Abstract
Dietary arsenic (As) contamination is a major public health issue. In the Middle East, the food supply relies primarily on the import of food commodities. Among different age groups the main source of As exposure is grains and grain-based food products, particularly rice and rice-based dietary products. Rice and rice products are a rich source of core macronutrients and act as a chief energy source across the world. The rate of rice consumption ranges from 250 to 650 g per day per person in South East Asian countries. The source of carbohydrates through rice is one of the leading causes of human As exposure. The Gulf population consumes primarily rice and ready-to-eat cereals as a large proportion of their meals. Exposure to arsenic leads to an increased risk of non-communicable diseases such as dysbiosis, obesity, metabolic syndrome, diabetes, chronic kidney disease, chronic heart disease, cancer, and maternal and fetal complications. The impact of arsenic-containing food items and their exposure on health outcomes are different among different age groups. In the Middle East countries, neurological deficit disorder (NDD) and autism spectrum disorder (ASD) cases are alarming issues. Arsenic exposure might be a causative factor that should be assessed by screening the population and regulatory bodies rechecking the limits of As among all age groups. Our goals for this review are to outline the source and distribution of arsenic in various foods and water and summarize the health complications linked with arsenic toxicity along with identified modifiers that add heterogeneity in biological responses and suggest improvements for multi-disciplinary interventions to minimize the global influence of arsenic. The development and validation of diverse analytical techniques to evaluate the toxic levels of different As contaminants in our food products is the need of the hour. Furthermore, standard parameters and guidelines for As-containing foods should be developed and implemented.
1. Introduction
As is a naturally found crystalline metalloid with ubiquitous distribution throughout the earth’s crust. As exposure to the human food chain ecosystem comprises air, water, food, and soil. Daily diet contamination depends on the inorganic or organic forms, oxidation state, water solubility, and food matrix. The differentiation and categorization of different foods as sources of inorganic and organic As contamination in daily life is an important issue. Industrialization, urbanization, and anthropogenic activities such as glassware, industrial chemicals, lead alloys, and pharmaceuticals manufacturing processes are prime causes of arsenic exposure in the environment. Heavy metal concentrations in all-natural water reservoirs exceed the cut-off of WHO’s safe limits for human health [1,2].
It is found that both organic and inorganic forms have no taste, odor, or color. Pentavalent arsenic (As (V) or arsenate) and trivalent arsenic (As (III) or arsenite), both in inorganic and organic forms, are present in natural ecosystems [3,4,5]. Inorganic arsenic (As (III) and As (V) or a combination of both) in ground water and biological activity transformation converts into arsenobetaine and different arsenosugars. Moreover, lipid-soluble arsenic forms, namely arsenolipids, have also been detected in fish and algae. Different As species can be seen in Table 1.

Table 1.
Different species of arsenic and their distribution in various foods with toxicities.
The demand for food supply and agricultural growth leads to the use of arsenic in various fertilizers and insecticides, which are aggravating factors of heavy metals in soils and crops, including calcium arsenite and copper acetoarsenite (Paris green) as pesticides and other herbicides such as methylarsenic acid and dimethylarsenic acid [15,16]. Staple food, beverage, and seafood consumption are increasing in day-to-day life. Only a few studies have correlated the relationship between diet and As concentration and some directly measured water arsenic intake [3,17]. In the Bangladeshi population, it has been reported that two food groups, namely cereals (53–60%) and vegetables (25%), contribute to inorganic arsenic (iAs) exposure [18]. Similarly, 54–85% of total arsenic exposure is found due to rice and other foods in the United States [19]. As per physiological and nutrition requirements, children and adolescents are at more risk of arsenic toxicities due to high energy and fluid allowances per body weight [20,21]. As in infants’ cereal and baby food (USA) gains too much attention because there are no specific regulations or guidelines, but the safe limit is 0.2 mg/kg for infants and young children [22,23]. According to the WHO, arsenic is among the most deadly substances impacting human wellbeing and is highly poisonous in its inorganic form. Long-term exposure increases the risk of cancer, skin lesions, pigmentation, cardiovascular disease, and diabetes. In utero and early childhood exposure to arsenic leads to poor cognitive function and increases teenage mortality [24,25,26,27].
2. Facts of Arsenic Exposure
2.1. Arsenic Ingestion through Water Ecosystem
The biggest hazard to the health care system from arsenic is groundwater contamination. As mobilization in groundwater is related to geologic setting and sedimentary components that regulate the geochemistry and As release from bedrocks into groundwater [28,29]. Mahmoud et al. (2018) assessed the quality (physicochemical parameters and heavy metals) of household drinking water gathered at four sampling points in Abu Dhabi’s Baniyas district and the United Arab Emirates [29]. Primary research revealed that several households had amounts of As, Cd, and Pb that exceeded the overall permissible limit set by UAE drinking water guidelines. Furthermore, as opposed to the other sampling points, the main water supply, the samples had a higher heavy metals concentration. Overall, the principal components analysis (PCA) showed that certain physical parameters could have contributed to high heavy metal levels. In a few samples of Kuwait marine water and sediments, it was observed that most heavy metals such as Cr, As, Ni, Hg, and Zn were within toxic limits with the exception of Cd, Cu, and Pb [30]. Okati et al. (2021) evaluated Hg, As, and Se levels in six Oman sea fish species and assessed the health risks of Hg and As toxicity, concluding that the consumption of such fish is not at high risk of As exposure [31]. Sabarathinam et al. (2019) compared Kuwait Bay seawater and revealed that the majority of heavy metals such as B, Li, Hg, Sr, Pb, Ba, Zn, Fe, Be, Mn, Co, Cd, Cr, Se, Ni, Al, V, Mo and As were present in higher concentrations [32].
Molamohyeddin et al. (2017) investigated the content of heavy metals in the surface sediments of Chabahar Bay and Oman. At certain stations, the arsenic level was higher than the effect range low (ERL) [33]. Statistical studies showed that organic matter and mud play an important part in metal dispersion. Similarly, Agah et al. (2016) found that the metal contents of the sediments was in the order listed: Al > Fe > Cr > V > Ni > Zn > Cu > As > Pb > Co [33]. As values of greater amounts were observed in certain regions, reflecting anthropogenic inputs. In another study, AI-Kalbani et al. (2001) evaluated the water quality of nine Aflaj samples in Al Jabal Al Akhdar, Oman [34]. Mg, Mb, and As concentrations in some Aflaj water samples were only just over the cap. Al-Farraj et al. (2013) evaluated the distribution of As and related hydrogeochemical parameters in 27 randomized aquifer-based boreholes in Saudi Arabia’s AI-Kharj agricultural area [35]. The contents of As were detected in every area with 92.5 percent of boreholes yielding concentrations that were greater than the WHO’s allowable maximum concentration of 10 μg/L. The maximum concentration was calculated to be 122 μg/L. Long-term exposure to high levels of iAs in drinking water has been linked with skin disorders and increased risk of diabetes, high blood pressure, and several types of cancer. iAs and As compounds are considered to be carcinogenic [36,37].
Ghrefat et al. (2016) analyzed the overall abundance and spatial distribution of many metals in the groundwater and farmland soils in Saudi Arabia’s Gulf of Aqaba (GoA) [38]. The metal amounts analyzed in the groundwater samples were below the WHO’s permissible limits. Similarly, Bu-Olayan et al. (2001) observed low As levels in the majority of marine organisms off the coast of Kuwait [39]. Further, in other studies, the quantities and concentrations of trace metals on the surface of seawater along the eastern coast of Saudi Arabia were investigated. It was observed that the concentrations of Zn, Fe, Cu, Se, and B were decreasing northward. Although As, Cd, Mn, and Pb levels were increased in the northern GoA. The amount of metal in the water was determined by nearby geologic materials [40]. In other studies, seawater was collected from 23 separate locations identified as fishing areas of the Kingdom of Bahrain’s territorial water for the purpose of creating a benchmark and evaluating marine pollution due to heavy metals [40]. The findings indicated that the coastal waters seem to be of good quality as the concentrations of metals recorded were much lower than those of the United Kingdom water quality standards, except for Cu at all sites and Hg at the Masoor site. De Mora et al. (2004) assessed heavy metal contaminants in the Gulf and Gulf of Oman using aquatic biota (fish and various bivalves) and coastal sediment collected in Bahrain, Oman, Qatar, and the UAE. Sediment metal loadings were typically not noteworthy, though hotspots of different heavy metals were noted in Bahrain (Hg, Cu, Pb, Zn) and UAE’s east coast (Co, As, Ni, Cr). As and Hg concentrations in sediments were usually low and overall Hg levels in top predator fish widely eaten in the area were 0.5 μg/g and posed no hazard to human health. Certain bivalve species from the area had extremely high As concentrations (up to 156 μg/g), though it cannot be confirmed that As is more likely produced from natural causes rather than anthropogenic pollution [41]. Moreover, Al-Awadi et al. (2003) found that As concentrations in the water samples from all 42 wells were lower than the detection limit of 0.005 mg/L. Various abnormal concentrations of As can be seen in different coastal areas of diverse countries in Table 2.

Table 2.
Various concentrations of arsenic in the water of different Middle East Countries.
2.2. Arsenic Ingestion through Dietary Contaminant Food
The dietary and food contamination of iAs is a matter of concern due to its carcinogenic potential. Children’s dietary components are mainly based on a staple diet, rice and infants’ formulas, with high iAs content. Gu et al. (2020) reported that 75% of rice-based infant food from Australia contains high iAs (0.1 mg/kg) due to rice and whole-grain mix cereals [48]. A similar finding was noticed by Hernández-Martínez and Navarro-Blasco (2013) in Spain and Juskelis et al. (2013) in American infants’ cereals. Another study carried out in Ireland and the UK reveals that formula-fed infants had higher concentrations of urinary dimethylamine (DMA) and methylmalonic acid (MMA) than exclusively or partially breastfed infants due to high exposure [49,50]. These As metabolites in post-weaning infants were found due to a complementary food formula based on rice cereals that were higher than the EU limit of 0.1 mg/kg [10]. Middle East regions, especially Gulf countries, have diets mainly consisting of rice-based foods. In another review, infants’ cereals in the Middle Eastern market showed exponential growth due to some social factors such as ready-made cereals, ready-to-eat restaurant practices, food processing technology, and the use of different additives in manufacturing processes, which, in Middle Eastern countries, are the main cause of dietary heavy metals contamination. The total iAs contamination of daily-use food can be seen in Table 3.

Table 3.
Total inorganic arsenic contamination in different foods available in our daily life with their toxic limits and detected values.
As-rich soil, water, geochemical activity, and pesticides lead to the contamination of the food chain. Arsenic exposure in nearly 1 billion of the population is through food and >200 million of the population through drinking water beyond the exposure limit of 10 μg/L [70]. As toxicities through food depend on food choice, culture, age group, and dietary restrictions. The consumption of a single food throughout the year without seasonal variety might be a worsening factor of toxic exposure. The ingestion of As through rice and legumes is considered a public health problem [3]. Rice has the highest capacity to absorb arsenic as compared to other cereal grains such as barley, wheat, oat, rye, and corn. Risk assessment was analyzed by the EFSA [71]. The European population concluded that cereals and processed foods are the primary cause of iAs exposure in the population, while water, rice, and dairy products exhibit a major role in iAs exposure in infants and toddlers. Other seafood and beverages such as apple juice have also been considered as a source of iAs toxic exposure. Lynch et al. (2014) estimated mean values in four different food groups, including seaweed/algae at 11,000 μg/kg, seafood at 130 μg/kg, rice at 130 μg/kg, and other cereal-related products at 92 μg/kg [72]. Arsenate (iAsV) or arsenite (iAsIII) are inorganic forms of arsenic present in food and drinking water—after ingestion, iAsV is converted to iAsIII—whereas seafood contains organic forms of arsenic, arsenobetaine and arsenocholine [7]. Root vegetables accumulate the highest As content and the lowest edible parts [73]. As contamination in various types of food and beverages in combination plays a major role in the progression of dangerous diseases such as cancer. Cancer has turned out to be an alarming public health concern for the whole world [74,75,76,77]. Consequently, the EFSA and JECFA reported a benchmark dose level (BMDL): 0.3–8 mg/kg body weight per day can be the cause of various kinds of cancer such as lung, skin, and bladder cancers.
3. Arsenic Cellular Metabolism
iAs present in the human body is generally excreted through urine and bile [78]. Urinary arsenic measurements (iAs%, MMA%, and DMA%) act as indicators of arsenic metabolism and methylation capacity [79,80]. Trans-cellular and paracellular pathways are major modes of iAs transportation [81]. Cellular metabolism includes methylation in four forms, namely monomethylarsonic acid (MMAV), monomethylarsonous acid (MMAIII), dimethylarsinic acid (DMAV), and dimethylarsinous acid (DMAIII). Among the different forms, MMAIII is reported as the most cytotoxic [82]. MMAIII species can inhibit mitochondrial I and III processes by electron escape through the electron transport chain, leading to the production of reactive oxygen species (ROS) and reactive nitrogen (RNS). Free radical production leads to DNA damage and impaired gene expression [83]. Enzymatic methylation occurs by way of the primary enzyme involved in As metabolism, called arsenic (3+) methyltransferase (AS3MT), and endogenous reducing agents such as thioredoxin (Trx) and glutathione (GSH) [84,85].
Arsenic has an affinity with thiol groups, inhibiting the catalytic activity of an enzyme by binding with thiol-containing active sites. GSH plays an important role in transforming arsenate (AsV) to arsenite (AsIII); the arsenite form has a shorter half-life in comparison to arsenate. Antioxidants act as electron donors during the reduction of pentavalent to trivalent arsenic due to their high affinity to GSH [86]. Arsenic–thiol interaction consequences include MMAIII inhibiting GSH reductase and thioredoxin reductase [87]. Arsenate produces glucose-6-arsenate and 6-arsenogluconate by the substitution of phosphate in glucose and gluconate and forms glucose-6-arsenate and 6-arsenogluconate, analogous to glucose-6-phosphate and 6-phospho-gluconate, respectively. Glucose-6-arsenate binds to glucose-6-phosphate dehydrogenase and a high concentration of arsenate inhibits hexokinase activity through negative feedback mechanisms during glycolysis. Arsenic inhibits the conversion of pyruvate to acetyl coenzyme A (acetyl-coA), which leads to diminished cellular glucose uptake, gluconeogenesis, the oxidation of fatty acid, and further acetyl-CoA production. Mitochondria is an important cellular target by arsenite and free radical production, lipid peroxidation, H2O2 production, and mitochondrial swelling. Arsenic induces the formation of superoxide anion radicals such as singlet oxygen, the peroxyl radical, hydroxyl radicals, NO, H2O2, dimethyl-arsinic-peroxyl radicals, and dimethylarsinic radicals in a dose-dependent manner and consequently leads to health complications [88].
4. Arsenic-induced Health Hazards
4.1. Major Organ Damage and Chronic Disease Development
Human body organs are typically distressed by As poisoning. Major organs susceptible to As toxicity are the kidneys, lungs, liver, and skin. Severe As toxicity leads to coma and death. Target organ damage (TOD) depends on the ingested arsenite (As+3) content in the body. Firstly, an initial sign of skin changes involves its binding with keratin and accumulation in hair and nails. The appearance of keratosis is a common early sign of arsenic exposure. Recently, squamous cell carcinoma (SQCC), melanosis, and keratosis to Bowen’s disease have been reported in Asian countries, especially in India, Nepal, and Bangladesh [89,90]. Increased monomethylarsonate (MMA)% and decreased dimethylarsinate (DMA)% of arsenic species are directly proportional to a higher risk of bladder, pulmonary, and skin cancers. Carcinogenic and chromatin alteration have been exhibited due to transcription initiation and gene sequencing. The cellular genomic modification of deoxyribonucleic acid (DNA) methylation and the post-transcriptional modification (PTMs) of histone proteins lead to tumors and benign dysplasia. Arsenic induces miRNA gene expression that affects polymerase elongation and the recruitment of splicing regulatory factors and leads to carcinogenicity [8,91].
Drinking water iAs is nearly 80–90% absorbed by the intestine and protein transporters of arsenic such as aquaporin-10, GLUT-5, and organic anion-transporting polypeptides (OATPB) in the gut epithelium [92]. As exposure can lead to developing a risk of nonalcoholic fatty liver diseases among adolescents [93]. As is a promoter of inflammation, oxidative stress, and endothelial dysfunction by different mechanisms including the activation of transcription factors such as protein-1 and nuclear factor κβ [94,95]. The mechanisms of carcinogenesis take place through multiple pathways, including the perturbation of gut microbiota, genotoxicity, and epigenetic dysregulation [96,97,98]. The conjugation of arsenic with glutathione forms arsenic triglutathione, and the methylation of arsenic produces dimethylarsenic glutathione and enters bile and the bloodstream. This unstable species changes into the volatile compound dimethylarsine. As is transported via RBCs and is stored as protein-bound trivalent dimethyl arsenicals in different organs of the body [7]. As exhibits carcinogenic effects in the liver and produces hepatocellular carcinoma through DNA repair inhibition and the development of micronuclei and epigenetic dysregulation [96,99]. Different As species-linked health effects have been mentioned in Table 4.

Table 4.
Various arsenic species and associated health hazards.
Chronic exposure to As produces harmful effects on the immune system. Immune responses depend on the proliferation of T and B cells as well as macrophages. Chronic exposure to As may cause immunosuppression by affecting cellular and humoral immunity [102,117]. It has been found that immunosuppression due to decreased T-cell proliferation is linked with low cytokine secretion, tumor necrosis factor (TNF)-α, interferon-γ, IL-2, IL-10, IL-5, and IL-4. A similar study states that chronic exposure leads to high serum immunoglobulin IgA, IgG, and IgE, which may lead to the development of respiratory complications such as pneumonia, allergic bronchitis, and chronic obstructive pulmonary disease [118]. Chronic exposure to As leads also to several other clinical manifestations such as hypercalciuria, glomerulonephritis, acute tubular necrosis, albuminuria, nephrocalcinosis, and renal papillae necrosis [81]. The development of incipient nephropathy and the incidence of chronic kidney disease (CKD) have been reported due to As-induced injury of the nephron [119]. As nephritis and renal damage occur due to direct podocyte injury and endothelial dysfunction and increase the expression of the vascular cell adhesion molecule 1 (VCAM-1) [120,121]. The direct effects of arsenic species and metabolites on chronic diseases with their respective mechanisms are listed in Table 5.

Table 5.
Direct effects of arsenic species and their metabolites with respective mechanisms in different chronic diseases.
As affects the gluconeogenesis in muscle cells by inhibiting glucose transporters and suppressing glucose metabolism regulatory genes. Hence, the glycolytic pathway and mitochondrial energy production are altered [132,133]. In experimental studies of mice, it has been reported that As decreases the functional capacity of muscle and destroys muscle progenitor cells [134]. Muscle damage occurs by inhibiting muscle repair and increasing the nuclear factor kappa light-chain enhancer of activated B cells (NF-κB), along with inflammation signaling, a long healing time, and fibrosis. Consequently, As exposure contributes to sarcopenia progression [135].
As acute toxicity generates oxidative stress and pro-inflammatory reactions in the epithelial cells of the intestine in in vivo studies [136,137]. Therefore, reactive oxygen species (ROS) damage the cytoskeleton and cause the loss of tight junction proteins such as claudin-5 and occludin in the blood–brain barrier [138]. iAs species might be liable to increase paracellular transport at tight junctions [139]. The high permeability of intestinal junctions is related to intestinal abnormalities such as dysbiosis, colitis, Crohn’s disease, ulcerative colitis, and other gastrointestinal complications [140]. High As levels increase colonies of pathogenic bacteria in dysbiosis, whereas low levels directly increase intestinal commensal bacteria. Therefore, gut microbiome health depends on a variety of species of probiotic bacteria [141]. A study conducted on children exposed to high As shows plenty of proteobacteria in stool samples [142]. A recent study on the Bangladeshi population demonstrated the toxic effect of high As on the species and flora of gut bacteria and the high number of pathogens [143]. Furthermore, it has also been seen that different forms of As species cross the blood–brain barrier, reducing neurotransmitters, including mono-amines and those associated with the cholinergic, dopaminergic, and glutamatergic systems, leading to damaged synaptic transmission [144]. Glutamate receptor expression inhibition may cause changes in synaptic plasticity, for instance, the long-term potentiation of learning and memory linked with enhancing extracellular glutamate levels [145]. Various diseases linked with As toxicity are depicted in Figure 1.
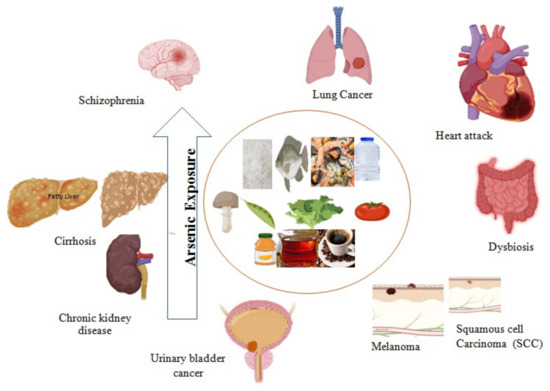
Figure 1.
Arsenic exposure leads to acute to chronic complications such as chronic kidney disease, cardiovascular disease, schizophrenia, fatty liver, cirrhosis, dysbiosis, and different types of cancer.
4.2. Effects on Maternal Health
A recent study of As exposure and gestational diabetes mellitus (GDM) showed their strong positive links to each other [146]. Sung et al. reported a negative metabolic effect of As toxicity in GDM [126]. iAs changes glucose homeostasis by switching phosphates in adenosine triphosphate (ATP) synthesis and impairing ATP-dependent insulin. Furthermore, arsenate conjugates with the disulfide bridges of insulin, insulin receptors, glucose transporters (GLUTs), and glucose metabolism enzymes. Peroxisome proliferator-activated receptor γ (PPARγ) plays a significant role in the expression of insulin activation. As free radical damage interferes with the signal transduction and gene expression of β-cells, leading to the development of diabetes. As activates superoxide and binds with uncoupling protein 2 (UCP2), consequently decreasing insulin secretion [147]. UCP2 acts as a negative regulator for the secretion of insulin and also mediates proton leakage across the inner mitochondrial membrane [148].
In a research study on high exposure to As in Bangladeshi women, it was concluded that As exposure is the cause of cervical cancer (squamous cell carcinoma) [149]. Further, in another study of Bangladeshi women, it was found that As is also responsible for anemia. A prevalence of anemia has been found in the reproductive female age group and was linked to arsenicosis skin lesions [150]. High exposure to As also leads to early menopause of two years compared to low- or normal-limit exposed females’ menopausal stages [151]. In vivo and in vitro research of iAs exposure has revealed that As can attach to human and animal hemoglobin and can alter morphology, cell shape, levels of hemoglobin, and heme metabolism. Absorbed arsenic is transported through portal circulation via RBCs and WBCs. In another study, a 2–3-fold higher prevalence of anemia has been reported with a low dose of As-exposed drinking water compared to normal potable water, and pregnant women were more susceptible than non-pregnant women [152,153].
Ahmed et al. reported the effect on cellular innate immunity and immunosuppression of prenatal As exposure in Bangladeshi women [154]. The immune-suppressive effect on T and B cells as well as macrophages is due to the reduced expression of major histocompatibility complex (MHC) class II molecules, CD69, interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α). Inflammatory cascades of cytokine release low lymphocyte proliferation and IL-2 secretion, leading to inflammation, macrophage adhesion, phagocytosis, the increased apoptosis of peripheral blood mononuclear cells (PBMC), reduced ROS stimulus by PBMC, and stop the progress of the immunogenic response in hosts [102]. The disruption of estrogen receptors and the suppression of the signaling pathway of estrogen is linked to breast cancer, and As is a potential metallo-estrogen and acts as a medium to promote breast cancer [155,156,157]. Marciniak et al. (2020) reported in a Poland study that high-dose arsenic exposure increases the risk of breast cancer by 13 times compared to normal women [158].
4.3. Effects on Fetal and Neonatal Health
As exposure’s adverse effects have been reported also in neonatal health. It has been observed that arsenic exposure during the last trimester of pregnancy directly affects newborn telomere length (TL). Telomeres are DNA–protein structures; they are present at the end of each strand of DNA and defend the genome from nucleolytic degradation, interchromosomal fusion, and unnecessary recombination. Thus, telomeres exhibit a significant role to preserve information within the genome. Prenatal arsenic toxicity is the cause of newborn telomerase elongation and may suggest a new approach to neonatal health hazards [159]. Exposure to arsenic has harmful genotoxicity in newborns, DNA strand breaks, and increased MN frequency in cord blood. Increased arsenic maternal biomarkers are associated with genetic defects in newborns. Milton et al. (2017) reported the effect of As on spontaneous abortion and stillbirth, which are increased by up to 2–3-fold, and the risk of complications is 6-fold higher than in unexposed women. The ingestion of high arsenic content in food and water reduces methylation, which leads to folate deficiency and high homocysteine in urine, significantly contributing to congenital malformations and placental abruption [160,161,162].
Neural tube defects (NTDs) and insufficient neuron growth, as well as self-regulation in newborns, have been reported due to maternal As exposure [163,164]. Similar effects on newborns with high arsenic exposure were also reported in a Turkish study [165]. Deficits in memory, attention, and IQ from early life exposures are also noted with exposure to As [166]. Quansah et al. (2015) analyzed prospective birth cohort study results that indicate increased risks of spontaneous abortion, stillbirth, and neonatal and infant mortality among populations highly exposed to arsenic in drinking water. Chronic iAs exposure leads to placental insufficiency complications including preterm delivery and intrauterine growth retardation (IUGR) [154]. During pregnancy, As inorganic forms and their methylated metabolites cross the placenta and enter cord blood, leading to altered immune cell and gene expression in the cord blood of a highly exposed mother [167,168,169,170]. Moreover, other fetal complications also reported include slow fetal growth, low birth weight, and the effect of neuronal development in early life [89,171]. Many case-control and observation studies on As exposure have shown that it delays cognitive function and causes low intelligence quotients [172,173]. Children up to five years of age are more susceptible to arsenic exposure due to the high consumption of baby foods and more demand for energy and carbohydrate-rich diets. As-associated maternal and fetal complications can be seen in Figure 2.

Figure 2.
Arsenic-induced maternal health complications such as anemia, early menopause, cervical and breast cancer, and child health complications including neural tube defects, DNA fragmentation, intrauterine growth retardation, and autism.
5. Arsenic Screening
The most consistent method to examine arsenic exposure is with a urine test. Urine and blood tissue are convenient screening methods for the detection of heavy metals. More than 90% of arsenic exposure is detected through urine samples. The accumulation of As in hair and finger or toenails is considered chronic exposure due to the binding of sulfhydryl groups of keratin found in hair and nails and deposition takes approximately 2 weeks [173]. Hair and nails grow slowly due to multiple phages and the toxic accumulation of heavy metals, and toxic accumulation takes more than 3–6 months [174]. Sample hair and nails quantify the chronic exposure of iAs, while blood and urine measurements indicate a short duration of exposure because the half-life of arsenic in blood is 2–6 h and 4 days in urine [47,119,120]. Wang et al. (2017) examined a salivary screening detection method. Saliva collection is easy and convenient for all age groups and is found to be appropriate for children and menstruating women [79].
There are different toxic levels of iAs that have been identified in various samples such as scalp hair arsenic (1.0 < 3.0 mg/kg), toenail arsenic (>0.5 μg/g), total organic blood arsenic (>130 nmol/L), urinary arsenic (>100 μg/L), and spot urine sample arsenic (>50 μg/L), considered as upper abnormal limits [175]. An oral intake of 100–300 mg (1–5 mg/kg BW) of iAs in humans usually leads to death within 1 h, if untreated [175,176].
6. Food Safety and Policy Interventions
Rice is a major part of the Saudi diet but is not domestically produced. It is estimated that 1.45 million metric tons (MMT) of rice were consumed in the kingdom of Saudi Arabia (KSA) in 2015–2016 [177]. The maximum allowable concentrations of iAs, as per the European Commission regulations (European Commission, 2015), are 0.25 and 0.20 mg/kg for brown and white rice, respectively. Several Asian countries such as India and Bangladesh have not set any limits or regulations so far. Recently, around half of the rice brands in the UK have potentially exceeded the hazardous limits and are unsafe for infants and young children [178]. The arsenic content of rice varies depending on geographic and water sources. Paddies cultivated in the United States (US) have higher total As (tAs) and low iAs arsenic as compared to Indian and Bangladesh rice cultivations [179]. The recommendation of polished rice instead of brown rice and basmati rice varieties is an alternate approach to counter the risk of iAs exposure through rice [180,181]. The scientific cooking method of parboiled and absorbed (PBA) rice was investigated for the removal of 54% and 73% of iAs from brown and white rice, respectively; therefore, meal preparation is another safe approach for iAs exposure [178,182]. Dehusking, milling, parboiling, and cooking methods for raw and cooked rice are important steps to minimize As content. Meal preparation techniques, washing and rinsing time, and the temperature of boiling water determine the concentrations of arsenic species transformations [183]. Inorganic forms, arsenite (As3+) and arsenate (As5+) are the predominant forms of As present in rice and its products [23].
An effective mitigation strategy to minimize the soluble arsenite forms in whole grain/polished rice is by cooking with a high volume of water [184]. Practicing a traditional method of cooking involves washing several times and then cooking in a large volume of water followed by draining the extra water once the rice is cooked. When the rice is boiled using sufficient water where no extra water is left after the rice is cooked results in the presence of high As levels in cooked rice. Raab et al. determined tAs and iAs content in a variety of basmati, long grain, polished, and whole rice by washing, rinsing, and boiling/steaming methods in low-volume (rice-to-water ratio 1:2.5) and high-volume (ratio of water to rice: 1:6) uncontaminated water [185]. Findings suggested that a high volume of water during preparation reduces both tAs and iAs by 35% and 45%, respectively, in long-grain basmati rice as compared to raw rice [185]. Recent studies suggested that washing three times with deionized water reduces tAs content in both white as well as brown rice by up to 81–84% and 71–83%. A similar finding was documented by rinsing with a 10:1 water:rice ratio with deionized water, reducing iAs in all varieties of brown rice [186]. Continual-stream percolating water reduces As content in cooked rice. The percolation cooking method uses a continual stream of percolating water through a filter unit by a coffee maker machine, wherein the rice is placed in the filter unit instead of coffee and boiling water is passed through the filter unit continuously. A reduction in iAs has been reported in raw polished rice by 59% and wholegrain rice by 69%, respectively [187]. Various factors such as high washing, soaking, and cooking rice with arsenic-contaminated water affect the final dietary arsenic ingestion in meal preparation [188].
Critical control points during the cooking of rice and other grains in different settings and standard protocols should be developed. The awareness of adopting proper safe cooking measures should be highlighted among the community and culinary workers. The safe upper limit of As content on labeling might be helpful for consumers of imported rice and other grains in international food markets and especially in Middle Eastern countries. Different countries’ arsenic levels in rice and other foods are listed in Table 6.

Table 6.
Arsenic levels in various foods in different countries.
WHO Standards for Drinking Water Safety (1996) defined a tentative value of 0.01 mg/L for As in drinking water. A majority of European countries have implemented the WHO interim recommendation of 0.01 mg/L as their standard. In 1996, Australia introduced an even higher standard for As in drinking water of 0.007 mg/L [192]. The EPA interim maximum contaminant level for As in drinking water was 0.05 mg/L in the United States, but the EPA adopted a revised limit of 0.01 mg/L in drinking water in 2001. Since 2006, all drinking water delivery agencies in the United States have been adhering to the new 0.01 mg/L requirements. China, India, and Bangladesh are among the nations where the national limit for As in drinking water is 0.05 mg/L. Just about all nations in the Eastern Mediterranean Region lack a consistent plan for establishing, enforcing, and reviewing drinking water quality requirements. The issued guidelines were modified from WHO and international standards, but they were not tailored to local requirements [193].
7. Conclusions and Recommendation
The occurrence of As in food and water is a major health concern and substantial steps are required to overcome such challenging issues to obtain a healthy life and control health hazards associated with As and its various species. There are different species of As; some of them are highly toxic and some are nontoxic in nature, such as arsenate, arsenite, dimethylarsinate, methylarsonite, arsenobetaine, trimethylarsonio propionate, trimethylarsine oxide, etc. Certain effects of arsenic have been seen in human health but of more concern is maternal, fetal, and child health. Particularly, children up to five years of age are more vulnerable to As-sensitive effects due to more application of baby foods. Several As-associated complications in newborns and children have been reported, including spina bifida, deficits in memory and attention, stillbirth, slow fetal growth, low birth weight, delays in cognitive function, and neonatal and infant mortality. As exposure effects such as immunosuppression, anemia, GDM, breast cancer, cervical cancer, and early menopause have been seen in various women. It is the need of the hour to pay more attention to the mineral contents of the land used for the cultivation of cereals to avoid toxic arsenic exposure through plant sources. Specific guidelines on the acceptable concentrations of As in water and foods, particularly in baby foods, should be mentioned by different countries with toxic levels of different arsenic species. Furthermore, efforts are required to know the effects of food processing and the addition of different food processing aids or chemicals in different As species present in foods. The development and validation of analytical methods to measure the toxic levels of various As contaminants in different food products, particularly grains and grain-based products and rice and rice-based products, is the basic need of the entire world to conquer current As health hazards. Study of the toxicities of As in humans should be explored through various classifications on the basis of gender, age, and demographics. The scientific data of present information, particularly in the Middle East, provides novel insights on As toxic exposure and adverse health effects in different groups of people and levels of As in different foods and water. Furthermore, research and data collection are required to get present levels of As in different foods, including ready-to-eat food, baby food, and other exported food from different countries, water, vegetables, and fruits growing in As-rich land.
Author Contributions
Conceptualization and methodology, M.I.K., M.F.A., S.K.; Writing—original draft preparation, M.F.A., F.A., K.R.H., S.W.; Writing—review and editing, F.A., S.W., I.A., A.A.A.; writing original draft preparation, I.A., K.R.H., A.A.A. Conceptualization referencing, M.I.K., M.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Scientific Research Deanship at King Khalid University, Abha, Saudi Arabia for their financial support through the Large Research Group Project under grant number (RGP.02-87-43).
Conflicts of Interest
The authors confirm that this article content has no conflict of interest.
References
- Ezemonye, L.I.; Adebayo, P.O.; Enuneku, A.A.; Tongo, I.; Ogbomida, E. Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin River, Nigeria. Toxicol. Rep. 2019, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social determinants of health and diabetes: A scientific review. Diabetes Care 2021, 44, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S.; Arcella, D.; Cascio, C.; Gómez Ruiz, J.Á. Chronic dietary exposure to inorganic arsenic. EFSA J. 2021, 19, e06380. [Google Scholar]
- Chou, C.-H.; Harper, C. Toxicological Profile for Arsenic. 2007. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/657856 (accessed on 1 March 2022).
- ATSDR (Agency for Toxic Substances and Disease Registry). Prepared by Clement International Corp., under contract 2000, 205, 88-0608.
- Wang, J.; Hu, W.; Yang, H.; Chen, F.; Shu, Y.; Zhang, G.; Liu, J.; Liu, Y.; Li, H.; Guo, L. Arsenic concentrations, diversity and co-occurrence patterns of bacterial and fungal communities in the feces of mice under sub-chronic arsenic exposure through food. Environ. Int. 2020, 138, 105600. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hirano, S. Metabolism of arsenic and its toxicological relevance. Arch. Toxicol. 2013, 87, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Eleazer, R.; Rea, M.; Fondufe-Mittendorf, Y. Epigenomic reprogramming in inorganic arsenic-mediated gene expression patterns during carcinogenesis. Rev. Environ. Health 2017, 32, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.; di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. Scientific Opinion on marine biotoxins in shellfish—Palytoxin group. EFSA J. 2009, 7, 1393. [Google Scholar]
- Signes-Pastor, A.J.; Woodside, J.V.; McMullan, P.; Mullan, K.; Carey, M.; Karagas, M.R.; Meharg, A.A. Levels of infants’ urinary arsenic metabolites related to formula feeding and weaning with rice products exceeding the EU inorganic arsenic standard. PLoS ONE 2017, 12, e0176923. [Google Scholar] [CrossRef]
- Molin, M.; Ulven, S.M.; Meltzer, H.M.; Alexander, J. Arsenic in the human food chain, biotransformation and toxicology–Review focusing on seafood arsenic. J. Trace Elem. Med. Biol. 2015, 31, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Yoshinaga, J.; Narukawa, T. Dietary intake and urinary excretion of methylated arsenicals of Japanese adults consuming marine foods and rice. Food Addit. Contam. Part A 2021, 38, 622–629. [Google Scholar] [CrossRef]
- Tibon, J.; Silva, M.; Sloth, J.J.; Amlund, H.; Sele, V. Speciation analysis of organoarsenic species in marine samples: Method optimization using fractional factorial design and method validation. Anal. Bioanal. Chem. 2021, 413, 3909–3923. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.F.; Jackson, B.P. Concentrations and speciation of arsenic in New England seaweed species harvested for food and agriculture. Chemosphere 2016, 163, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, C.; Stiboller, M.; Glabonjat, R.A.; Rieger, J.; Paton, L.; Francesconi, K.A. Transport of arsenolipids to the milk of a nursing mother after consuming salmon fish. J. Trace Elem. Med. Biol. 2020, 61, 126502. [Google Scholar] [CrossRef] [PubMed]
- Bencko, V.; Foong, F.Y.L. The history of arsenical pesticides and health risks related to the use of Agent Blue. Ann. Agric. Environ. Med. 2017, 24, 312–316. [Google Scholar] [CrossRef]
- Gilbert-Diamond, D.; Cottingham, K.L.; Gruber, J.F.; Punshon, T.; Sayarath, V.; Gandolfi, A.J.; Baker, E.R.; Jackson, B.P.; Folt, C.L.; Karagas, M.R. Rice consumption contributes to arsenic exposure in US women. Proc. Natl. Acad. Sci. USA 2011, 108, 20656–20660. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.K.; Shaheen, N.; Islam, M.S.; Habibullah-Al-Mamun, M.; Islam, S.; Islam, M.M.; Kundu, G.K.; Bhattacharjee, L. A comprehensive assessment of arsenic in commonly consumed foodstuffs to evaluate the potential health risk in Bangladesh. Sci. Total Environ. 2016, 544, 125–133. [Google Scholar] [CrossRef]
- Kurzius-Spencer, M.; O’rourke, M.K.; Hsu, C.-H.; Hartz, V.; Harris, R.B.; Burgess, J.L. Measured versus modeled dietary arsenic and relation to urinary arsenic excretion and total exposure. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 442–449. [Google Scholar] [CrossRef]
- Guillod-Magnin, R.; Brüschweiler, B.J.; Aubert, R.; Haldimann, M. Arsenic species in rice and rice-based products consumed by toddlers in Switzerland. Food Addit. Contam. Part A 2018, 35, 1164–1178. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, H.; Takata, A. Arsenic metabolism differs between child and adult patients during acute arsenic poisoning. Toxicol. Appl. Pharmacol. 2021, 410, 115352. [Google Scholar] [CrossRef]
- Shibata, T.; Meng, C.; Umoren, J.; West, H. Risk assessment of arsenic in rice cereal and other dietary sources for infants and toddlers in the US. Int. J. Environ. Res. Public Health 2016, 13, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubadda, F.; Jackson, B.P.; Cottingham, K.L.; Van Horne, Y.O.; Kurzius-Spencer, M. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Sci. Total Environ. 2017, 579, 1228–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100, p. 11. [Google Scholar]
- Lee, C.-H.; Yu, C.-L.; Liao, W.-T.; Kao, Y.-H.; Chai, C.-Y.; Chen, G.-S.; Yu, H.-S. Effects and interactions of low doses of arsenic and UVB on keratinocyte apoptosis. Chem. Res. Toxicol. 2004, 17, 1199–1205. [Google Scholar] [CrossRef]
- Chen, W.-J.; Davis, E.M.; Stoner, J.A.; Robledo, C.; Goodman, J.R.; Garwe, T.; Janitz, A.E.; Xu, C.; Hwang, J.; Peck, J.D. Urinary total arsenic and arsenic methylation capacity in pregnancy and gestational diabetes mellitus: A case-control study. Chemosphere 2021, 271, 129828. [Google Scholar] [CrossRef]
- Sarkar, M.; Pal, S.C. Human health hazard assessment for high groundwater arsenic and fluoride intact in Malda district, Eastern India. Groundw. Sustain. Dev. 2021, 13, 100565. [Google Scholar] [CrossRef]
- Mahmoud, M.T.; Hamouda, M.A.; Al Kendi, R.R.; Mohamed, M.M. Health risk assessment of household drinking water in a district in the UAE. Water 2018, 10, 1726. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Chidambaram, S. Assessment of trace inorganic contaminates in water and sediment to address its impact on common fish varieties along Kuwait Bay. Environ. Geochem. Health 2021, 43, 855–883. [Google Scholar] [CrossRef]
- Okati, N.; Moghadam, M.S.; Einollahipeer, F. Mercury, arsenic and selenium concentrations in marine fish species from the Oman Sea, Iran, and health risk assessment. Toxicol. Environ. Health Sci. 2021, 13, 25–36. [Google Scholar] [CrossRef]
- Sabarathinam, C.; Bhandary, H.; Al-Khalid, A. A geochemical analogy between the metal sources in Kuwait Bay and territorial sea water of Kuwait. Environ. Monit. Assess. 2019, 191, 1–19. [Google Scholar] [CrossRef]
- Molamohyeddin, N.; Ghafourian, H.; Sadatipour, S.M. Contamination assessment of mercury, lead, cadmium and arsenic in surface sediments of Chabahar Bay. Mar. Pollut. Bull. 2017, 124, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Al-Kalbani, M.S.; Price, M.F.; Ahmed, M.; Abahussain, A.; O’Higgins, T.; Argyll, U. Water quality assessment of Aflaj in the Mountains of Oman. Environ. Nat. Resour. Res. 2016, 6, 99. [Google Scholar] [CrossRef] [Green Version]
- Al-Farraj, A.S.; Al-Wabel, M.I.; El-Saeid, M.H.; El-Naggar, A.H.; Ahmed, Z. Evaluation of groundwater for arsenic contamination using hydrogeochemical properties and multivariate statistical methods in Saudi Arabia. J. Chem. 2013, 2013, 812365. [Google Scholar] [CrossRef] [Green Version]
- Pakzad, D.; Akbari, V.; Sepand, M.R.; Aliomrani, M. Risk of neurodegenerative disease due to tau phosphorylation changes and arsenic exposure via drinking water. Toxicol. Res. 2021, 10, 325–333. [Google Scholar] [CrossRef]
- Al-Forkan, M.; Wali, F.B.; Khaleda, L.; Alam, M.J.; Chowdhury, R.H.; Datta, A.; Rahman, M.Z.; Hosain, N.; Maruf, M.F.; Chowdhury, M.A.Q. Association of arsenic-induced cardiovascular disease susceptibility with genetic polymorphisms. Sci. Rep. 2021, 11, 6263. [Google Scholar] [CrossRef]
- Ghrefat, H.; El Waheidi, M.; Batayneh, A.; Nazzal, Y.; Zumlot, T.; Mogren, S. Pollution assessment of arsenic and other selected elements in the groundwater and soil of the Gulf of Aqaba, Saudi Arabia. Environ. Earth Sci. 2016, 75, 229. [Google Scholar] [CrossRef]
- Bu-Olayan, A.; Thomas, B. Arsenic levels in the marine ecosystem off the Kuwait coast, Arabian Gulf. Environmentalist 2001, 21, 71–75. [Google Scholar] [CrossRef]
- Al-Taani, A.A.; Batayneh, A.; Nazzal, Y.; Ghrefat, H.; Elawadi, E.; Zaman, H. Status of trace metals in surface seawater of the Gulf of Aqaba, Saudi Arabia. Mar. Pollut. Bull. 2014, 86, 582–590. [Google Scholar] [CrossRef]
- De Mora, S.; Fowler, S.W.; Wyse, E.; Azemard, S. Distribution of heavy metals in marine bivalves, fish and coastal sediments in the Gulf and Gulf of Oman. Mar. Pollut. Bull. 2004, 49, 410–424. [Google Scholar] [CrossRef]
- Agah, H.; Bastami, K.D.; Fumani, N.S. Ecological risk, source and preliminary assessment of metals in the surface sediments of Chabahar Bay, Oman Sea. Mar. Pollut. Bull. 2016, 107, 383–388. [Google Scholar] [CrossRef]
- El-Sorogy, A.S.; Youssef, M.; Al-Kahtany, K.; Al-Otaiby, N. Assessment of arsenic in coastal sediments, seawaters and molluscs in the Tarut Island, Arabian Gulf, Saudi Arabia. J. Afr. Earth Sci. 2016, 113, 65–72. [Google Scholar] [CrossRef]
- Youssef, M.; El-Sorogy, A.; Al Kahtany, K.; Al Otiaby, N. Environmental assessment of coastal surface sediments at Tarut Island, Arabian Gulf (Saudi Arabia). Mar. Pollut. Bull. 2015, 96, 424–433. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, M.M.; El Nasr, A.K.O.A.; Hamouda, M.S. Quality assessment of groundwater at south Al Madinah Al Munawarah area, Saudi Arabia. Environ. Earth Sci. 2013, 70, 1525–1538. [Google Scholar] [CrossRef]
- Juma, H.A.; Al-Madany, I.M. Concentration of heavy metals in the territorial sea water of the Kingdom of Bahrain, Arabian Gulf. Arab. Gulf J. Sci. Res. 2008, 26, 19–32. [Google Scholar]
- Al-Awadi, E.; Mukhopadhyay, A.; Akber, A.; Hadi, K. Distribution of selected trace constituents in the ground water of Kuwait. Adv. Environ. Res. 2003, 7, 367–380. [Google Scholar] [CrossRef]
- Gu, Z.; de Silva, S.; Reichman, S.M. Arsenic concentrations and dietary exposure in rice-based infant food in Australia. Int. J. Environ. Res. Public Health 2020, 17, 415. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Martínez, R.; Navarro-Blasco, I. Survey of total mercury and arsenic content in infant cereals marketed in Spain and estimated dietary intake. Food Control 2013, 30, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Juskelis, R.; Li, W.; Nelson, J.; Cappozzo, J.C. Arsenic speciation in rice cereals for infants. J. Agric. Food Chem. 2013, 61, 10670–10676. [Google Scholar] [CrossRef]
- Shraim, A.M. Rice is a potential dietary source of not only arsenic but also other toxic elements like lead and chromium. Arab. J. Chem. 2017, 10, S3434–S3443. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.H.; Rahman, M.M.; Correll, R.; Naidu, R. Arsenic and other elemental concentrations in mushrooms from Bangladesh: Health risks. Int. J. Environ. Res. Public Health 2018, 15, 919. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, N.; Irfan, N.M.; Khan, I.N.; Islam, S.; Islam, M.S.; Ahmed, M.K. Presence of heavy metals in fruits and vegetables: Health risk implications in Bangladesh. Chemosphere 2016, 152, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, E.; Jinadasa, B. Arsenic and cadmium concentrations in legumes and cereals grown in the North Central Province, Sri Lanka and assessment of their health risk. Int. J. Food Contam. 2019, 6, 3. [Google Scholar] [CrossRef]
- Kowalska, G.; Pankiewicz, U.; Kowalski, R. Determination of the level of selected elements in canned meat and fish and risk assessment for consumer health. J. Anal. Methods Chem. 2020, 2020, 2148794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkoda, J. Arsenic in food of animal origin-exposure assessment. Ochr. Sr. I Zasobów Nat. 2009, 41, 128–134. [Google Scholar]
- Ashraf, M.W.; Mian, A. Levels of mercury and arsenic contamination in popular fish and shrimp brands consumed in Saudi Arabia. Bull. Chem. Soc. Ethiop. 2019, 33, 573–578. [Google Scholar] [CrossRef]
- Krishnakumar, P.K.; Qurban, M.A.; Stiboller, M.; Nachman, K.E.; Joydas, T.V.; Manikandan, K.P.; Mushir, S.A.; Francesconi, K.A. Arsenic and arsenic species in shellfish and finfish from the western Arabian Gulf and consumer health risk assessment. Sci. Total Environ. 2016, 566, 1235–1244. [Google Scholar] [CrossRef]
- Simsek, O.; Gültekin, R.; Öksüz, O.; Kurultay, S. The effect of environmental pollution on the heavy metal content of raw milk. Food 2000, 44, 360–363. [Google Scholar] [CrossRef]
- Gebeyehu, H.R.; Bayissa, L.D. Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PLoS ONE 2020, 15, e0227883. [Google Scholar] [CrossRef] [Green Version]
- Massadeh, A.M.; Allah, A.; Al-Massaedh, T.; Kharibeh, S. Determination of selected elements in canned food sold in Jordan markets. Environ. Sci. Pollut. Res. 2018, 25, 3501–3509. [Google Scholar] [CrossRef]
- Salama, K.F.; Randhawa, M.A.; Al Mulla, A.A.; Labib, O.A. Heavy metals in some date palm fruit cultivars in Saudi Arabia and their health risk assessment. Int. J. Food Prop. 2019, 22, 1684–1692. [Google Scholar] [CrossRef] [Green Version]
- Maduabuchi, J.-M.; Adigba, E.; Nzegwu, C.; Oragwu, C.; Okonkwo, I.; Orisakwe, O.E. Arsenic and chromium in canned and non-canned beverages in Nigeria: A potential public health concern. Int. J. Environ. Res. Public Health 2007, 4, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathabad, A.E.; Shariatifar, N.; Moazzen, M.; Nazmara, S.; Fakhri, Y.; Alimohammadi, M.; Azari, A.; Khaneghah, A.M. Determination of heavy metal content of processed fruit products from Tehran’s market using ICP-OES: A risk assessment study. Food Chem. Toxicol. 2018, 115, 436–446. [Google Scholar] [CrossRef]
- Savić, S.R.; Petrović, S.M.; Stamenković, J.J.; Petronijević, Ž.B. The presence of minerals in clear orange juices. Adv. Technol. 2015, 4, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Bazeyad, A.Y.; Al-Sarar, A.S.; Rushdi, A.I.; Hassanin, A.S.; Abobakr, Y. Levels of heavy metals in a multifloral Saudi honey. Environ. Sci. Pollut. Res. 2019, 26, 3946–3953. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.; Genuis, S.J.; Rodushkin, I. The benefits and risks of consuming brewed tea: Beware of toxic element contamination. J. Toxicol. 2013, 2013, 370460. [Google Scholar] [CrossRef] [Green Version]
- Al Nouri, D.; Al Abdulkarim, B.; Arzoo, S.; Bakeet, Z. Quality characteristics of commonly consumed drinking water in Riyadh and effect of domestic treatments on its chemical constituents. J. Food Nutr. Res. 2014, 2, 25–33. [Google Scholar]
- Shraim, A.; Alsuhaimi, A.; Al-Muzaini, K.; Kurdi, K.; Al-Ameen, H. Quality assessment of groundwater of Almadinah Almunawarah City. Global NEST J. 2013, 15, 374–383. [Google Scholar]
- Stanton, B.A.; Caldwell, K.; Congdon, C.B.; Disney, J.; Donahue, M.; Ferguson, E.; Flemings, E.; Golden, M.; Guerinot, M.L.; Highman, J. MDI Biological Laboratory arsenic summit: Approaches to limiting human exposure to arsenic. Curr. Environ. Health Rep. 2015, 2, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Authority, E. Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014, 8, 36–37. [Google Scholar]
- Lynch, H.N.; Greenberg, G.I.; Pollock, M.C.; Lewis, A.S. A comprehensive evaluation of inorganic arsenic in food and considerations for dietary intake analyses. Sci. Total Environ. 2014, 496, 299–313. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, F.; Deacon, C.; Chen, H.-j.; Raab, A.; Meharg, A.A. Arsenic accumulation and phosphorus status in two rice (Oryza sativa L.) cultivars surveyed from fields in South China. Environ. Pollut. 2010, 158, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount the cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F. Ganoderma lucidum: A Macro Fungus with Phytochemicals and Their Pharmacological Properties. In Plant and Human Health; Springer: Berlin, Germany, 2019; Volume 2, pp. 491–515. [Google Scholar]
- Wahab, S.; Alshahrani, M.Y.; Ahmad, M.F.; Abbas, H. Current trends and future perspectives of nanomedicine for the management of colon cancer. Eur. J. Pharmacol. 2021, 910, 174464. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Xie, S.; Hafeez, M.A.; Wang, X.; Hussain, H.I.; Iqbal, Z.; Pan, Y.; Iqbal, M.; Shabbir, M.A.; Yuan, Z. Metabolism and toxicity of arsenicals in mammals. Environ. Toxicol. Pharmacol. 2016, 48, 214–224. [Google Scholar] [CrossRef]
- Wang, D.; Shimoda, Y.; Wang, S.; Wang, Z.; Liu, J.; Liu, X.; Jin, H.; Gao, F.; Tong, J.; Yamanaka, K. Total arsenic and speciation analysis of saliva and urine samples from individuals living in a chronic arsenicosis area in China. Environ. Health Prev. Med. 2017, 22, 45. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, Y.; Liu, J.; Wang, D.; Zheng, Q.; Sun, G. Differences of urinary arsenic metabolites and methylation capacity between individuals with and without skin lesions in Inner Mongolia, Northern China. Int. J. Environ. Res. Public Health 2014, 11, 7319–7332. [Google Scholar] [CrossRef] [Green Version]
- Roggenbeck, B.A.; Banerjee, M.; Leslie, E.M. Cellular arsenic transport pathways in mammals. J. Environ. Sci. 2016, 49, 38–58. [Google Scholar] [CrossRef]
- Moe, B.; Peng, H.; Lu, X.; Chen, B.; Chen, L.W.; Gabos, S.; Li, X.-F.; Le, X.C. Comparative cytotoxicity of fourteen trivalent and pentavalent arsenic species determined using real-time cell sensing. J. Environ. Sci. 2016, 49, 113–124. [Google Scholar] [CrossRef]
- Hubaux, R.; Becker-Santos, D.D.; Enfield, K.S.; Rowbotham, D.; Lam, S.; Lam, W.L.; Martinez, V.D. Molecular features in arsenic-induced lung tumors. Mol. Cancer 2013, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dheeman, D.S.; Packianathan, C.; Pillai, J.K.; Rosen, B.P. Pathway of human AS3MT arsenic methylation. Chem. Res. Toxicol. 2014, 27, 1979–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roggenbeck, B.A.; Leslie, E.M.; Walk, S.T.; Schmidt, E.E. Redox metabolism of ingested arsenic: Integrated activities of microbiome and host on toxicological outcomes. Curr. Opin. Toxicol. 2019, 13, 90–98. [Google Scholar] [CrossRef]
- Flora, S.J. Toxic metals: Health effects, and therapeutic measures. J. Biomed. Ther. Sci. 2014, 1, 48–64. [Google Scholar]
- Styblo, M.; Serves, S.V.; Cullen, W.R.; Thomas, D.J. Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem. Res. Toxicol. 1997, 10, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Mehta, A.; Flora, S.J. Reversal of arsenic-induced hepatic apoptosis with combined administration of DMSA and its analogues in guinea pigs: Role of glutathione and linked enzymes. Chem. Res. Toxicol. 2008, 21, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, D.; Rahman, M.M.; Ahamed, S.; Dutta, R.N.; Pati, S.; Mukherjee, S.C. Arsenic groundwater contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere 2016, 152, 520–529. [Google Scholar] [CrossRef]
- Hu, Y.; Xiao, T.; Zhang, A. Associations between and risks of trace elements related to skin and liver damage induced by arsenic from coal burning. Ecotoxicol. Environ. Saf. 2021, 208, 111719. [Google Scholar] [CrossRef]
- Chervona, Y.; Arita, A.; Costa, M. Carcinogenic metals and the epigenome: Understanding the effect of nickel, arsenic, and chromium. Metallomics 2012, 4, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, M.; Barrios, J.A.; Vélez, D.; Devesa, V. In vitro study of transporters involved in intestinal absorption of inorganic arsenic. Chem. Res. Toxicol. 2012, 25, 446–453. [Google Scholar] [CrossRef]
- Frediani, J.K.; Naioti, E.A.; Vos, M.B.; Figueroa, J.; Marsit, C.J.; Welsh, J.A. Arsenic exposure and risk of nonalcoholic fatty liver disease (NAFLD) among US adolescents and adults: An association modified by race/ethnicity, NHANES 2005–2014. Environ. Health 2018, 17, 6. [Google Scholar] [CrossRef] [Green Version]
- Bunderson, M.; Coffin, J.D.; Beall, H.D. Arsenic induces peroxynitrite generation and cyclooxygenase-2 protein expression in aortic endothelial cells: Possible role in atherosclerosis. Toxicol. Appl. Pharmacol. 2002, 184, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Factor-Litvak, P.; Howe, G.R.; Graziano, J.H.; Brandt-Rauf, P.; Parvez, F.; Van Geen, A.; Ahsan, H. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: A population-based, cross-sectional study. Am. J. Epidemiol. 2007, 165, 541–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustaffa, E.; Stoccoro, A.; Bianchi, F.; Migliore, L. Genotoxic and epigenetic mechanisms in arsenic carcinogenicity. Arch. Toxicol. 2014, 88, 1043–1067. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wang, N.; Qin, W. Gut microbiota and hepatocellular carcinoma. Gastrointest. Tumors 2015, 2, 33–40. [Google Scholar] [CrossRef]
- Lu, K.; Abo, R.P.; Schlieper, K.A.; Graffam, M.E.; Levine, S.; Wishnok, J.S.; Swenberg, J.A.; Tannenbaum, S.R.; Fox, J.G. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: An integrated metagenomics and metabolomics analysis. Environ. Health Perspect. 2014, 122, 284–291. [Google Scholar] [CrossRef]
- Sinha, D.; Roy, M. Antagonistic role of tea against sodium arsenite-induced oxidative DNA damage and inhibition of DNA repair in Swiss albino mice. J. Environ. Pathol. Toxicol. Oncol. 2011, 30, 311–312. [Google Scholar] [CrossRef]
- D’Ippoliti, D.; Santelli, E.; De Sario, M.; Scortichini, M.; Davoli, M.; Michelozzi, P. Arsenic in drinking water and mortality for cancer and chronic diseases in Central Italy, 1990-2010. PLoS ONE 2015, 10, e0138182. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; de Silva, P.M.C. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Dangleben, N.L.; Skibola, C.F.; Smith, M.T. Arsenic immunotoxicity: A review. Environ. Health 2013, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.Y.A.; Briedé, J.J.; van Herwijnen, M.; Krauskopf, J.; Jennen, D.G.; Malik, R.N.; Kleinjans, J.C. Integrating SNPs-based genetic risk factor with blood epigenomic response of differentially arsenic-exposed rural subjects reveals disease-associated signaling pathways. Environ. Pollut. 2022, 292, 118279. [Google Scholar] [CrossRef]
- Martinez, V.D.; Vucic, E.A.; Adonis, M.; Gil, L.; Lam, W.L. Arsenic biotransformation as a cancer promoting factor by inducing DNA damage and disruption of repair mechanisms. Mol. Biol. Int. 2011, 2011, 718974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, M.; Prakash, C.; Sehwag, S.; Kumar, V. Protective effect of hydroxytyrosol in arsenic-induced mitochondrial dysfunction in rat brain. J. Biochem. Mol. Toxicol. 2017, 31, e21906. [Google Scholar] [CrossRef] [PubMed]
- Akbal, A.; Yılmaz, H.; Tutkun, E. Arsenic exposure associated with decreased bone mineralization in male. Aging Male 2014, 17, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Dwivedi, S.; Yadav, S.S.; Yadav, R.S.; Khattri, S. Anti-diabetic effect of Emblica officinalis (Amla) against arsenic induced metabolic disorder in mice. Indian J. Clin. Biochem. 2020, 35, 179–187. [Google Scholar] [CrossRef]
- Carlson, P.; van Beneden, R.J. Arsenic exposure alters expression of cell cycle and lipid metabolism genes in the liver of adult zebrafish (Danio rerio). Aquat. Toxicol. 2014, 153, 66–72. [Google Scholar] [CrossRef]
- Afolabi, O.K.; Wusu, A.D.; Ogunrinola, O.O.; Abam, E.O.; Babayemi, D.O.; Dosumu, O.; Onunkwor, O.; Balogun, E.; Odukoya, O.O.; Ademuyiwa, O. Arsenic-induced dyslipidemia in male albino rats: Comparison between trivalent and pentavalent inorganic arsenic in drinking water. BMC Pharmacol. Toxicol. 2015, 16, 15. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.; Bastos, D.; Sertorio, M.; Santos, F.; Ervilha, L.; de Oliveira, L.; Machado-Neves, M. Combined effects of arsenic exposure and diabetes on male reproductive functions. Andrology 2019, 7, 730–740. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Sun, X.; Guo, Q.; Xia, W.; Wu, Y.; Li, J.; Xu, S.; Li, Y. Arsenic exposure and metabolism in relation to blood pressure changes in pregnant women. Ecotoxicol. Environ. Saf. 2021, 222, 112527. [Google Scholar] [CrossRef]
- Faita, F.; Cori, L.; Bianchi, F.; Andreassi, M.G. Arsenic-induced genotoxicity and genetic susceptibility to arsenic-related pathologies. Int. J. Environ. Res. Public Health 2013, 10, 1527–1546. [Google Scholar] [CrossRef] [Green Version]
- Tokar, E.J.; Benbrahim-Tallaa, L.; Ward, J.M.; Lunn, R.; Sams, R.L.; Waalkes, M.P. Cancer in experimental animals exposed to arsenic and arsenic compounds. Crit. Rev. Toxicol. 2010, 40, 912–927. [Google Scholar] [CrossRef]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 100052. [Google Scholar] [CrossRef]
- Xu, P.; Liu, A.; Li, F.; Tinkov, A.A.; Liu, L.; Zhou, J.-C. Associations between metabolic syndrome and four heavy metals: A systematic review and meta-analysis. Environ. Pollut. 2021, 273, 116480. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, G.-X.; Williams, P.N.; Nunes, L.; Zhu, Y.-G. Inorganic arsenic in Chinese food and its cancer risk. Environ. Int. 2011, 37, 1219–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, R.; Ghosh, P.; Banerjee, N.; Das, J.; Sau, T.; Banerjee, A.; Roy, S.; Ganguly, S.; Chatterjee, M.; Mukherjee, A. Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Hum. Exp. Toxicol. 2008, 27, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Islam, L.N.; Nurun Nabi, A.; Rahman, M.M.; Zahid, M.S.H. Association of respiratory complications and elevated serum immunoglobulins with drinking water arsenic toxicity in human. J. Environ. Sci. Health Part A 2007, 42, 1807–1814. [Google Scholar] [CrossRef]
- Robles-Osorio, M.L.; Sabath-Silva, E.; Sabath, E. Arsenic-mediated nephrotoxicity. Ren. Fail. 2015, 37, 542–547. [Google Scholar] [CrossRef]
- Li, Z.; Piao, F.; Liu, S.; Wang, Y.; Qu, S. Subchronic exposure to arsenic trioxide-induced oxidative DNA damage in kidney tissue of mice. Exp. Toxicol. Pathol. 2010, 62, 543–547. [Google Scholar] [CrossRef]
- Hossain, E.; Ota, A.; Takahashi, M.; Karnan, S.; Damdindorj, L.; Konishi, Y.; Konishi, H.; Hosokawa, Y. Arsenic upregulates the expression of angiotensin II Type I receptor in mouse aortic endothelial cells. Toxicol. Lett. 2013, 220, 70–75. [Google Scholar] [CrossRef]
- Li, L.; Bi, Z.; Wadgaonkar, P.; Lu, Y.; Zhang, Q.; Fu, Y.; Thakur, C.; Wang, L.; Chen, F. Metabolic and Epigenetic Reprogramming in the Arsenic-Induced Cancer Stem Cells. In Proceedings of the Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2019; pp. 10–18. [Google Scholar]
- Muzaffar, S.; Khan, J.; Srivastava, R.; Gorbatyuk, M.S.; Athar, M. Mechanistic understanding of the toxic effects of arsenic and warfare arsenicals on human health and environment. Cell Biol. Toxicol. 2022, 1–26. [Google Scholar] [CrossRef]
- Xu, L.; Polya, D.A.; Li, Q.; Mondal, D. Association of low-level inorganic arsenic exposure from rice with age-standardized mortality risk of cardiovascular disease (CVD) in England and Wales. Sci. Total Environ. 2020, 743, 140534. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Jarouliya, U.; Prasad, G.; Flora, S.; Bisen, P.S. Arsenic-induced abnormalities in glucose metabolism: Biochemical basis and potential therapeutic and nutritional interventions. World J. Transl. Med. 2014, 3, 96–111. [Google Scholar] [CrossRef]
- Sung, T.-C.; Huang, J.-W.; Guo, H.-R. Association between arsenic exposure and diabetes: A meta-analysis. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-González, M.; Osorio-Yáñez, C.; Gaspar-Ramírez, O.; Pavković, M.; Ochoa-Martinez, A.; López-Ventura, D.; Medeiros, M.; Barbier, O.; Pérez-Maldonado, I.; Sabbisetti, V. Environmental exposure to arsenic and chromium in children is associated with kidney injury molecule-1. Environ. Res. 2016, 150, 653–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, L.-I.; Hsieh, F.-I.; Wang, Y.-H.; Lai, T.-S.; Wu, M.-M.; Chen, C.-J.; Chiou, H.-Y.; Hsu, K.-H. Arsenic exposure from drinking water and the incidence of CKD in low to moderate exposed areas of Taiwan: A 14-year prospective study. Am. J. Kidney Dis. 2017, 70, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, L.; Sun, Q.; Yang, Q.; Xue, J.; Shi, M.; Tang, H.; Zhang, J.; Liu, Q. MicroRNA-191 blocking the translocation of GLUT4 is involved in arsenite-induced hepatic insulin resistance through inhibiting the IRS1/AKT pathway. Ecotoxicol. Environ. Saf. 2021, 215, 112130. [Google Scholar] [CrossRef] [PubMed]
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Gandolfi, A.J.; Ornelas-Aguirre, J.M.; Torres-Sánchez, L.; Cebrian, M.E. Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol. Appl. Pharmacol. 2014, 280, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.K.; Keltie, E.; Sweeney, E.; Weerasinghe, S.; MacPherson, K.; Kim, J.S. Toenail speciation biomarkers in arsenic-related disease: A feasibility study for investigating the association between arsenic exposure and chronic disease. Ecotoxicol. Environ. Saf. 2022, 232, 113269. [Google Scholar] [CrossRef]
- Díaz-Villaseñor, A.; Burns, A.L.; Hiriart, M.; Cebrián, M.E.; Ostrosky-Wegman, P. Arsenic-induced alteration in the expression of genes related to type 2 diabetes mellitus. Toxicol. Appl. Pharmacol. 2007, 225, 123–133. [Google Scholar] [CrossRef]
- Sarker, M.; Tony, S.R.; Siddique, A.E.; Karim, M.; Haque, N.; Islam, Z.; Islam, M.; Khatun, M.; Islam, J.; Hossain, S. Arsenic secondary methylation capacity is inversely associated with arsenic exposure-related muscle mass reduction. Int. J. Environ. Res. Public Health 2021, 18, 9730. [Google Scholar] [CrossRef]
- Ambrosio, F.; Brown, E.; Stolz, D.; Ferrari, R.; Goodpaster, B.; Deasy, B.; Distefano, G.; Roperti, A.; Cheikhi, A.; Garciafigueroa, Y. Arsenic induces sustained impairment of skeletal muscle and muscle progenitor cell ultrastructure and bioenergetics. Free. Radic. Biol. Med. 2014, 74, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ferrari, R.; Beezhold, K.; Stearns-Reider, K.; D’Amore, A.; Haschak, M.; Stolz, D.; Robbins, P.D.; Barchowsky, A.; Ambrosio, F. Arsenic promotes NF-κB-mediated fibroblast dysfunction and matrix remodeling to impair muscle stem cell function. Stem Cells 2016, 34, 732–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calatayud, M.; Devesa, V.; Vélez, D. Differential toxicity and gene expression in Caco-2 cells exposed to arsenic species. Toxicol. Lett. 2013, 218, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Gimeno-Alcañiz, J.V.; Devesa, V.; Vélez, D. Proinflammatory effect of trivalent arsenical species in a co-culture of Caco-2 cells and peripheral blood mononuclear cells. Arch. Toxicol. 2015, 89, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Schreibelt, G.; Kooij, G.; Reijerkerk, A.; van Doorn, R.; Gringhuis, S.I.; van der Pol, S.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Piontek, J. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007, 21, 3666–3676. [Google Scholar] [CrossRef] [Green Version]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta Biomembr. 2009, 1788, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Ahmad, M.F.; Patel, M.; Adnan, M.; Sulieman, A.M.E. Probiotic Fermented Foods and Health Promotion. In African Fermented Food Products-New Trends; Springer: Berlin/Heildeberg, Germany, 2022; pp. 59–88. [Google Scholar]
- Dong, X.; Shulzhenko, N.; Lemaitre, J.; Greer, R.L.; Peremyslova, K.; Quamruzzaman, Q.; Rahman, M.; Hasan, O.S.I.; Joya, S.A.; Golam, M. Arsenic exposure and intestinal microbiota in children from Sirajdikhan, Bangladesh. PLoS ONE 2017, 12, e0188487. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Yang, L.; Islam, M.T.; Jasmine, F.; Kibriya, M.G.; Nahar, J.; Barmon, B.; Parvez, F.; Sarwar, G.; Ahmed, A. The role of gut microbiome and its interaction with arsenic exposure in carotid intima-media thickness in a Bangladesh population. Environ. Int. 2019, 123, 104–113. [Google Scholar] [CrossRef]
- Yadav, R.S.; Shukla, R.K.; Sankhwar, M.L.; Patel, D.K.; Ansari, R.W.; Pant, A.B.; Islam, F.; Khanna, V.K. Neuroprotective effect of curcumin in arsenic-induced neurotoxicity in rats. Neurotoxicology 2010, 31, 533–539. [Google Scholar] [CrossRef]
- Ramos-Chávez, L.A.; Rendón-López, C.R.; Zepeda, A.; Silva-Adaya, D.; Del Razo, L.M.; Gonsebatt, M.E. Neurological effects of inorganic arsenic exposure: Altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front. Cell. Neurosci. 2015, 2015, 21. [Google Scholar] [CrossRef] [Green Version]
- Salmeri, N.; Villanacci, R.; Ottolina, J.; Bartiromo, L.; Cavoretto, P.; Dolci, C.; Lembo, R.; Schimberni, M.; Valsecchi, L.; Viganò, P. Maternal arsenic exposure and gestational diabetes: A systematic review and meta-analysis. Nutrients 2020, 12, 3094. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol. Appl. Pharmacol. 2004, 197, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, N.; Jonasson, M.E.; Hunt, K.L.; Xiang, B.; Cooper, S.; Wheeler, M.B.; Dolinsky, V.W.; Doucette, C.A. Uncoupling protein 2 regulates daily rhythms of insulin secretion capacity in MIN6 cells and isolated islets from male mice. Mol. Metab. 2017, 6, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.G.; Queen, Z.J.; Cherry, N. Histopathology of cervical cancer and arsenic concentration in well water: An ecological analysis. Int. J. Environ. Res. Public Health 2017, 14, 1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kile, M.L.; Faraj, J.M.; Ronnenberg, A.G.; Quamruzzaman, Q.; Rahman, M.; Mostofa, G.; Afroz, S.; Christiani, D.C. A cross sectional study of anemia and iron deficiency as risk factors for arsenic-induced skin lesions in Bangladeshi women. BMC Public Health 2016, 16, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunus, F.M.; Rahman, M.J.; Alam, M.Z.; Hore, S.K.; Rahman, M. Relationship between arsenic skin lesions and the age of natural menopause. BMC Public Health 2014, 14, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surdu, S.; Bloom, M.S.; Neamtiu, I.A.; Pop, C.; Anastasiu, D.; Fitzgerald, E.F.; Gurzau, E.S. Consumption of arsenic-contaminated drinking water and anemia among pregnant and non-pregnant women in northwestern Romania. Environ. Res. 2015, 140, 657–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breton, C.V.; Houseman, E.A.; Kile, M.L.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Christiani, D.C. Gender-specific protective effect of hemoglobin on arsenic-induced skin lesions. Cancer Epidemiol. Prev. Biomark. 2006, 15, 902–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Khoda, S.M.-e.; Rekha, R.S.; Gardner, R.M.; Ameer, S.S.; Moore, S.; Ekström, E.-C.; Vahter, M.; Raqib, R. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ. Health Perspect. 2011, 119, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Davey, J.C.; Bodwell, J.E.; Gosse, J.A.; Hamilton, J.W. Arsenic as an endocrine disruptor: Effects of arsenic on estrogen receptor–mediated gene expression in vivo and in cell culture. Toxicol. Sci. 2007, 98, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Chatterji, U. Arsenic abrogates the estrogen-signaling pathway in the rat uterus. Reprod. Biol. Endocrinol. 2010, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquino, N.B.; Sevigny, M.B.; Sabangan, J.; Louie, M.C. The role of cadmium and nickel in estrogen receptor signaling and breast cancer: Metalloestrogens or not? J. Environ. Sci. Health Part C 2012, 30, 189–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciniak, W.; Derkacz, R.; Muszyńska, M.; Baszuk, P.; Gronwald, J.; Huzarski, T.; Cybulski, C.; Jakubowska, A.; Falco, M.; Dębniak, T. Blood arsenic levels and the risk of familial breast cancer in Poland. Int. J. Cancer 2020, 146, 2721–2727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Liu, B.; Zhang, L.; Wu, M.; Wang, L.; Cao, Z.; Zhang, B.; Li, Y.; Wang, Y.; Xu, S. Association of prenatal exposure to arsenic with newborn telomere length: Results from a birth cohort study. Environ. Res. 2019, 175, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Pilsner, J.R.; Hall, M.N.; Liu, X.; Ilievski, V.; Slavkovich, V.; Levy, D.; Factor-Litvak, P.; Yunus, M.; Rahman, M.; Graziano, J.H. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLoS ONE 2012, 7, e37147. [Google Scholar] [CrossRef] [Green Version]
- Milton, A.H.; Hussain, S.; Akter, S.; Rahman, M.; Mouly, T.A.; Mitchell, K. A review of the effects of chronic arsenic exposure on adverse pregnancy outcomes. Int. J. Environ. Res. Public Health 2017, 14, 556. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Pan, A.; Hu, F.B.; Ma, X. Folic acid supplementation, birth defects, and adverse pregnancy outcomes in Chinese women: A population-based mega-cohort study. Lancet 2016, 388, S91. [Google Scholar] [CrossRef]
- Mazumdar, M.; Hasan, M.O.S.I.; Hamid, R.; Valeri, L.; Paul, L.; Selhub, J.; Rodrigues, E.G.; Silva, F.; Mia, S.; Mostofa, M.G. Arsenic is associated with reduced effect of folic acid in myelomeningocele prevention: A case control study in Bangladesh. Environ. Health 2015, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, R.P.; Fujiwara, T.; Umezaki, M.; Watanabe, C. Association of cord blood levels of lead, arsenic, and zinc with neurodevelopmental indicators in newborns: A birth cohort study in Chitwan Valley, Nepal. Environ. Res. 2013, 121, 45–51. [Google Scholar] [CrossRef]
- Demir, N.; Başaranoğlu, M.; Huyut, Z.; Değer, İ.; Karaman, K.; Şekeroğlu, M.R.; Tuncer, O. The relationship between mother and infant plasma trace element and heavy metal levels and the risk of neural tube defect in infants. J. Matern. Fetal Neonatal Med. 2019, 32, 1433–1440. [Google Scholar] [CrossRef]
- Tolins, M.; Ruchirawat, M.; Landrigan, P. The developmental neurotoxicity of arsenic: Cognitive and behavioral consequences of early life exposure. Ann. Glob. Health 2014, 80, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, K.C.; Li, Z.; Farzan, S.; Koestler, D.; Robbins, D.; Fei, D.L.; Malipatlolla, M.; Maecker, H.; Enelow, R.; Korrick, S. In utero arsenic exposure and fetal immune repertoire in a US pregnancy cohort. Clin. Immunol. 2014, 155, 188–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzan, S.F.; Korrick, S.; Li, Z.; Enelow, R.; Gandolfi, A.J.; Madan, J.; Nadeau, K.; Karagas, M.R. In utero arsenic exposure and infant infection in a United States cohort: A prospective study. Environ. Res. 2013, 126, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.-B.; Hsi, H.-C.; Fan, C.-H.; Chien, L.-C. Fetal exposure to environmental neurotoxins in Taiwan. PLoS ONE 2014, 9, e109984. [Google Scholar] [CrossRef] [Green Version]
- Vahter, M. Effects of arsenic on maternal and fetal health. Annu. Rev. Nutr. 2009, 29, 381–399. [Google Scholar] [CrossRef]
- Bae, S.; Kamynina, E.; Farinola, A.F.; Caudill, M.A.; Stover, P.J.; Cassano, P.A.; Berry, R.; Peña-Rosas, J.P. Provision of folic acid for reducing arsenic toxicity in arsenic-exposed children and adults. Cochrane Database Syst. Rev. 2017, CD012649. [Google Scholar] [CrossRef]
- Arroyo, H.A.; Fernández, M.C. Tóxicos ambientales y su efecto sobre el neurodesarrollo. Medicina 2013, 73. [Google Scholar]
- Yorifuji, T.; Kato, T.; Ohta, H.; Bellinger, D.C.; Matsuoka, K.; Grandjean, P. Neurological and neuropsychological functions in adults with a history of developmental arsenic poisoning from contaminated milk powder. Neurotoxicol. Teratol. 2016, 53, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Normandin, L.; Ayotte, P.; Levallois, P.; Ibanez, Y.; Courteau, M.; Kennedy, G.; Chen, L.; Le, X.C.; Bouchard, M. Biomarkers of arsenic exposure and effects in a Canadian rural population exposed through groundwater consumption. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the US population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Pullella, K.; Kotsopoulos, J. Arsenic exposure and breast cancer risk: A re-evaluation of the literature. Nutrients 2020, 12, 3305. [Google Scholar] [CrossRef] [PubMed]
- Arabia, S. Grain and Feed Annual 2016, United States Department of Agriculture. Foreign Agriculture Service. Available online: www.gain.fas.usda.gov (accessed on 3 March 2022).
- Menon, M.; Sarkar, B.; Hufton, J.; Reynolds, C.; Reina, S.V.; Young, S. Do arsenic levels in rice pose a health risk to the UK population? Ecotoxicol. Environ. Saf. 2020, 197, 110601. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.Y.; Deacon, C.; Villada, A.; Cambell, R.C.; Sun, G.; Zhu, Y.-G.; Feldmann, J. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci. Technol. 2009, 43, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Rahman, M.M.; Duan, L.; Islam, M.; Kuchel, T.; Naidu, R. Variation in arsenic bioavailability in rice genotypes using swine model: An animal study. Sci. Total Environ. 2017, 599, 324–331. [Google Scholar] [CrossRef]
- Mwale, T.; Rahman, M.M.; Mondal, D. Risk and benefit of different cooking methods on essential elements and arsenic in rice. Int. J. Environ. Res. Public Health 2018, 15, 1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, M.; Dong, W.; Chen, X.; Hufton, J.; Rhodes, E.J. Improved rice cooking approach to maximise arsenic removal while preserving nutrient elements. Sci. Total Environ. 2021, 755, 143341. [Google Scholar] [CrossRef]
- Bundschuh, J.; Nath, B.; Bhattacharya, P.; Liu, C.-W.; Armienta, M.A.; López, M.V.M.; Lopez, D.L.; Jean, J.-S.; Cornejo, L.; Macedo, L.F.L. Arsenic in the human food chain: The Latin American perspective. Sci. Total Environ. 2012, 429, 92–106. [Google Scholar] [CrossRef]
- Sun, G.; Williams, P.; Carey, A.; Zhu, Y.; Deacon, C.; Raab, A.; Feldmann, J.; Meharg, A.; Islam, R. Elevated arsenic in rice bran products used as food supplements and in food aid programs. Environ. Sci. Technol. 2008, 42, 7542–7546. [Google Scholar] [CrossRef]
- Raab, A.; Baskaran, C.; Feldmann, J.; Meharg, A.A. Cooking rice in a high water to rice ratio reduces inorganic arsenic content. J. Environ. Monit. 2009, 11, 41–44. [Google Scholar] [CrossRef]
- Carey, M.; Jiujin, X.; Gomes Farias, J.; Meharg, A.A. Rethinking rice preparation for highly efficient removal of inorganic arsenic using percolating cooking water. PLoS ONE 2015, 10, e0131608. [Google Scholar] [CrossRef]
- Gray, P.J.; Conklin, S.D.; Todorov, T.I.; Kasko, S.M. Cooking rice in excess water reduces both arsenic and enriched vitamins in the cooked grain. Food Addit. Contam. Part A 2016, 33, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.R.; Das, A.; Joardar, M.; De, A.; Mridha, D.; Das, R.; Rahman, M.M.; Roychowdhury, T. Flow of arsenic between rice grain and water: Its interaction, accumulation and distribution in different fractions of cooked rice. Sci. Total Environ. 2020, 731, 138937. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, T.; Bhattacharjee, P.; Paul, S. Recent advances in arsenic research: Significance of differential susceptibility and sustainable strategies for mitigation. Front. Public Health 2020, 8, 464. [Google Scholar] [CrossRef]
- Moxness Reksten, A.; Rahman, Z.; Kjellevold, M.; Garrido Gamarro, E.; Thilsted, S.H.; Pincus, L.M.; Aakre, I.; Ryder, J.; Ariyawansa, S.; Nordhagen, A. Metal Contents in Fish from the Bay of Bengal and Potential Consumer Exposure—The EAF-Nansen Programme. Foods 2021, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Nachman, K.E.; Ginsberg, G.L.; Miller, M.D.; Murray, C.J.; Nigra, A.E.; Pendergrast, C.B. Mitigating dietary arsenic exposure: Current status in the United States and recommendations for an improved path forward. Sci. Total Environ. 2017, 581, 221–236. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).