Eggs Improve Plasma Biomarkers in Patients with Metabolic Syndrome Following a Plant-Based Diet—A Randomized Crossover Study
Abstract
:1. Introduction
2. Materials and Methods
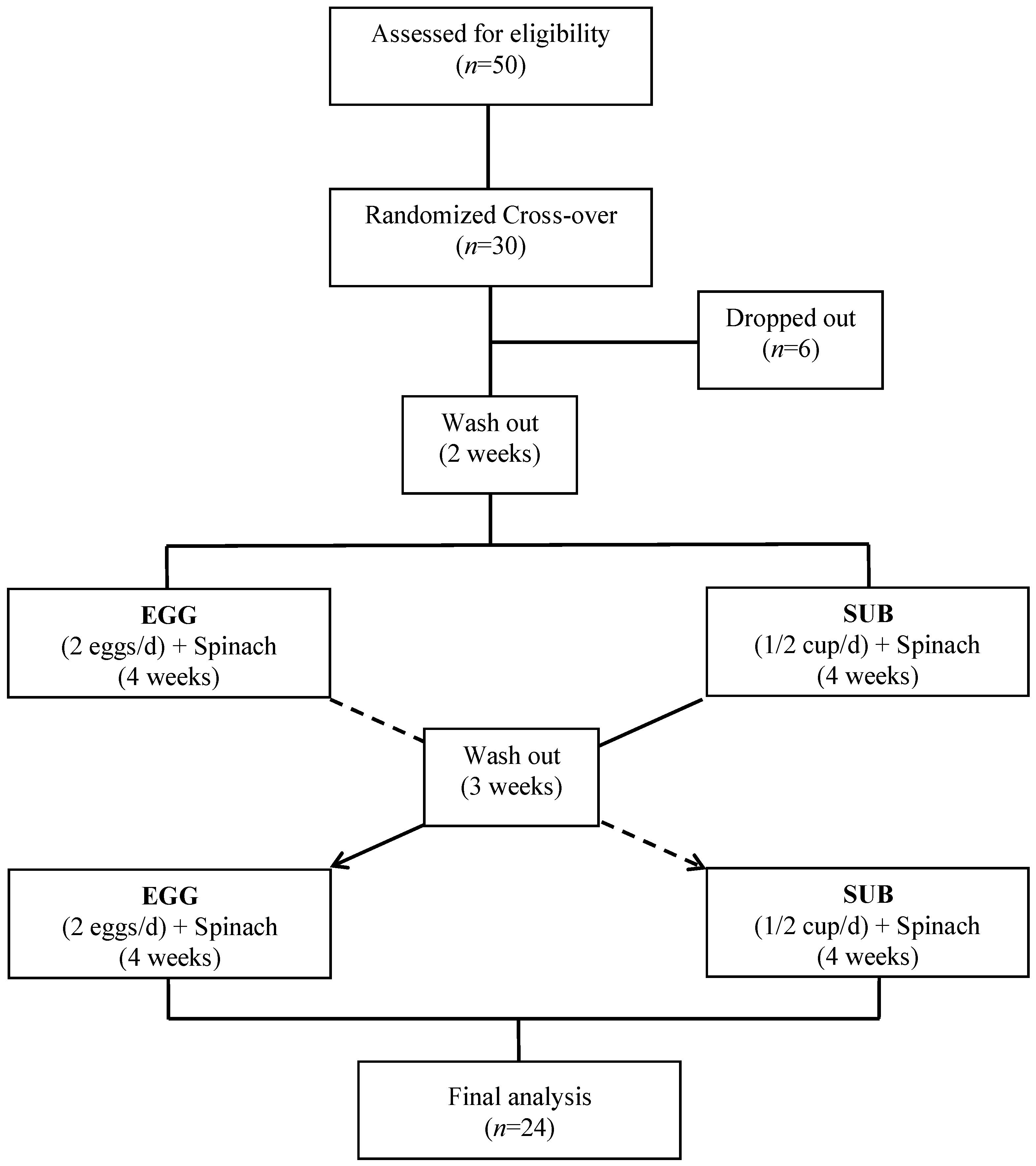
2.1. Experimental Design
2.2. Diet Analysis
2.3. Blood Collection and Processing,
2.4. Anthropometrics and Blood Pressure (BP)
2.5. Plasma Lipids
2.6. Plasma Glucose, Insulin, HOMA-IR and MetS-Z Score
2.7. Lipoprotein Particle Size and Subfractions
2.8. Plasma Choline and TMAO
2.9. Plasma Lutein and Zeaxanthin
2.10. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Dietary Intake
3.3. BMI and Weight Anthropometrics, Blood Pressure, Plasma Lipids and Glucose
3.4. Lipoprotein Particle Size and Subfractions
3.5. Plasma Choline, Metabolites and TMAO
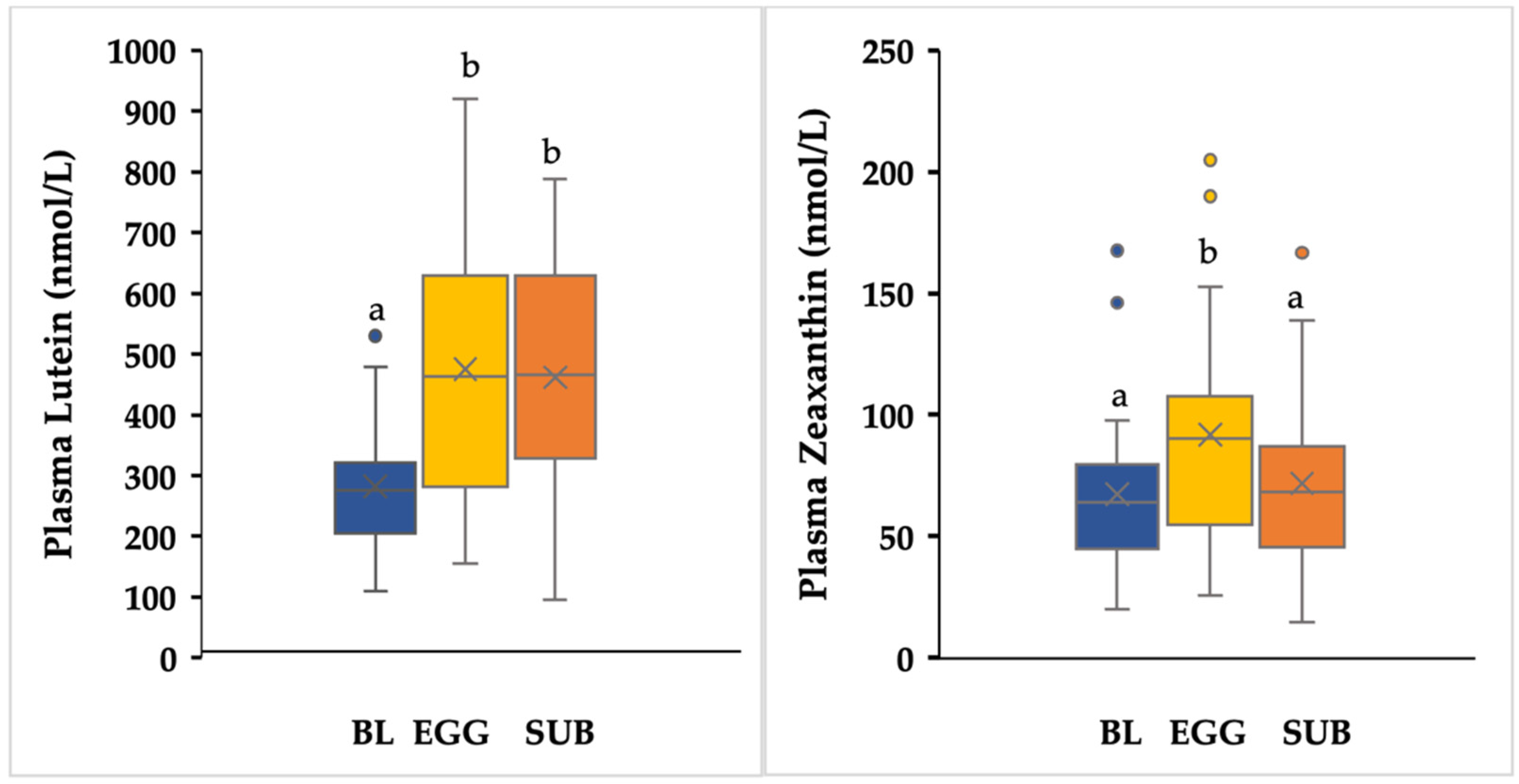
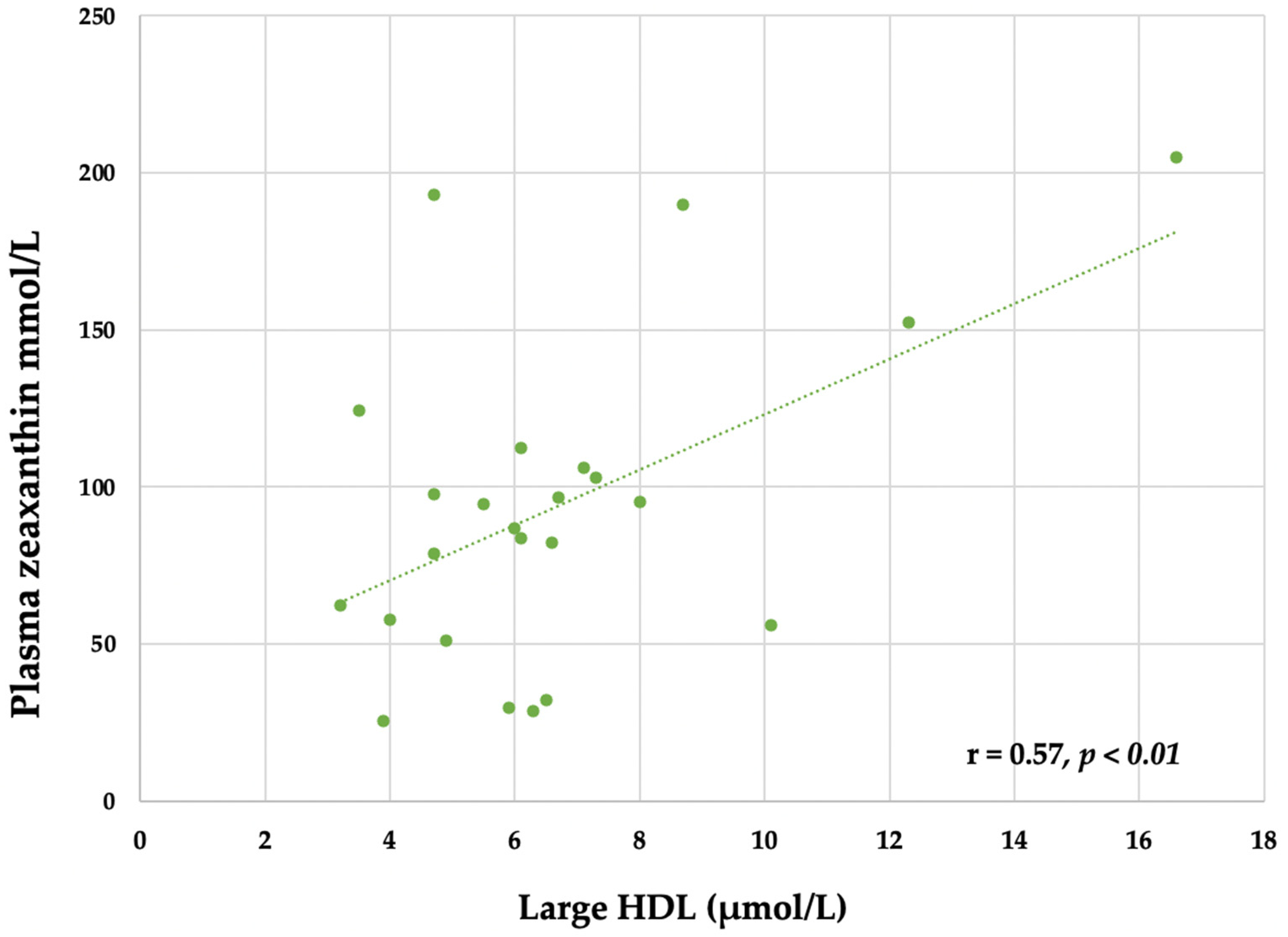
3.6. Plasma Lutein and Zeaxanthin
4. Discussion
4.1. Dietary Changes Associated with Egg Intake
4.2. Anthropometrics and Blood Pressure
4.3. Lipoprotein Modifications and Egg Intake
4.4. Carotenoids, Choline and Egg Intake
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinheiro, C.; Leite, J.C.; Negrão, R.; Keating, E. Vegetarian Diets as a Possible Therapeutic Approach to Patients with Metabolic Syndrome. Porto Biomed. J. 2020, 5, e098. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan Diet Health Benefits in Metabolic Syndrome. Nutrients 2021, 13, 817. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Goff, L.M.; Bell, J.D.; So, P.-W.; Dornhorst, A.; Frost, G.S. Veganism and Its Relationship with Insulin Resistance and Intramyocellular Lipid. Eur. J. Clin. Nutr. 2005, 59, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.-K.; Lin, Y.-L.; Chen, C.-L.; Ouyang, C.-M.; Wu, Y.-T.; Chi, Y.-C.; Huang, K.-C.; Yang, W.-S. Reduced Risk for Metabolic Syndrome and Insulin Resistance Associated with Ovo-Lacto-Vegetarian Behavior in Female Buddhists: A Case-Control Study. PLoS ONE 2013, 8, e71799. [Google Scholar] [CrossRef] [Green Version]
- DiMarco, D.M.; Missimer, A.; Murillo, A.G.; Lemos, B.S.; Malysheva, O.V.; Caudill, M.A.; Blesso, C.N.; Fernandez, M.L. Intake of up to 3 Eggs/Day Increases HDL Cholesterol and Plasma Choline While Plasma Trimethylamine-N-Oxide Is Unchanged in a Healthy Population. Lipids 2017, 52, 255–263. [Google Scholar] [CrossRef]
- Andersen, C.J.; Blesso, C.N.; Lee, J.; Barona, J.; Shah, D.; Thomas, M.J.; Fernandez, M.L. Egg Consumption Modulates HDL Lipid Composition and Increases the Cholesterol-Accepting Capacity of Serum in Metabolic Syndrome. Lipids 2013, 48, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volk, B.; Volek, J.S.; Fernandez, M.L. Effects of carbohydrate restriction and dietary cholesterol provided by eggs on clinical risk factors of metabolic syndrome. J. Clin. Lipidol. 2013, 7, 463–471. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Bolling, B.W.; Fernandez, M.L. Egg Intake Improves Carotenoid Status by Increasing Plasma HDL Cholesterol in Adults with Metabolic Syndrome. Food Funct. 2013, 4, 213–221. [Google Scholar] [CrossRef]
- Niesor, E.J. Will Lipidation of ApoA1 through Interaction with ABCA1 at the Intestinal Level Affect the Protective Functions of HDL? Biology 2015, 4, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Kent, L.; Morton, D.; Rankin, P.; Ward, E.; Grant, R.; Gobble, J.; Diehl, H. The Effect of a Low-Fat, Plant-Based Lifestyle Intervention (CHIP) on Serum HDL Levels and the Implications for Metabolic Syndrome Status—A Cohort Study. Nutr. Metab. 2013, 10, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, M.L.; Andersen, C.J. 1. The Good Egg, the Forgotten Benefits: Protein, Carotenoids, Choline and Glycemic Index. In Human Health Handbooks; Wageninen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 15–34. [Google Scholar]
- Zhang, Y.; Knol, L.L.; Tan, L. Association between Dietary Lutein/Zeaxanthin Intake and Metabolic Syndrome among US Females: An Analysis of National Health and Examination Surveys 2015–2018. Curr. Dev. Nutr. 2021, 5, nzab123. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, W.; Cao, Y.; He, L.; Guan, K.; Ling, W.; Chen, Y. Higher Serum Carotenoid Concentrations Associated with a Lower Prevalence of the Metabolic Syndrome in Middle-Aged and Elderly Chinese Adults. Br. J. Nutr. 2014, 112, 2041–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morganti, P.; Fabrizi, G.; Bruno, C. Protective Effects of Oral Antioxidants on Skin and Eye Function. Skinmed 2004, 3, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W. Vitamins E and C, β-Carotene, and Other Carotenoids as Antioxidants. Am. J. Clin. Nutr. 1995, 62, 1315S–1321S. [Google Scholar] [CrossRef]
- Kim, J.E.; Leite, J.O.; DeOgburn, R.; Smyth, J.A.; Clark, R.M.; Fernandez, M.L. A Lutein-Enriched Diet Prevents Cholesterol Accumulation and Decreases Oxidized LDL and Inflammatory Cytokines in the Aorta of Guinea Pigs. J. Nutr. 2011, 141, 1458–1463. [Google Scholar] [CrossRef] [Green Version]
- Vishwanathan, R.; Goodrow-Kotyla, E.F.; Wooten, B.R.; Wilson, T.A.; Nicolosi, R.J. Consumption of 2 and 4 Egg Yolks/d for 5 Wk Increases Macular Pigment Concentrations in Older Adults with Low Macular Pigment Taking Cholesterol-Lowering Statins. Am. J. Clin. Nutr. 2009, 90, 1272–1279. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, A.J.; Gerweck, C.; Barbato, D.; Nicolosi, R.J.; Handelman, G.J.; Curran-Celentano, J. A 12-Wk Egg Intervention Increases Serum Zeaxanthin and Macular Pigment Optical Density in Women. J. Nutr. 2006, 136, 2568–2573. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-R.; Zou, Z.-Y.; Xiao, X.; Huang, Y.-M.; Wang, X.; Lin, X.-M. Effects of Lutein Supplement on Serum Inflammatory Cytokines, ApoE and Lipid Profiles in Early Atherosclerosis Population. J. Atheroscler. Thromb. 2013, 20, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary Carotenoids, Vitamins A, C, and E, and Advanced Age-Related Macular Degeneration. JAMA J. Am. Med. Assoc. 1994, 272, 1413–1420. [Google Scholar] [CrossRef]
- Murphy, M.M.; Barraj, L.M.; Herman, D.; Bi, X.; Cheatham, R.; Randolph, R.K. Phytonutrient Intake by Adults in the United States in Relation to Fruit and Vegetable Consumption. J. Acad. Nutr. Diet 2012, 112, 222–229. [Google Scholar] [CrossRef] [PubMed]
- USDA Nutrient Data Laboratory|Food and Nutrition Information Center|NAL|USDA. Available online: https://www.nal.usda.gov/fnic/usda-nutrient-data-laboratory (accessed on 27 April 2022).
- Chung, H.Y.; Rasmussen, H.M.; Johnson, E.J. Lutein Bioavailability Is Higher from Lutein-Enriched Eggs than from Supplements and Spinach in Men. J. Nutr. 2004, 134, 1887–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herron, K.L.; McGrane, M.M.; Waters, D.; Lofgren, I.E.; Clark, R.M.; Ordovas, J.M.; Fernandez, M.L. The ABCG5 Polymorphism Contributes to Individual Responses to Dietary Cholesterol and Carotenoids in Eggs. J. Nutr. 2006, 136, 1161–1165. [Google Scholar] [CrossRef] [Green Version]
- Schaeffer, J.L.; Tyczkowski, J.K.; Parkhurst, C.R.; Hamilton, P.B. Carotenoid Composition of Serum and Egg Yolks of Hens Fed Diets Varying in Carotenoid Composition. Poult. Sci. 1988, 67, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Smolders, L.; DeWit, N.J.W.; Balvers, M.G.J.; Obeid, R.; Vissers, M.M.M.; Esser, D. Natural Choline from Egg Yolk Phospholipids Is More Efficiently Absorbed Compared with Choline Bitartrate; Outcomes of a Randomized Trial in Healthy Adults. Nutrients 2019, 11, 2758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; Caudill, M.A.; Fernandez, M.L. Effects of Egg Consumption and Choline Supplementation on Plasma Choline and Trimethylamine-N-Oxide in a Young Population. J. Am. Coll. Nutr. 2018, 37, 716–723. [Google Scholar] [CrossRef]
- Zeisel, S.H. A Brief History of Choline. Ann. Nutr. Metab. 2012, 61, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Grizales, A.M.; Patti, M.-E.; Lin, A.P.; Beckman, J.A.; Sahni, V.A.; Cloutier, E.; Fowler, K.M.; Dreyfuss, J.M.; Pan, H.; Kozuka, C.; et al. Metabolic Effects of Betaine: A Randomized Clinical Trial of Betaine Supplementation in Prediabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3038–3049. [Google Scholar] [CrossRef]
- Tiihonen, K.; Saarinen, M.T. Effect of Dietary Betaine on Metabolic Syndrome Risk Factors in Asian. J. Diabetes Metab. 2016, 7, 4172. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Guo, X.; Li, K.; Li, S.; Li, D. Effect of Betaine on Reducing Body Fat—A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 2480. [Google Scholar] [CrossRef] [Green Version]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A Small Molecule of Great Expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.P. Betaine Supplementation Decreases Plasma Homocysteine in Healthy Adult Participants: A Meta-Analysis. J. Chiropr. Med. 2013, 12, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonn, E. Homocysteine in the Prevention of Ischemic Heart Disease, Stroke and Venous Thromboembolism: Therapeutic Target or Just Another Distraction? Curr. Opin. Intern. Med. 2007, 6, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of Metabolic Syndrome. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Gurka, M.J.; Lilly, C.L.; Oliver, M.N.; DeBoer, M.D. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: A confirmatory factor analysis and a resulting continuous severity score. Metabolism 2014, 63, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein Particle Analysis by Nuclear Magnetic Resonance Spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef]
- Holm, P.I.; Ueland, P.M.; Kvalheim, G.; Lien, E.A. Determination of Choline, Betaine, and Dimethylglycine in Plasma by a High-Throughput Method Based on Normal-Phase Chromatography–Tandem Mass Spectrometry. Clin. Chem. 2003, 49, 286–294. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; West, A.; Perry, C.; Malysheva, O.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Stabler, S.; Allen, R.; et al. Maternal Choline Intake Modulates Maternal and Fetal Biomarkers of Choline Metabolism in Humans. Am. J. Clin. Nutr. 2012, 95, 1060–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biddle, M.J.; Lennie, T.A.; Bricker, G.V.; Kopec, R.E.; Schwartz, S.J.; Moser, D.K. Lycopene Dietary Intervention. J. Cardiovasc. Nurs. 2015, 30, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Timmers, C.D.; Francis, D.M.; Lesinski, G.B.; Clinton, S.K.; et al. Enhanced Bioavailability of Lycopene When Consumed as Cis-Isomers from Tangerine Compared to Red Tomato Juice, a Randomized, Cross-over Clinical Trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missimer, A.; DiMarco, D.; Andersen, C.; Murillo, A.; Vergara-Jimenez, M.; Fernandez, M. Consuming Two Eggs per Day, as Compared to an Oatmeal Breakfast, Decreases Plasma Ghrelin While Maintaining the LDL/HDL Ratio. Nutrients 2017, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Ratliff, J.C.; Leite, J.O.; DeOgburn, R.; Puglisi, M.; VanHeest, J.; Fernandez, M.L. Consuming eggs for breakfast influences plasma glucose and ghrelin, while reducing caloric intake during the next 24 hours in adult men. Nutr. Res. 2010, 30, 96–103. [Google Scholar] [CrossRef]
- Thomas, M.S.; DiBella, M.; Blesso, C.N.; Malysheva, O.; Caudill, M.; Sholola, M.; Cooperstone, J.L.; Fernandez, M.L. Comparison between Egg Intake versus Choline Supplementation on Gut Microbiota and Plasma Carotenoids in Subjects with Metabolic Syndrome. Nutrients 2022, 14, 1179. [Google Scholar] [CrossRef]
- Hu, T.; Yao, L.; Reynolds, K.; Niu, T.; Li, S.; Whelton, P.; He, J.; Bazzano, L. The Effects of a Low-Carbohydrate Diet on Appetite: A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 476–488. [Google Scholar] [CrossRef] [Green Version]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary Carbohydrate Restriction as the First Approach in Diabetes Management: Critical Review and Evidence Base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-Carbohydrate Nutrition and Metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Shi, Z. Higher Egg Consumption Associated with Increased Risk of Diabetes in Chinese Adults—China Health and Nutrition Survey. Br. J. Nutr. 2021, 126, 110–117. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.-P.; Chen, S.; Li, Y.; Schwab, A.L.; Stampfer, M.J.; Sacks, F.M.; Rosner, B.; Willett, W.C.; Hu, F.B.; Bhupathiraju, S.N. Egg Consumption and Risk of Cardiovascular Disease: Three Large Prospective US Cohort Studies, Systematic Review, and Updated Meta-Analysis. BMJ 2020, 368, m513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Mohan, V.; Lear, S.; Swaminathan, S.; Wielgosz, A.; Seron, P.; Avezum, A.; Lopez-Jaramillo, P.; et al. Association of Egg Intake with Blood Lipids, Cardiovascular Disease, and Mortality in 177,000 People in 50 Countries. Am. J. Clin. Nutr. 2020, 111, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhuang, P.; Zhang, Y.; Zhan, C.; Zhang, Y.; Jiao, J. Egg and Dietary Cholesterol Consumption and Mortality Among Hypertensive Patients: Results from a Population-Based Nationwide Study. Front. Nutr. 2021, 8, 739533. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Lv, J.; Guo, Y.; Bian, Z.; Si, J.; Yang, L.; Chen, Y.; Zhou, Y.; Zhang, H.; Liu, J.; et al. Associations of Egg Consumption with Cardiovascular Disease in a Cohort Study of 0.5 Million Chinese Adults. Heart 2018, 104, 1756–1763. [Google Scholar] [CrossRef] [Green Version]
- Drouin-Chartier, J.-P.; Schwab, A.L.; Chen, S.; Li, Y.; Sacks, F.M.; Rosner, B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; Hu, F.B.; et al. Egg Consumption and Risk of Type 2 Diabetes: Findings from 3 Large US Cohort Studies of Men and Women and a Systematic Review and Meta-Analysis of Prospective Cohort Studies. Am. J. Clin. Nutr. 2020, 112, 619–630. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volek, J.S.; Fernandez, M.L. Whole Egg Consumption Improves Lipoprotein Profiles and Insulin Sensitivity to a Greater Extent than Yolk-Free Egg Substitute in Individuals with Metabolic Syndrome. Metabolism 2013, 62, 400–410. [Google Scholar] [CrossRef]
- Dibella, M.; Thomas, M.S.; Alyousef, H.; Millar, C.; Blesso, C.; Malysheva, O.; Caudill, M.A.; Fernandez, M.L. Choline Intake as Supplement or as a Component of Eggs Increases Plasma Choline and Reduces Interleukin-6 without Modifying Plasma Cholesterol in Participants with Metabolic Syndrome. Nutrients 2020, 12, 3120. [Google Scholar] [CrossRef]
- Cockerill, G.W.; Rye, K.-A.; Gamble, J.R.; Vadas, M.A.; Barter, P.J. High-Density Lipoproteins Inhibit Cytokine-Induced Expression of Endothelial Cell Adhesion Molecules. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1987–1994. [Google Scholar] [CrossRef]
- Navab, M.; Imes, S.S.; Hama, S.Y.; Hough, G.P.; Ross, L.A.; Bork, R.W.; Valente, A.J.; Berliner, J.A.; Drinkwater, D.C.; Laks, H. Monocyte Transmigration Induced by Modification of Low Density Lipoprotein in Cocultures of Human Aortic Wall Cells Is Due to Induction of Monocyte Chemotactic Protein 1 Synthesis and Is Abolished by High Density Lipoprotein. J. Clin. Investig. 1991, 88, 2039–2046. [Google Scholar] [CrossRef]
- Navab, M.; Berliner, J.A.; Subbanagounder, G.; Hama, S.; Lusis, A.J.; Castellani, L.W.; Reddy, S.; Shih, D.; Shi, W.; Watson, A.D.; et al. HDL and the Inflammatory Response Induced by LDL-Derived Oxidized Phospholipids. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Sawrey-Kubicek, L.; Zhu, C.; Bardagjy, A.S.; Rhodes, C.H.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole Egg Consumption Compared with Yolk-Free Egg Increases the Cholesterol Efflux Capacity of High-Density Lipoproteins in Overweight, Postmenopausal Women. Am. J. Clin. Nutr. 2019, 110, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, G.; Waters, D.; Ratliff, J.; Puglisi, M.; Clark, R.M.; Volek, J.S.; Fernandez, M.L. Eggs Distinctly Modulate Plasma Carotenoid and Lipoprotein Subclasses in Adult Men Following a Carbohydrate-Restricted Diet. J. Nutr. Biochem. 2010, 21, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, J.C.; Mutungi, G.; Puglisi, M.J.; Volek, J.S.; Fernandez, M.L. Eggs Modulate the Inflammatory Response to Carbohydrate Restricted Diets in Overweight Men. Nutr. Metab. 2008, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- DiMarco, D.M.; Norris, G.H.; Millar, C.; Blesso, C.; Fernandez, M.L. Intake of up to 3 eggs per day is associated with changes in HDL function and increased plasma antioxidants in healthy young adults. J. Nutr. 2017, 147, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.M.; Waters, D.; Clark, R.M.; Contois, J.H.; Fernandez, M.L. Plasma LDL and HDL Characteristics and Carotenoid Content Are Positively Influenced by Egg Consumption in an Elderly Population. Nutr. Metab. 2006, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Goodrow, E.F.; Wilson, T.A.; Houde, S.C.; Vishwanathan, R.; Scollin, P.A.; Handelman, G.; Nicolosi, R.J. Consumption of One Egg per Day Increases Serum Lutein and Zeaxanthin Concentrations in Older Adults without Altering Serum Lipid and Lipoprotein Cholesterol Concentrations. J. Nutr. 2006, 136, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- Burns-Whitmoré, B.L.; Haddad, E.H.; Sabaté, J.; Jaceldo-Siegl, K.; Tanzman, J.; Rajaram, S. Effect of N-3 Fatty Acid Enriched Eggs and Organic Eggs on Serum Lutein in Free-Living Lacto-Ovo Vegetarians. Eur. J. Clin. Nutr. 2010, 64, 1332–1337. [Google Scholar] [CrossRef]
- Kelly, E.R.; Plat, J.; Haenen, G.R.M.M.; Kijlstra, A.; Berendschot, T.T.J.M. The Effect of Modified Eggs and an Egg-Yolk Based Beverage on Serum Lutein and Zeaxanthin Concentrations and Macular Pigment Optical Density: Results from a Randomized Trial. PLoS ONE 2014, 9, e92659. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; 2020. Available online: DietaryGuidelines.gov (accessed on 27 April 2022).
- Wallace, T.C.; Fulgoni, V.L. Assessment of Total Choline Intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.A. Choline: An Essential Nutrient for Public Health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H.; Mar, M.-H.; Howe, J.C.; Holden, J.M. Concentrations of Choline-Containing Compounds and Betaine in Common Foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; George, P.M.; Atkinson, W.; Molyneux, S.L.; Elmslie, J.L.; Slow, S.; Richards, A.M.; Chambers, S.T. Plasma Lipids and Betaine Are Related in an Acute Coronary Syndrome Cohort. PLoS ONE 2011, 6, e21666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; De Alteriis, G.; Tenore, G.; Colao, A.; Savastano, S. Trimethylamine-N-Oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| EGG (2 Large Eggs) | SUB (½ cup Egg Substitute) | Spinach (70 gm) | |
|---|---|---|---|
| Cholesterol (mg) | 370 | 0 | 0 |
| Choline (mg) | 294–285 | 0 | 13.5 |
| Lutein + Zeaxanthin (mg) | 0.2–0.3 | 0 | 20.3 |
| Parameter | Values |
|---|---|
| Age (years) | 49.3 ± 8 |
| Gender (F/M) | 13/11 |
| Race (Caucasians/African Americans) | 21/4 |
| BMI (kg/m2) | 34.3 ± 4.6 |
| Gender Female (%) | 54% |
| Waist Circumference (cm) | 112.5 ± 11.9 |
| Systolic Blood pressure (mmHg) | 183 ± 27.6 |
| Diastolic Blood pressure (mmHg) | 86.6 ± 5.6 |
| HDL cholesterol (mg/dL) | 42.1 ± 10.3 |
| Triglycerides (mg/dL) | 155 ± 68 |
| Glucose (mg/dL) | 103 ± 12 |
| Dietary Component | Baseline | EGG | SUB |
|---|---|---|---|
| Energy (Kcal) 2 | 1677 ± 573 a | 1798 ± 579 a | 1699 ± 512 a |
| Total fat (%) | 35.6 ± 6.5 a | 40.9 ± 7.2 b | 35.4 ± 6.4 a |
| Total CHO (%) | 49.6 ± 4.7 a | 43.7 ± 7.7 b | 47.1 ± 7.9 ab |
| Total protein (%) | 13.3 ± 3.0 a | 14.8 ± 2.9 b | 15.4 ± 2.8 b |
| SFA (g) | 21.8 ± 2.8 a | 29.2 ± 1.3 b | 24.2 ± 11.2 ab |
| MUFA (g) | 23.5 ± 9.3 a | 28.6 ± 10.9 b | 23.4 ± 9.1 a |
| PUFA (g) | 16.6 ± 5.8 | 18.2 ± 7.7 | 17.1 ± 7.5 |
| TFA (g) | 1.5 ± 0.8 | 1.9 ± 1.5 | 1.9 ± 1.3 |
| Cholesterol (mg) | 102 ± 86 a | 438 ± 135 b | 143 ± 115 a |
| Omega-3 fatty acids (g) | 1.6 ± 0.7 a | 2.0 ± 0.9 b | 2.0 ± 0.7 b |
| Added sugars (g) | 45.7 ± 36.2 b | 32.4 ± 30.0 a | 32.0 ± 21.2 a |
| Glycemic Index | 57.5 ± 4.3 | 56.5 ± 4.9 | 55.8 ± 4.8 |
| Glycemic Load | 112 ± 45 | 102 ± 42 | 101 ± 33 |
| Fiber (g) | 23.7 ± 8.7 | 21.6 ± 6.3 | 25.3 ± 8.6 |
| Alpha-carotene | 526 ± 489 | 452 ± 618 | 512 ± 594 |
| Beta-carotene (µg) | 4084 ± 2890 a | 6357 ± 2527 b | 7684 ± 3387 b |
| Lutein + Zeaxanthin (µg) | 3151 ± 4382 a | 9190 ± 1527 b | 9179 ± 2188 b |
| Choline (mg) | 200.7 ± 82.9 a | 436.0 ± 96.9 b | 226.7 ± 109.4 a |
| Betaine (mg) | 120.7 ± 65.7 a | 170.7 ± 65.6 b | 177.5 ± 82 b |
| Vitamin A (µg) | 1207 ± 538 a | 1643 ± 493 b | 1748 ± 640 b |
| Vitamin D (µg) | 3.3 ± 2.3 a | 5.4 ± 2.3 c | 4.3 ± 1.7 b |
| Vitamin E (mg) | 11.3 ± 6.3 | 11.8 ± 4 | 12.7 ± 3.9 |
| Vitamin B2 (mg) | 1.9 ± 0.8 a | 2.4 ± 0.7 b | 3.1 ± 0.5 c |
| Vitamin B12 (mg) | 2.9 ± 2.2 ab | 3.4 ± 1 b | 2.8 ± 1.4 a |
| Sodium (mg) | 2646.5 ± 1114.5 | 3189.5 ± 1099 | 3081 ± 994.2 |
| Selenium (µg) | 78.6 ± 38.9 a | 106.6 ± 29.4 b | 101.0 ± 29.3 b |
| Physical activity (min) | 53.2 ± 25.4 | 51.5 ± 21 | 47.8 ± 19.8 |
| Parameters | Baseline | EGG | SUB |
|---|---|---|---|
| Body weight (Kg) | 99.4 ± 19.6 b | 98.5 ± 19.2 a | 99.6 ± 20.1 b |
| BMI (kg/m2) | 34.3 ± 4.8 b | 33.8 ± 4.6 a | 34.7 ± 4.6 b |
| Waist circumference (cm) | 112.5 ± 11.9 | 113.4 ± 13.3 | 113.3 ± 12.7 |
| Diastolic BP (mm Hg) | 86.6 ± 5.6 | 86.2 ± 8.4 | 86.7 ± 6.6 |
| Systolic BP (mm Hg) | 183.0 ± 27.6 | 185.3 ± 29.0 | 179.1 ± 24.6 |
| HDL cholesterol (mg/dL) | 42.1 ± 10.3 b | 43.3 ± 10.7 a | 41.5 ± 10.1 b |
| Triglycerides (mg/dL) | 155 ± 68 | 149 ± 58 | 156 ± 66 |
| LDL cholesterol (mg/dL) | 109.9 ± 26.6 | 112.3 ± 25.9 | 108.1 ± 19.8 |
| LDL/HDL ratio | 2.75 ± 0.88 | 2.72 ± 0.77 | 2.72 ± 0.73 |
| Glucose (mg/dL) | 103 ± 12 | 93 ± 11 | 92 ± 9 |
| Insulin (pmol/L) | 67.68 ± 34 | 67.42 ± 34.66 | 71.3 ± 39.46 |
| HOMA-IR | 2.61 ± 1.41 | 2.62 ± 1.54 | 2.71 ± 1.62 |
| MetS-Z score | 0.75 ± 0.40 | 0.70 ± 0.49 | 0.74 ± 0.50 |
| Lipoprotein Concentration | Baseline | EGG | SUB |
|---|---|---|---|
| Total VLDL (nmol/L) | 62.0 ± 17.8 | 68.7 ± 31.5 | 68.0 ± 25.4 |
| Large VLDL (nmol/L) | 8.7 ± 5.0 | 8.7 ± 5.7 | 10.9 ± 8.1 |
| Medium VLDL (nmol/L) | 23.2 ± 14.8 | 25.9 ± 19.9 | 22.8 ± 12.7 |
| Small VLDL (nmol/L) | 30.2 ± 11.6 | 35.0 ± 14.5 | 34.7 ± 20.3 |
| Total LDL (nmol/L) | 1118.3 ± 263.8 | 1138.8 ± 255.9 | 1147.8 ± 226.8 |
| IDL (nmol/L) | 233.5 ± 126.3 | 213.4 ± 117.8 | 208.3 ± 115.8 |
| Large LDL (nmol/L) | 137.0 ± 116.6 | 164.0 ± 152.7 | 187.0 ± 142.0 |
| Small LDL (nmol/L) | 747.9 ± 209.4 | 755.6 ± 243.2 | 766.4 ± 203.7 |
| Total HDL (μmol/L) | 35.9 ± 5.5 a | 37.6 ± 7.1 b | 35.7 ± 6.0 a |
| Large HDL (μmol/L) | 6.1 ± 2.3 a | 6.6 ± 3.0 b | 5.9 ± 2.3 a |
| Medium HDL (μmol/L) | 11.0 ± 4.3 | 10.3 ± 4.4 | 9.7 ± 5.1 |
| Small HDL (μmol/L) | 18.8 ± 5.5 | 20.0 ± 5.2 | 20.1 ± 6.7 |
| VLDL size (nm) | 55.6 ± 7.4 | 54.5 ± 8.4 | 53.9 ± 10.4 |
| LDL size (nm) | 20.2 ± 0.5 | 20.3 ± 0.6 | 20.3 ± 0.5 |
| HDL size (nm) | 9.2 ± 0.4 | 9.2 ± 0.4 | 9.1 ± 0.4 |
| Parameters (μmol/L) | Baseline | EGG | SUB |
|---|---|---|---|
| Choline | 8.33 ± 2.08 a | 10.54 ± 2.8 b | 9.84 ± 3.17 a |
| Betaine | 35.94 ± 9.8 a | 43.4 ± 11.7 a | 39.1 ± 13.5 a |
| DMG | 2.25 ± 0.98 a | 3.06 ± 1.91 b | 2.8 ± 2.44 ab |
| Methionine | 30.6 ± 5.07 | 29.77 ± 8.3 | 30.4 ± 7.7 |
| TMAO | 2.3 ± 1.4 a | 2.8 ± 1.2 ab | 3.0 ± 2.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, M.S.; Puglisi, M.; Malysheva, O.; Caudill, M.A.; Sholola, M.; Cooperstone, J.L.; Fernandez, M.L. Eggs Improve Plasma Biomarkers in Patients with Metabolic Syndrome Following a Plant-Based Diet—A Randomized Crossover Study. Nutrients 2022, 14, 2138. https://doi.org/10.3390/nu14102138
Thomas MS, Puglisi M, Malysheva O, Caudill MA, Sholola M, Cooperstone JL, Fernandez ML. Eggs Improve Plasma Biomarkers in Patients with Metabolic Syndrome Following a Plant-Based Diet—A Randomized Crossover Study. Nutrients. 2022; 14(10):2138. https://doi.org/10.3390/nu14102138
Chicago/Turabian StyleThomas, Minu S., Michael Puglisi, Olga Malysheva, Marie A. Caudill, Maria Sholola, Jessica L. Cooperstone, and Maria Luz Fernandez. 2022. "Eggs Improve Plasma Biomarkers in Patients with Metabolic Syndrome Following a Plant-Based Diet—A Randomized Crossover Study" Nutrients 14, no. 10: 2138. https://doi.org/10.3390/nu14102138
APA StyleThomas, M. S., Puglisi, M., Malysheva, O., Caudill, M. A., Sholola, M., Cooperstone, J. L., & Fernandez, M. L. (2022). Eggs Improve Plasma Biomarkers in Patients with Metabolic Syndrome Following a Plant-Based Diet—A Randomized Crossover Study. Nutrients, 14(10), 2138. https://doi.org/10.3390/nu14102138









