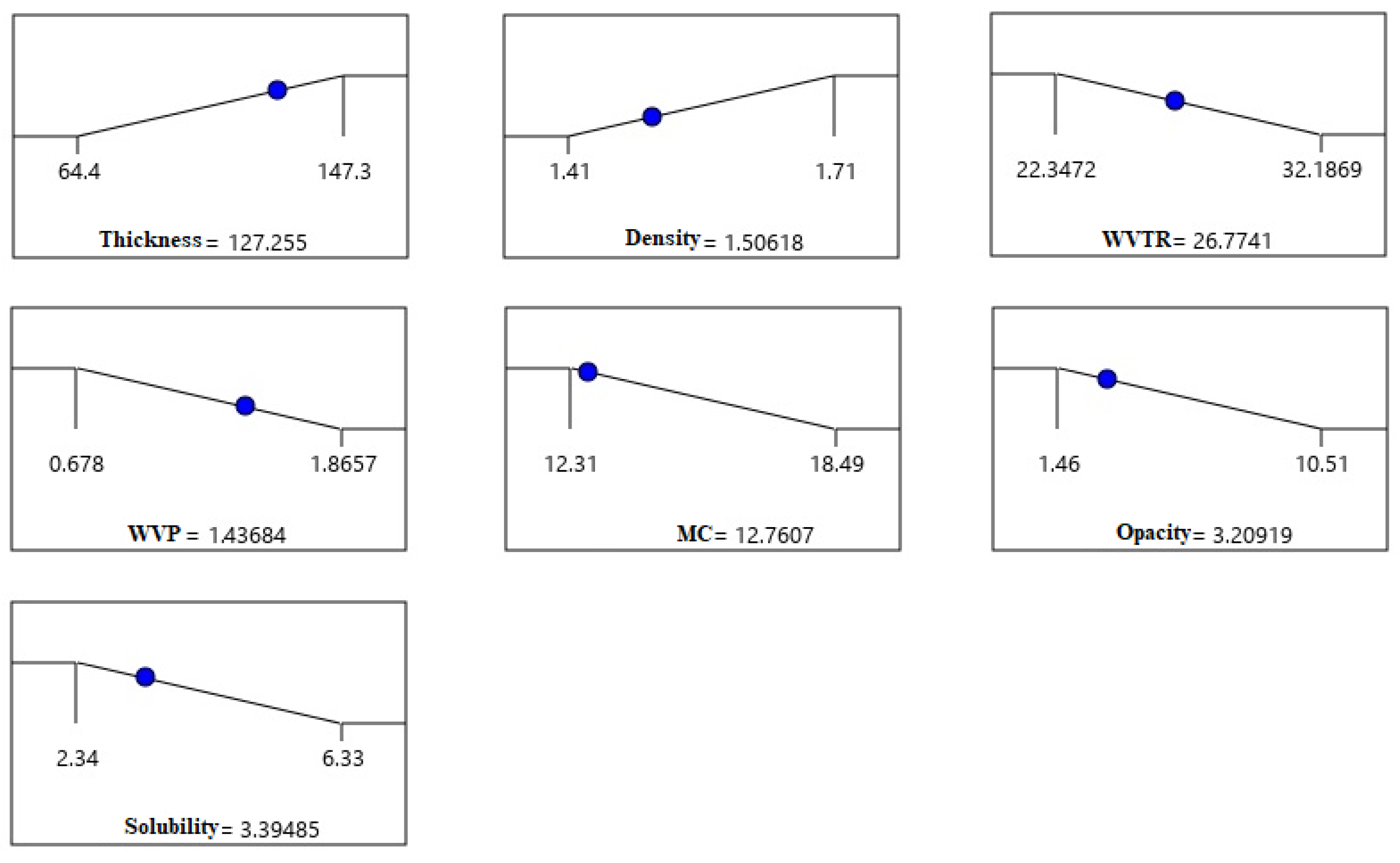
3.1.2. Response Surface Analysis
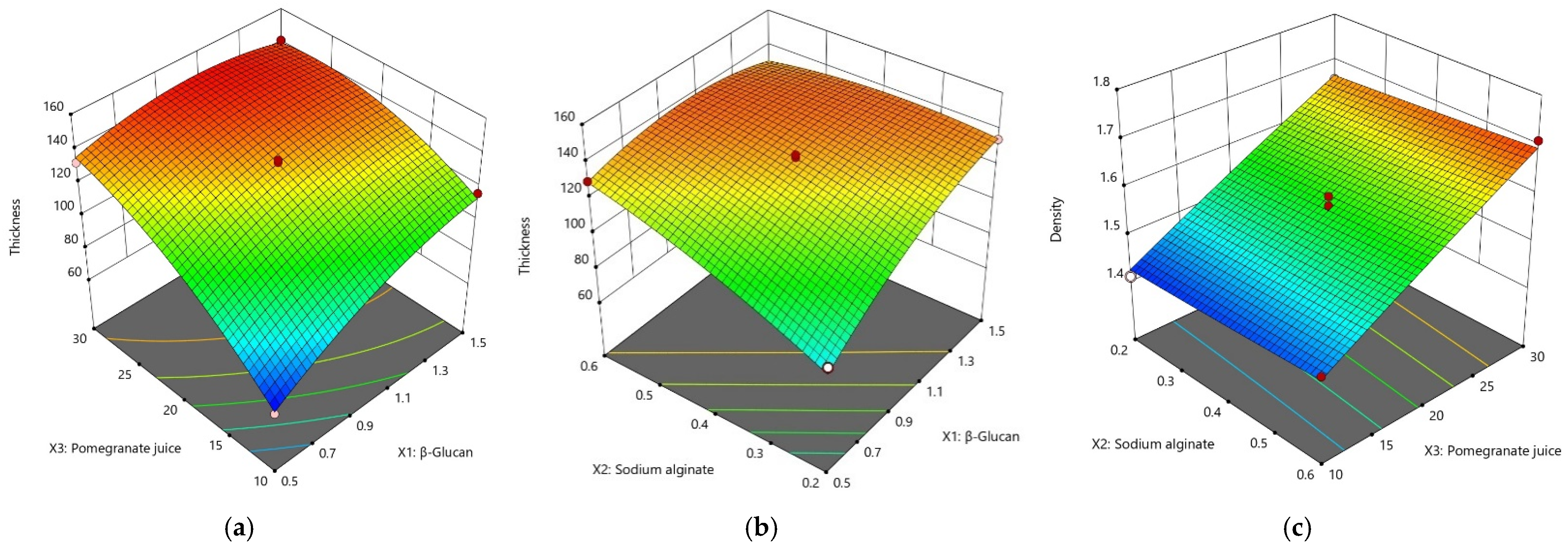
- Thickness and density
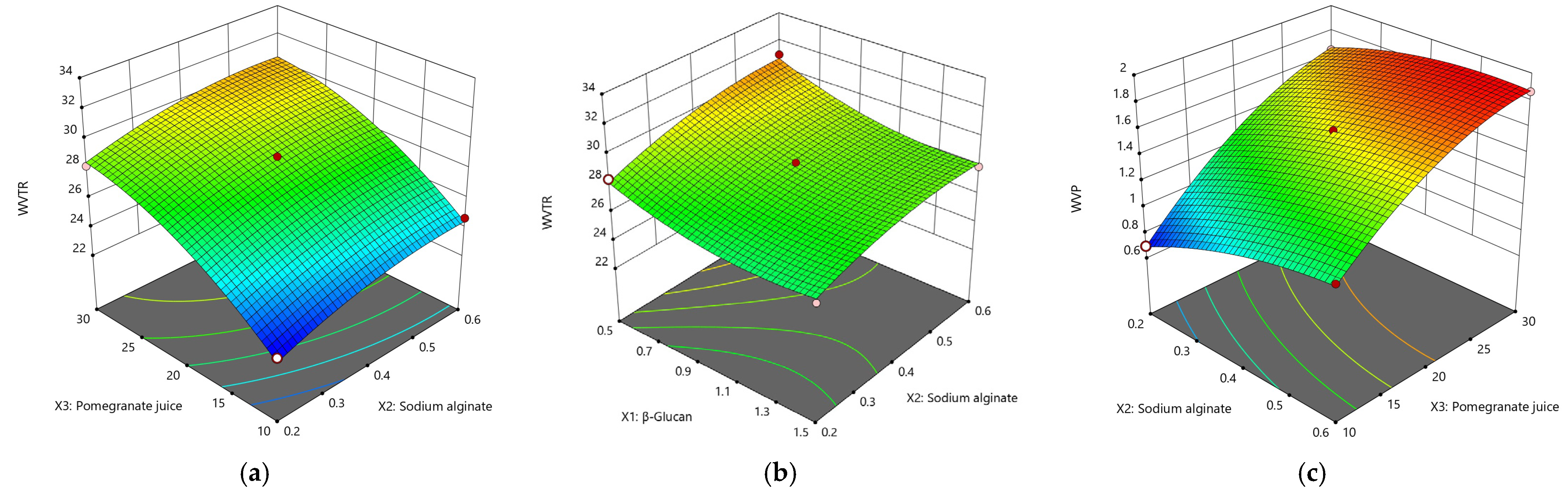
- Water vapor transmission rate (WVTR)
R2 = 0.9824; p < 0.05; F-value = 30.92
- Water vapor permeability (WVP)
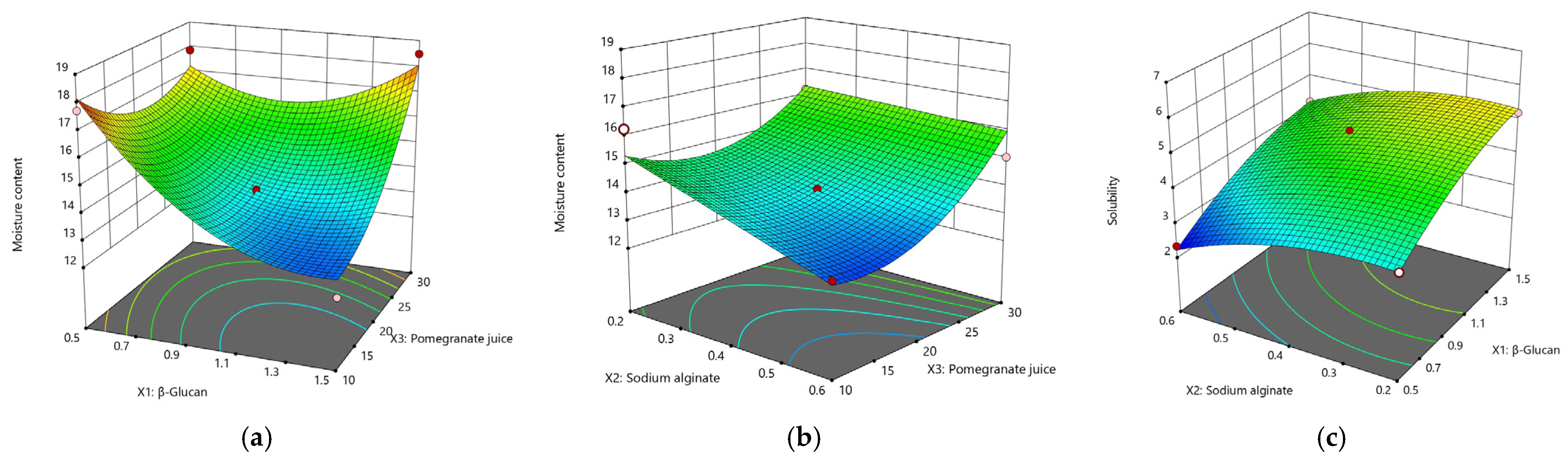
- Moisture content (MC)
- Film solubility
- Opacity and color
3.1.3. Water Activity (aw)
3.1.4. FT-IR Spectroscopy
3.2. Optimization of Process Parameters for Optimal Film Development
3.2.1. Validation and Confirmation of Computationally Predicted Data
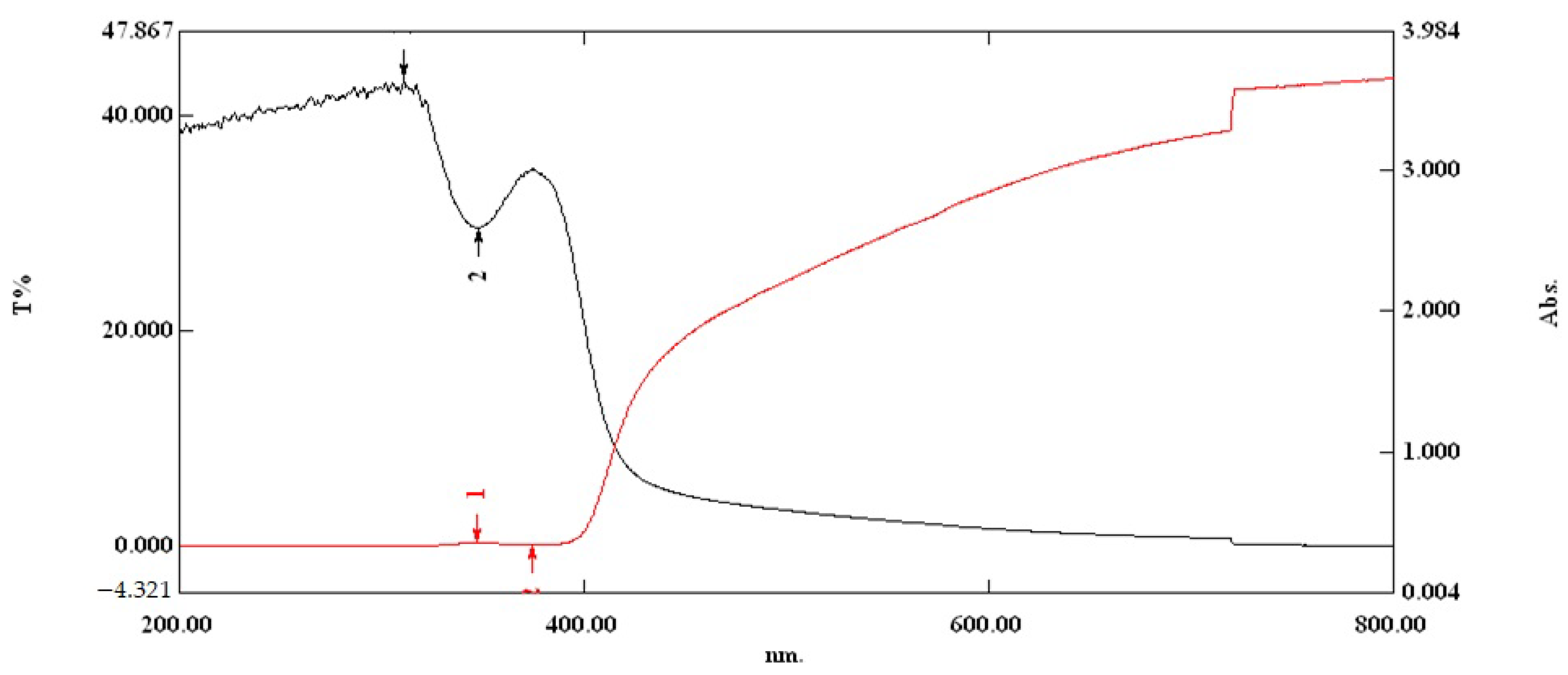
3.2.2. Evaluation of the Optical Properties on Optimal Film in the UV-VIS Region
3.2.3. Microbiological Analysis of Optimal Sample
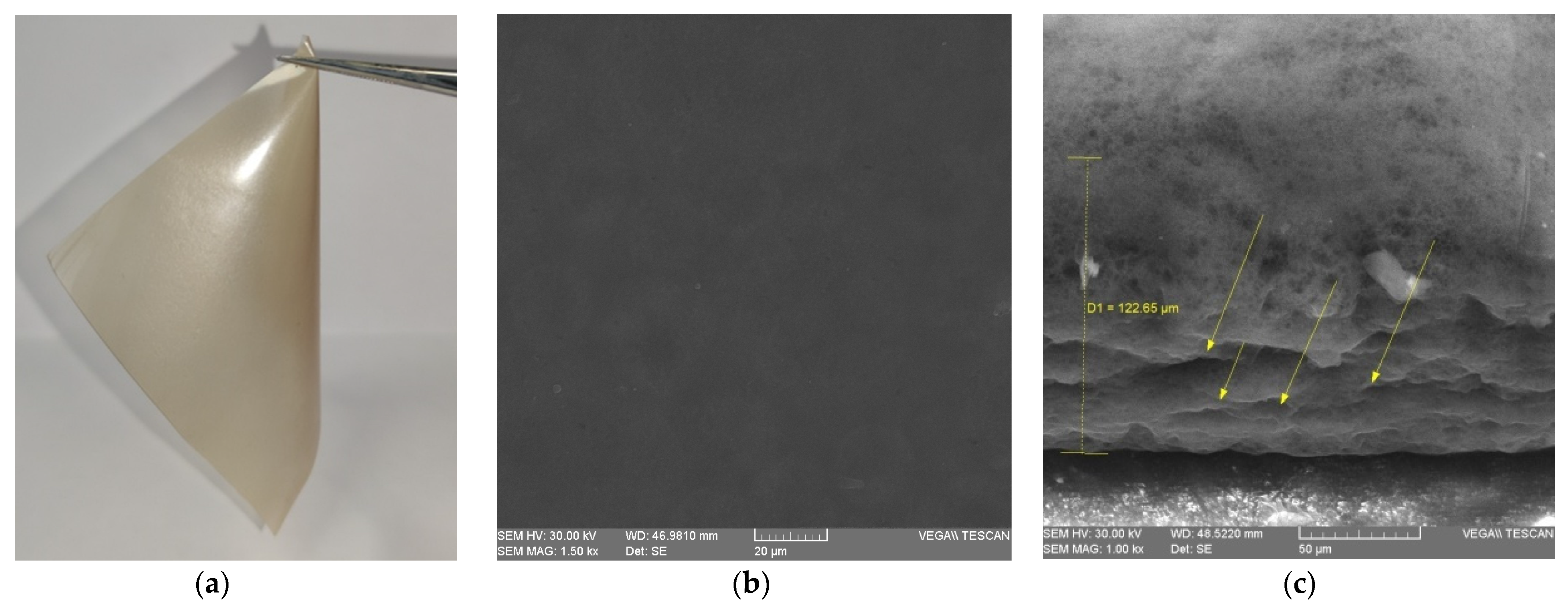
3.2.4. Scanning Electron Microscopy (SEM)
3.3. The Packaging Concept of Dry Powdered Pharmaceuticals for Special Medical Purposes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Kroner, Z. The Relationship between Alzheimer’s Disease and Diabetes: Type 3 Diabetes. Altern. Med. Rev. 2009, 14, 373–379. [Google Scholar]
- Mellitus, D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2005, 28, S5–S10. [Google Scholar]
- Lawrence, R.D. Types of human diabetes. Br. Med. J. 1951, 1, 373. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef]
- Arora, K.; Tomar, P.C.; Mohan, V. Diabetic neuropathy: An insight on the transition from synthetic drugs to herbal therapies. J. Diabetes Metab. Disord. 2021, 20, 1773–1784. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Debeaufort, F. Bioactive edible films for food applications: Influence of the bioactive compounds on film structure and properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 1137–1153. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, D.; Regenstein, J.M.; Xia, W.; Dong, J. A comprehensive review on natural bioactive films with controlled release characteristics and their applications in foods and pharmaceuticals. Trends Food Sci. Technol. 2021, 112, 690–707. [Google Scholar] [CrossRef]
- Nogueira, G.F.; de Oliveira, R.A.; Velasco, J.I.; Fakhouri, F.M. Methods of incorporating plant-derived bioactive compounds into films made with agro-based polymers for application as food packaging: A brief review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alias, A.R.; Wan, M.K.; Sarbon, N.M. Emerging materials and technologies of multi-layer film for food packaging application: A review. Food Control 2022, 136, 108875. [Google Scholar] [CrossRef]
- Mandeep, K.; Rana, A.C.; Nimrata, S. Fast Dissolving Films: An Innovative Drug Delivery System. Int. J. Pharm. Res. Allied Sci. 2013, 2, 14–24. [Google Scholar]
- Kogan, G.; Alföldi, J.; Masler, L. 13C-nmr spectroscopic investigation of two yeast cell wall β-D-glucans. Biopolym. Orig. Res. Biomol. 1988, 27, 1055–1063. [Google Scholar] [CrossRef]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of fungal β-glucan in host defense signaling by dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Nedra, D.; Castro, S.P.M.; Paulín, E.G.L.; Kwiatkowski, S.; Edgar, S.; Reyes, R.E.; González, C.R.; Jiménez, R.C.; Herrera, O.; Andrade, A.A.; et al. The Complex World of Polysaccharides; BoD–Books on Demand: Paris, France, 2012; ISBN 9789535108191. [Google Scholar]
- European Commission. Commission Implementing Decision (EU) 2017/2078 of 10 November 2017 Authorising an Extension of Use of Yeast Beta-Glucans as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (Notified Under Document C(2017) 7391). Off. J. Eur. Union 2017, 2009, 12–15. [Google Scholar]
- Cao, Y.; Sun, Y.; Zou, S.; Li, M.; Xu, X. Orally administered baker’s yeast β-glucan promotes glucose and lipid homeostasis in the livers of obesity and diabetes model mice. J. Agric. Food Chem. 2017, 65, 9665–9674. [Google Scholar] [CrossRef]
- Karumuthil-Melethil, S.; Gudi, R.; Johnson, B.M.; Perez, N.; Vasu, C. Fungal β-glucan, a Dectin-1 ligand, promotes protection from type 1 diabetes by inducing regulatory innate immune response. J. Immunol. 2014, 193, 3308–3321. [Google Scholar] [CrossRef] [Green Version]
- Richter, J.; Závorková, M.; Vetvicka, V.; Liehneová, I.; Kral, V.; Dobiasova, L.R. Effects of β-glucan and vitamin D supplementation on inflammatory parameters in patients with diabetic retinopathy. J. Diet. Suppl. 2019, 16, 369–378. [Google Scholar] [CrossRef]
- Baliga, M.S.; Shivashankara, A.R.; Shetty, C.B.; Thilakchand, K.R.; Periera, N.; Palatty, P.L. Antidiabetic effects of Punica granatum L, (Pomegranate): A review. In Bioactive Food as Dietary Interventions for Diabetes; Elsevier: Amsterdam, The Netherlands, 2012; pp. 355–369. ISBN 9780123971531. [Google Scholar] [CrossRef]
- Arun, N.; Singh, D.P. Punica granatum: A review on pharmacological and therapeutic properties. Int. J. Pharm. Sci. Res. 2012, 3, 1240. [Google Scholar]
- El-Nemr, S.E.; Ismail, I.A.; Ragab, M. Chemical composition of juice and seeds of pomegranate fruit. Food/Nahrung 1990, 34, 601–606. [Google Scholar] [CrossRef]
- Wang, R.; Ding, Y.; Liu, R.; Xiang, L.; Du, L. Pomegranate: Constituents, bioactivities and pharmacokinetics. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 77–87. [Google Scholar]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Józwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective effects of pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banihani, S.; Swedan, S.; Alguraan, Z. Pomegranate and type 2 diabetes. Nutr. Res. 2013, 33, 341–348. [Google Scholar] [CrossRef]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef]
- Guerrero-Solano, J.A.; Jaramillo-Morales, O.A.; Velázquez-González, C.; la O-Arciniega, D.; Castañeda-Ovando, A.; Betanzos-Cabrera, G.; Bautista, M. Pomegranate as a potential alternative of pain management: A review. Plants 2020, 9, 419. [Google Scholar] [CrossRef] [Green Version]
- Medjakovic, S.; Jungbauer, A. Pomegranate: A fruit that ameliorates metabolic syndrome. Food Funct. 2013, 4, 19–39. [Google Scholar] [CrossRef]
- Eltabakh, M.; Kassab, H.; Badawy, W.; Abdin, M.; Abdelhady, S. Active Bio-composite Sodium Alginate/Maltodextrin Packaging Films for Food Containing Azolla pinnata Leaves Extract as Natural Antioxidant. J. Polym. Environ. 2022, 30, 1355–1365. [Google Scholar] [CrossRef]
- Abrisham, M.; Noroozi, M.; Panahi-Sarmad, M.; Arjmand, M.; Goodarzi, V.; Shakeri, Y.; Golbaten-Mofrad, H.; Dehghan, P.; Sahzabi, A.S.; Sadri, M. The role of polycaprolactone-triol (PCL-T) in biomedical applications: A state-of-the-art review. Eur. Polym. J. 2020, 131, 109701. [Google Scholar] [CrossRef]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Vetvicka, V. Comparison of Immunological Effects of Commercially Available β-Glucans: Part III. Int. Clin. Pathol. J. 2016, 2, 00046. [Google Scholar] [CrossRef]
- Thanardkit, P.; Khunrae, P.; Suphantharika, M.; Verduyn, C. Glucan from spent brewer’s yeast: Preparation, analysis and use as a potential immunostimulant in shrimp feed. World J. Microbiol. Biotechnol. 2002, 18, 527–539. [Google Scholar] [CrossRef]
- Zechner-Krpan, V.; Petravić-Tominac, V.; Gospodarić, I.; Sajli, L.; Daković, S.; Filipović-Grčić, J. Characterization of β-glucans isolated from brewer’s yeast and dried by different methods. Food Technol. Biotechnol. 2010, 48, 189–197. [Google Scholar]
- Liu, X.Y.; Wang, Q.; Cui, S.W.; Liu, H.Z. A new isolation method of β-d-glucans from spent yeast Saccharomyces cerevisiae. Food Hydrocoll. 2008, 22, 239–247. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Spent Brewer’s yeast as a source of insoluble β-glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Petravić-Tominac, V.; Zechner-Krpan, V.; Berković, K.; Galović, P.; Herceg, Z.; Srečec, S.; Špoljarić, I. Rheological properties, water-holding and oil-binding capacities of particulate β-glucans isolated from spent Brewer’s yeast by three different procedures. Food Technol. Biotechnol. 2011, 49, 56–64. [Google Scholar]
- Kopecká, M. Yeast and fungal cell-wall polysaccharides can self-assemble in vitro into an ultrastructure resembling in vivo yeast cell walls. J. Electron Microsc. 2013, 62, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Valovirta, I. Water Vapor Permeability and Thermal Conductivity as a Function of temperature and relative humidity. In Proceedings of the Performance of Exterior Envelopes of Whole Buildings, IX International Conference, Clearwater Beach, FL, USA, 5–10 December 2004. [Google Scholar]
- ASTM Standard Test Methods for Water Vapor Transmission of Materials 1. ASTM Int. 2018, i, 1–14.
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; de la Osa, O.; Wagner, J.R. Use of Residual Yeast Cell Wall for New Biobased Materials Production: Effect of Plasticization on Film Properties. Food Bioprocess Technol. 2018, 11, 1995–2007. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Musavi, M.; Oromiehie, A.R.; Rezayi, K.; Razmi, E.; Milani, J. Effect of plasticizing sugars on water vapor permeability, surface energy and microstructure properties of zein films. LWT Food Sci. Technol. 2007, 40, 1191–1197. [Google Scholar] [CrossRef]
- Nabi, M.; Jafar, D. Preparation and characterization of a novel biodegradable film based on sulfated polysaccharide extracted from seaweed Ulva intestinalis. Food Sci. Nutr. 2021, 9, 4108–4116. [Google Scholar] [CrossRef]
- Wexler, A. Vapor pressure formulation for water in range 0 to 100 Degrees C: A revision. J. Res. Natl. Bur. Stand. 1976, 80, 775. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.; Santos, R.; Viegas, C.; Sapata, M.M.; dos Santos, R.G.; Condeço, J.; Marques, A.C.; Bordado, J.C. Edible films to improve quality and shelf life of fresh tortillas. Int. J. Gastron. Food Sci. 2022, 27, 100480. [Google Scholar] [CrossRef]
- Oluwasina, O.O.; Olaleye, F.K.; Olusegun, S.J.; Oluwasina, O.O.; Mohallem, N.D.S. Influence of oxidized starch on physicomechanical, thermal properties, and atomic force micrographs of cassava starch bioplastic film. Int. J. Biol. Macromol. 2019, 135, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, M. Water content, water activity, water structure and the stability of foodstuffs. Food Control 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Sherafatkhah, S.; Ainaz, A.; Leila, A.; Narmela, R.; Hamed, A. Preparation and characterization of gelatin/β—Glucan nanocomposite film incorporated with ZnO nanoparticles as an active food packaging system. J. Polym. Environ. 2021, 29, 1143–1152. [Google Scholar] [CrossRef]
- Novák, M.; Synytsyaa, A.; Gedeonb, O.; Slepičkac, P.; Procházkab, V.; Synytsyad, A.; Blahovece, J.; Hejlováe, A.; Čopíkováa, J. Yeast β(1-3),(1-6)-d-glucan films: Preparation and characterization of some structural and physical properties. Carbohydr. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]
- Raj, B. Low density polyethylene/starch blend films for food packaging applications. Adv. Polym. Technol. J. Polym. Process. Inst. 2004, 23, 32–45. [Google Scholar] [CrossRef]
- Chan, D.I.; Víctor, M.; López, M.T.; Lourdes, M. De Preparation and characterization of chitosan—Based bioactive films incorporating Moringa oleifera leaves extract. J. Food Meas. Charact. 2021, 15, 4813–4824. [Google Scholar] [CrossRef]
- Costa, N.N.; Lopes, L.d.F.; Ferreira, D.F.; de Prado, E.M.L.; Severi, J.A.; Resende, J.A.; de Paula Careta, F.; Ferreira, M.C.P.; Carreira, L.G.; de Souza, S.O.L.; et al. Polymeric films containing pomegranate peel extract based on PVA/starch/PAA blends for use as wound dressing: In vitro analysis and physicochemical evaluation. Mater. Sci. Eng. C 2020, 109, 110643. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, K.; Heilmann, C.J.; Sorgo, A.G.; Purschke, G.; de Koster, C.G.; Klis, F.M.; Heinisch, J.J. A systematic study of the cell wall composition of Kluyveromyces lactis. Yeast 2010, 27, 647–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paola, M.G.; Paletta, R.; Lopresto, C.G.; Lio, G.E.; De Luca, A.; Chakraborty, S.; Calabr, V. Stability of Film-Forming Dispersions: Affects the Morphology and Optical Properties of Polymeric Films. Polymers 2021, 13, 1464. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R. Influence of Water Activity on Growth, Metabolic Activities and Survival of Yeasts and Molds. J. Food Prot. 1983, 46, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Novák, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef]
- Galichet, A.; Sockalingum, G.D.; Belarbi, A.; Manfait, M. FTIR spectroscopic analysis of Saccharomyces cerevisiae cell walls: Study of an anomalous strain exhibiting a pink-colored cell phenotype. FEMS Microbiol. Lett. 2001, 197, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Naser, A.A.; Zohri, H.M. Biotechnological β-glucan Production from Returned Baker’s Yeast and Yeast Remaining after Ethanol Fermentation. Egypt. Sugar J. 2019, 13, 29–43. [Google Scholar]
- Khan, A.A.; Gani, A.; Masoodi, F.A.; Amin, F.; Wani, I.A.; Khanday, F.A.; Gani, A. Structural, thermal, functional, antioxidant & antimicrobial properties of β-d-glucan extracted from baker’s yeast (Saccharomyces cereviseae)—Effect of γ-irradiation. Carbohydr. Polym. 2016, 140, 442–450. [Google Scholar]
- Wu, W.; Solval, K.M.; Chen, J. Inhibitory activity of aqueous extracts of pomegranate peel products and juice powder against Salmonella enterica. LWT 2022, 155, 112934. [Google Scholar] [CrossRef]
- Garcia, M.A.; Martino, M.N.; Zaritzky, N.E. Edible starch films and coatings characterization: Scanning electron microscopy, water vapor, and gas permeabilities. Scanning 1999, 21, 348–353. [Google Scholar] [CrossRef]
- Emmambux, M.N.; Stading, M. In situ tensile deformation of zein films with plasticizers and filler materials. Food Hydrocoll. 2007, 21, 1245–1255. [Google Scholar] [CrossRef]


| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| β–glucan (g), X1 | 0.5 | 1 | 1.5 |
| Sodium alginate (g), X2 | 0.2 | 0.4 | 0.6 |
| Pomegranate juice (mL), X3 | 10 | 20 | 30 |
| Runs | β–Glucan | Sodium Alginate | Pomegranate Juice | |||
|---|---|---|---|---|---|---|
| Code | Experimental (g) | Code | Experimental (g) | Code | Experimental (mL) | |
| 1 | −1 | 0.5 | −1 | 0.2 | 0 | 20 |
| 2 | −1 | 0.5 | 0 | 0.4 | −1 | 10 |
| 3 | −1 | 0.5 | 0 | 0.4 | 1 | 30 |
| 4 | −1 | 0.5 | 1 | 0.6 | 0 | 20 |
| 5 | 0 | 1 | −1 | 0.2 | −1 | 10 |
| 6 | 0 | 1 | −1 | 0.2 | 1 | 30 |
| 7 | 0 | 1 | 0 | 0.4 | 0 | 20 |
| 8 | 0 | 1 | 0 | 0.4 | 0 | 20 |
| 9 | 0 | 1 | 0 | 0.4 | 0 | 20 |
| 10 | 0 | 1 | 1 | 0.6 | −1 | 10 |
| 11 | 0 | 1 | 1 | 0.6 | 1 | 30 |
| 12 | 1 | 1.5 | −1 | 0.2 | 0 | 20 |
| 13 | 1 | 1.5 | 0 | 0.4 | −1 | 10 |
| 14 | 1 | 1.5 | 0 | 0.4 | 1 | 30 |
| 15 | 1 | 1.5 | 1 | 0.6 | 0 | 20 |
| Run | β–Glucan Wet Weight (17.32% w/w Determined) (g) | Sodium Alginate (g) | Pomegranate Juice (12.42% w/v Determined) (mL) | Glycerin (25% w/w of the Total Solid Weight) (g) | Distilled Water (Up to Total Volume) (mL) | Sample Code |
|---|---|---|---|---|---|---|
| 1 | 2.89 (0.5) | 0.2 | 20 | 0.80 | 150 | 0.5-0.2-20 |
| 2 | 2.89 (0.5) | 0.4 | 10 | 0.54 | 150 | 0.5-0.4-10 |
| 3 | 2.89 (0.5) | 0.4 | 30 | 1.16 | 150 | 0.5-0.4-30 |
| 4 | 2.89 (0.5) | 0.6 | 20 | 0.90 | 150 | 0.5-0.6-20 |
| 5 | 5.77 (1) | 0.2 | 10 | 0.61 | 150 | 1-0.2-10 |
| 6 | 5.77 (1) | 0.2 | 30 | 1.23 | 150 | 1-0.2-30 |
| 7 | 5.77 (1) | 0.4 | 20 | 0.97 | 150 | 1-0.4-20-1 |
| 8 | 5.77 (1) | 0.4 | 20 | 0.97 | 150 | 1-0.4-20-2 |
| 9 | 5.77 (1) | 0.4 | 20 | 0.97 | 150 | 1-0.4-20-3 |
| 10 | 5.77 (1) | 0.6 | 10 | 0.71 | 150 | 1-0.6-10 |
| 11 | 5.77 (1) | 0.6 | 30 | 1.33 | 150 | 1-0.6-30 |
| 12 | 8.66 (1.5) | 0.2 | 20 | 1.05 | 150 | 1.5-0.2-20 |
| 13 | 8.66 (1.5) | 0.4 | 10 | 0.79 | 150 | 1.5-0.4-10 |
| 14 | 8.66 (1.5) | 0.4 | 30 | 1.41 | 150 | 1.5-0.4-30 |
| 15 | 8.66 (1.5) | 0.6 | 20 | 1.15 | 150 | 1.5-0.6-20 |
| Run | Variables | Responses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β–Glucan (g) X1 | Sodium Alginate (g) X2 | Pomegranate Juice (mL) X3 | Thickness (µm) | Density | WVTR |
WVP | Moisture Content (%) | Opacity | Solubility (Minutes) | L | a* | b* | |
| 1 | 0.5 | 0.2 | 20 | 88.6 | 1.53 | 28.3377 | 1.0565 | 17.59 | 4.75 | 3.42 | 29.51 | 0.23 | 1.7 |
| 2 | 0.5 | 0.4 | 10 | 64.4 | 1.46 | 25.0188 | 0.6780 | 17.72 | 7.86 | 2.5 | 29.18 | 0.02 | 1.26 |
| 3 | 0.5 | 0.4 | 30 | 132.4 | 1.69 | 32.1869 | 1.7932 | 17.86 | 2.77 | 4.2 | 32.11 | 0.35 | 1.54 |
| 4 | 0.5 | 0.6 | 20 | 129.7 | 1.55 | 31.0122 | 1.6925 | 15.23 | 1.46 | 2.34 | 27.83 | −0.01 | 1.59 |
| 5 | 1 | 0.2 | 10 | 73.9 | 1.41 | 22.3472 | 0.6949 | 16.25 | 10.51 | 4.51 | 31.21 | −0.02 | 1.16 |
| 6 | 1 | 0.2 | 30 | 138.7 | 1.66 | 28.2248 | 1.6473 | 16.20 | 1.81 | 5.38 | 31.86 | 0.47 | 1.63 |
| 7 | 1 | 0.4 | 20 | 135.2 | 1.57 | 27.8893 | 1.5866 | 13.98 | 3.55 | 4.07 | 33 | 0.26 | 1.76 |
| 8 | 1 | 0.4 | 20 | 133.7 | 1.52 | 27.7534 | 1.5614 | 14.02 | 3.84 | 4.41 | 33.07 | 0.28 | 1.71 |
| 9 | 1 | 0.4 | 20 | 130.5 | 1.59 | 28.8807 | 1.5859 | 13.89 | 3.82 | 5.19 | 33.13 | 0.34 | 1.81 |
| 10 | 1 | 0.6 | 10 | 112.7 | 1.45 | 24.6596 | 1.1694 | 12.93 | 5.47 | 3.11 | 33.36 | 0 | 1.5 |
| 11 | 1 | 0.6 | 30 | 147.3 | 1.71 | 30.1003 | 1.8657 | 15.04 | 3.03 | 4.25 | 32.59 | 0.44 | 1.76 |
| 12 | 1.5 | 0.2 | 20 | 134.8 | 1.60 | 26.2584 | 1.4894 | 13.92 | 4.07 | 5.22 | 33.31 | 0.2 | 1.56 |
| 13 | 1.5 | 0.4 | 10 | 116.6 | 1.42 | 23.8945 | 1.1723 | 12.31 | 5.08 | 6.33 | 33.43 | −0.01 | 1.45 |
| 14 | 1.5 | 0.4 | 30 | 141.3 | 1.67 | 30.2471 | 1.7984 | 18.49 | 3.64 | 4.5 | 27.62 | 0.34 | 2.38 |
| 15 | 1.5 | 0.6 | 20 | 127.3 | 1.58 | 27.9846 | 1.4990 | 14.99 | 1.91 | 4.22 | 30.84 | 0.6 | 1.76 |
| Independent Variables | Dependent Variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Term Regression Coefficients | Thickness (µm) | Density | WVTR |
WVP | Moisture Content (%) | Opacity | Solubility (Minutes) | L | a* | b* | |
| Constant | b0 | 133.13 | 1.56 | 28.17 | 1.58 | 13.96 | 3.74 | 4.56 | 33.07 | 0.29 | 1.76 |
| β–glucan (X1) | b1 | 13.11 * | ns | −1.02 * | 0.09 * | −1.09 * | −0.26 | 0.97 * | 0.82 | 0.06 | 0.13 |
| Sodium alginate (SA) (X2) | b2 | 10.12 * | 0.01 | 1.07 * | 0.16 * | −0.72 | −1.16 * | −0.57 * | −0.15 | 0.01 | 0.07 |
| Pomegranate juice (X3) | b3 | 24.01 * | 0.12 * | 3.10 * | 0.42 * | 1.05 * | −2.21 * | 0.23 | −0.37 | 0.20 * | 0.24* |
| SA (X1X2) | b4 | −12.15 * | −0.01 | −0.23 | −0.15 * | 0.85 | 0.28 | 0.017 | −0.19 | 0.16 * | 0.07 |
| PJ (X1X3) | b5 | −10.82 * | ns | −0.20 | −0.12 * | 1.51 * | 0.91 * | −0.88 * | −2.19 * | ns | 0.16 |
| PJ (X2X3) | b6 | −7.55 * | ns | −0.10 | −0.06 * | 0.54 | 1.57 * | 0.06 | -0.35 | −0.01 | −0.05 |
| β–glucan (X12) | b7 | −8.75 * | ns | 0.86 * | −0.06 * | 1.48 * | −0.52 | −0.34 | −2.18 * | −0.04 | 0.01 |
| SA (X22) | b8 | −4.28 | ns | −0.64 | −0.07 * | −0.01 | −0.16 | −0.41 | −0.51 | ns | −0.12 |
| PJ (X32) | b9 | −10.70 * | ns | −1.20 * | −0.15 * | 1.15 * | 1.63 * | 0.17 | −0.29 | −0.07 | −0.12 |
| F-value | 62.28 | 9.98 | 30.92 | 109.58 | 7.29 | 12.34 | 6.51 | 1.85 | 4.35 | 2.83 | |
| p-value | 0.0001 | 0.0104 | 0.0007 | 0.0001 | 0.0207 | 0.0064 | 0.0264 | 0.2573ns | 0.0599ns | 0.1323ns | |
| R2 | 0.9912 | 0.9473 | 0.9824 | 0.9950 | 0.9292 | 0.9569 | 0.9214 | 0.7694 | 0.8868 | 0.8359 | |
| Sample Code | aw | Temperature of Measurement (°C) |
|---|---|---|
| 0.5-0.2-20 | 0.2148 (0.009) b | 25.00 |
| 0.5-0.4-10 | 0.2266 (0.012) b | 25.03 |
| 0.5-0.4-30 | 0.2288 (0.005) ab | 25.07 |
| 0.5-0.6-20 | 0.2294 (0.007) ab | 25.04 |
| 1-0.2-10 | 0.2386 (0.006) ab | 25.02 |
| 1-0.2-30 | 0.2615 (0.009) ab | 24.99 |
| 1-0.4-20-1 | 0.2745 (0.013) a | 25.40 |
| 1-0.4-20-2 | 0.2344 (0.009) ab | 25.10 |
| 1-0.4-20-3 | 0.2274 (0.023) ab | 25.02 |
| 1-0.6-10 | 0.2508 (0.010) ab | 25.01 |
| 1-0.6-30 | 0.2447 (0.006) ab | 25.00 |
| 1.5-0.2-20 | 0.2418 (0.001) ab | 25.07 |
| 1.5-0.4-10 | 0.2566 (0.004) ab | 25.07 |
| 1.5-0.4-30 | 0.2285 (0.024) b | 25.00 |
| 1.5-0.6-20 | 0.2557 (0.004) ab | 25.03 |
| Film Composition | Predicted Values | Actual Values | Std. Dev. | SE Pred. |
|---|---|---|---|---|
| 0.98 g β–Glucan, 0.6 g SA, 14.80 mL PJ | 1 g β–Glucan, 0.6 g SA, 14 mL PJ, 0.83 g Glycerin (1-0.6-14) | |||
| Thickness (µm) | 125.24 | 121.4 | 3.95 | 4.75 |
| cm−m) | 1.49 | 1.47 | 0.03 | 0.04 |
| WVTR (g×h−××m−m) | 26.3781 | 27.1224 | 0.61 | 0.74 |
| WVP (g×mm×kPa−××h−h×m−m) | 1.394 | 1.390 | 0.04 | 0.05 |
| Moisture content (%) | 12.69 | 14.59 | 0.85 | 1.03 |
| Opacity | 3.38 | 4.48 | 0.82 | 0.99 |
| Solubility (minutes) | 3.44 | 4.53 | 0.50 | 0.61 |
| L | 32.72 | 36.45 | 1.63 | 1.97 |
| a* | 0.17 | −0.05 | 0.11 | 0.13 |
| b* | 1.54 | 2.54 | 0.18 | 0.22 |
| aw | nd | 0.2287 | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramia, I.; Amariei, S. Formulation, Characterization and Optimization of β–Glucan and Pomegranate Juice Based Films for Its Potential in Diabetes. Nutrients 2022, 14, 2142. https://doi.org/10.3390/nu14102142
Avramia I, Amariei S. Formulation, Characterization and Optimization of β–Glucan and Pomegranate Juice Based Films for Its Potential in Diabetes. Nutrients. 2022; 14(10):2142. https://doi.org/10.3390/nu14102142
Chicago/Turabian StyleAvramia, Ionut, and Sonia Amariei. 2022. "Formulation, Characterization and Optimization of β–Glucan and Pomegranate Juice Based Films for Its Potential in Diabetes" Nutrients 14, no. 10: 2142. https://doi.org/10.3390/nu14102142
APA StyleAvramia, I., & Amariei, S. (2022). Formulation, Characterization and Optimization of β–Glucan and Pomegranate Juice Based Films for Its Potential in Diabetes. Nutrients, 14(10), 2142. https://doi.org/10.3390/nu14102142