Long-Term Effect of Gluten-Free Diets on Nutritional Status, Body Composition, and Associated Factors in Adult Saudi Females with Celiac Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Analysis of Dietary Intake
2.3. Anthropometric Measurement
2.4. Blood Parameters
2.5. Ethical Approval
2.6. Data Analysis
3. Results
3.1. Nutrient Intake and Blood Parameters
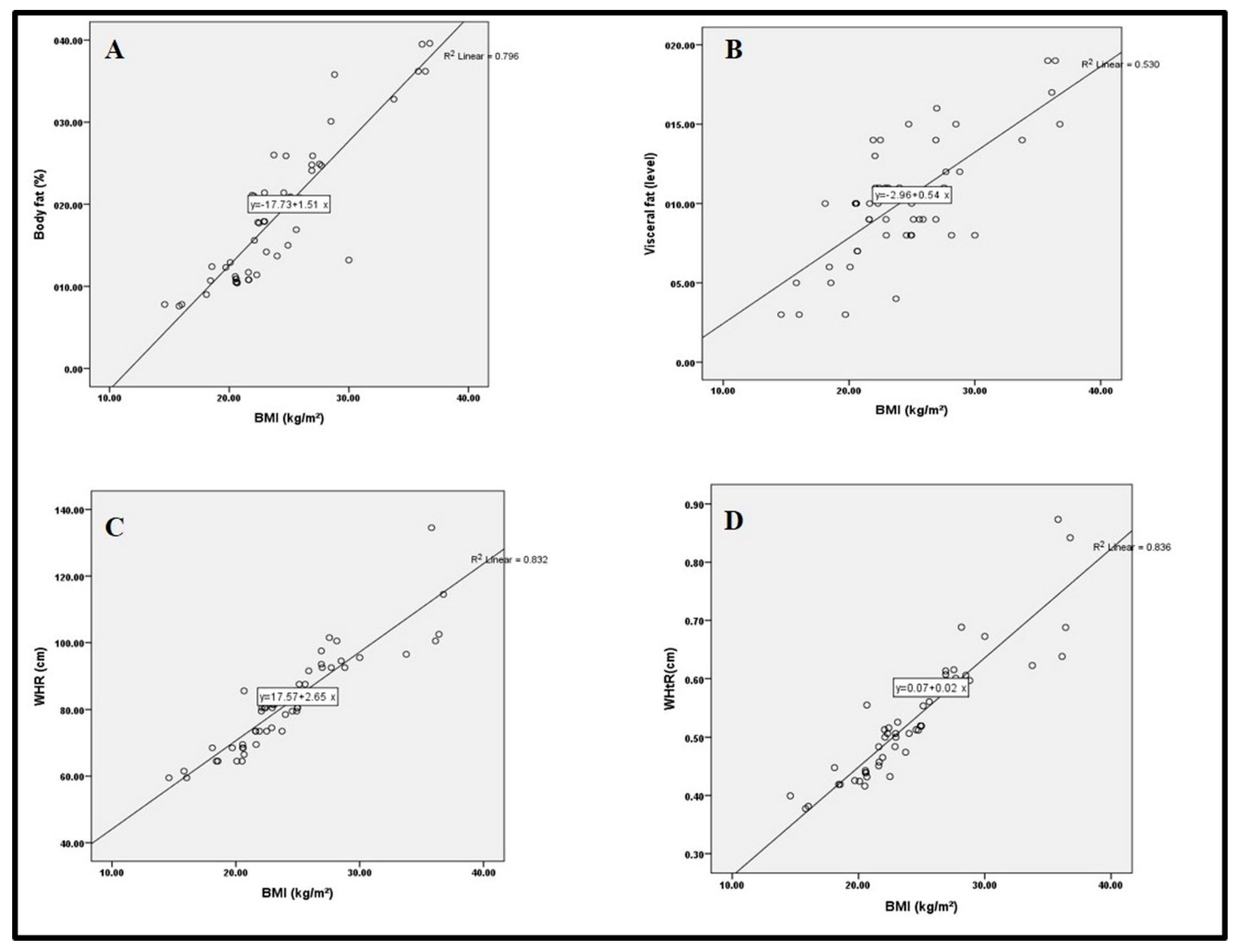
3.2. Anthropometric Measurements
3.3. Factors Associated with Nutritional Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, J.; Kumar, M.; Pandey, R.; Chauhan, N.S. Physiopathology and management of gluten-induced celiac disease. J. Food Sci. 2017, 82, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Metwally, A.; Toivola, P.; AlAhmary, K.; Bahkali, S.; AlKhathaami, A.; AlSaqabi, M.K.; Alosaimi, S.M. The epidemiology of CDin the general population and high-risk groups in Arab countries: A systematic review. BioMed Res. Int. 2020, 2020, 6865917. [Google Scholar] [CrossRef]
- García-Manzanares, A.; Lucendo, A.J. Nutritional and dietary aspects of celiac disease. Nutr. Clin. Pract. 2011, 26, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Hopman, E.G.; le Cessie, S.; von Blomberg, B.M.E.; Mearin, M.L. Nutritional management of the gluten-free diet in young people with CD in The Netherlands. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.; Geisel, T.; Maresch, C.; Krieger, K.; Stein, J. Inadequate nutrient intake in patients with celiac disease: Results from a German dietary survey. Digestion 2013, 87, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Della Valle, N.; Rosania, R.; Facciorusso, A.; Trotta, A.; Cantatore, F.P.; Francavilla, R. A comparison of the nutritional status between adult celiac patients on a long-term, strictly gluten-free diet and healthy subjects. Eur. J. Clin. Nutr. 2016, 70, 23–27. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Melini, V.; Melini, F. Gluten-free diet: Gaps and needs for a healthier diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [Green Version]
- Polzonetti, V.; Pucciarelli, S.; Vincenzetti, S.; Polidori, P. Dietary intake of vitamin D from dairy products reduces the risk of osteoporosis. Nutrients 2020, 12, 1743. [Google Scholar] [CrossRef]
- Lucendo, A.J.; García-Manzanares, A. Bone mineral density in adult coeliac disease: An updated review. Rev. Esp. Enferm. Dig. 2013, 105, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdimarsson, T.; Toss, G.; Löfman, O.; Ström, M. Three years’ follow-up of bone density in adult coeliac disease: Significance of secondary hyperparathyroidism. Scand. J. Gastroenterol. 2000, 35, 274–280. [Google Scholar] [CrossRef]
- Daly, R.M.; Ahlborg, H.G.; Ringsberg, K.; Gardsell, P.; Sernbo, I.; Karlsson, M.K. Association between changes in habitual physical activity and changes in bone density, muscle strength, and functional performance in elderly men and women. J. Am. Geriatr. Soc. 2008, 56, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Bardella, M.T.; Fredella, C.; Prampolini, L.; Molteni, N.; Giunta, A.M.; Bianchi, P.A. Body composition and dietary intakes in adult CD patients consuming a strict gluten-free diet. Am. J. Clin. Nutr. 2000, 72, 937–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churruca, I.; Miranda, J.; Lasa, A.; Bustamante, M.; Larretxi, I.; Simon, E. Analysis of body composition and food habits of Spanish celiac women. Nutrients 2015, 7, 5515–5531. [Google Scholar] [CrossRef] [Green Version]
- Tovoli, F.; Negrini, G.; Farì, R.; Guidetti, E.; Faggiano, C.; Napoli, L.; Granito, A. Increased risk of nonalcoholic fatty liver disease in patients with coeliac disease on a gluten-free diet: Beyond traditional metabolic factors. Aliment. Pharmacol. Ther. 2018, 48, 538–546. [Google Scholar] [CrossRef]
- Volta, U.; De Giorgio, R.; Granito, A.; Stanghellini, V.; Barbara, G.; Avoni, P.; Bianchi, F.B. Anti-ganglioside antibodies in coeliac disease with neurological disorders. Dig. Liver Dis. 2006, 38, 183–187. [Google Scholar] [CrossRef]
- Zauli, D.; Grassi, A.; Granito, A.; Foderaro, S.; De Franceschi, L.; Ballardini, G.; Volta, U. Prevalence of silent coeliac disease in atopics. Dig. Liver Dis. 2000, 32, 775–779. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity; World Health Organization: Geneva, Switzerland, 1997; p. 276. [Google Scholar]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist–Hip Ratio. Eur. J. Clin. Nutr. 2010, 64, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardio-metabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.E.; Rosell, C.M. Chemical composition and starch digestibility of different gluten-free breads. Plant Foods Hum. Nutr. 2011, 66, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Larretxi, I.; Simon, E.; Benjumea, L.; Miranda, J.; Bustamante, M.A.; Lasa, A.; Eizaguirre, F.J.; Churruca, I. Gluten-free-rendered products contribute to imbalanced diets in children and adolescents with celiac disease. Eur. J. Nutr. 2019, 58, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Safer, L.; Fadoua, H.; Zrour, S.; Bejia, I.; Touzi, M.; Najjar, M.F.; Saffar, H.; Bergaoui, N. Prevalence of bone loss in adult CD and associated factors: A control case study. Tunis Med. 2012, 90, 129–135. [Google Scholar] [PubMed]
- Demirin, H.; Ozhan, H.; Ucgun, T.; Celer, A.; Bulur, S.; Cil, H.; Yildirim, H.A. Normal range of mean platelet volume in healthy subjects: Insight from a large epidemiologic study. Thromb. Res. 2011, 128, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Ulkumen, B.A.; Pala, H.G.; Calik, E.; Koltan, S.O. Platelet distribution width (PDW): A putative marker for threatened preterm labour. Pak. J. Med. Sci. 2014, 30, 745. [Google Scholar]
- Choi, J.J.; McCarthy, M.W. Novel applications for serum procalcitonin testing in clinical practice. Expert Rev. Mol. Diagn. 2018, 18, 27–34. [Google Scholar] [CrossRef]
- Tavakkoli, A.; DiGiacomo, D.; Green, P.H.; Lebwohl, B. Vitamin D status and concomitant autoimmunity in celiac disease. J. Clin. Gastroenterol. 2013, 47, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Brambilla, P.; Picca, M.; Dilillo, D.; Meneghin, F.; Cravidi, C.; Tischer, M.C.; Vivaldo, T.; Bedogni, G.; Zuccotti, G.V. Changes of body mass index in celiac children on a gluten-free diet. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 177–182. [Google Scholar] [CrossRef]
- Tucker, E.; Rostami, K.; Prabhakaran, S.; Dulaimi, D.A. Patients with coeliac disease are increasingly overweight or obese on presentation. J. Gastrointest. Liver Dis. 2012, 21, 11–15. [Google Scholar]
- Valletta, E.; Fornaro, M.; Cipolli, M.; Conte, S.; Bissolo, F.; Danchielli, C. CD and obesity: Need for nutritional follow-up after diagnosis. Eur. J. Clin. Nutr. 2010, 64, 1371–1372. [Google Scholar] [CrossRef] [Green Version]
- Balamtekin, N.; Aksoy, Ç.; Baysoy, G.; Uslu, N.; Demir, H.; Saltık-Temizel, I.N.; Özen, H.; Gürakan, F.; Yüce, A. Is compliance with gluten-free diet sufficient? Diet composition of celiac patients. Turk. J. Pediatr. 2015, 57, 374–379. [Google Scholar]
- Addolorato, G.; Leggio, L.; D’Angelo, C.; Mirijello, A.; Ferrulli, A.; Cardone, S.; Vonghia, L.; Abenavoli, L.; Leso, V.; Nesci, A.; et al. Affective and psychiatric disorders in celiac disease. Dig. Dis. 2008, 26, 140–148. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Reutfors, J.; Osby, U.; Ekbom, A.; Montgomery, S.M. Coeliac disease and risk of mood disorders—A general population-based cohort study. J. Affect. Disord. 2007, 99, 117–126. [Google Scholar] [CrossRef]
- Addolorato, G.; Mirijello, A.; D’Angelo, C.; Leggio, L.; Ferrulli, A.; Vonghia, L.; Cardone, S.; Leso, V.; Miceli, A.; Gasbarrini, G. Social phobia in coeliac disease. Scand. J. Gastroenterol. 2008, 43, 410–415. [Google Scholar] [CrossRef]
- Roos, S.; Kärner, A.; Hallert, C. Psychological well-being of adult coeliac patients treated for 10 years. Dig. Liver Dis. 2006, 38, 177–180. [Google Scholar] [CrossRef]
- Häuser, W.; Stallmach, A.; Caspary, W.F.; Stein, J. Predictors of reduced health-related quality of life in adults with coeliac disease. Aliment. Pharmacol. Ther. 2007, 25, 569–578. [Google Scholar] [CrossRef]
- Joelson, A.M.; Geller, M.G.; Zylberberg, H.M.; Green, P.H.; Lebwohl, B. The effect of depressive symptoms on the association between gluten-free diet adherence and symptoms in celiac disease: Analysis of a patient powered research network. Nutrients 2018, 10, 538. [Google Scholar] [CrossRef] [Green Version]
- Arigo, D.; Anskis, A.M.; Smyth, J.M. Psychiatric comorbidities in women with celiac disease. Chronic Illn. 2012, 8, 45–55. [Google Scholar] [CrossRef]
- Zysk, W.; Głąbska, D.; Guzek, D. Social and emotional fears and worries influencing the quality of life of female celiac disease patients following a gluten-free diet. Nutrients 2018, 10, 1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| No. | Blood Parameter | Classification |
|---|---|---|
| 1 | White blood cells (WBC) | low < 4, normal 4–11, high > 11 |
| 2 | Red blood cell (RBC) | low < 3.80, normal 3.80–6.50, high > 6.50 |
| 3 | Hemoglobin (HGP) | low < 11.50, normal 11.50–17, high > 17 |
| 4 | Hematocrit (HCT) | low < 36, normal 36–54, high > 54 |
| 5 | Mean corpuscular volume (MCV) | low < 80, normal 80–96, high > 96 |
| 6 | Mean corpuscular hemoglobin (MCH) | low < 26, normal 26–34, high > 34 |
| 7 | Mean corpuscular hemoglobin concentration (MCHC) | low < 31, normal 31–37, high > 37 |
| 8 | Platelet count (PLT) | low < 150, normal 150–400, high > 400 |
| 9 | Red cell distribution width in fL (RDW-SD) | low < 37, normal 37–54, high > 54 |
| 10 | Red cell distribution width in percentage (RDW-CV) | Low < 11, normal 11–16, high > 16 |
| 11 | Platelet distribution width (PDW) | low < 12.2, normal 12.2–16.1, high > 16.1 |
| 12 | Mean platelet volume (MPV) | low < 7.5, normal 7.5–11.4, high > 11.4 |
| 13 | Platelet–large cell ratio (P-LCR) | low < 15, normal 15–30, high > 30 |
| 14 | Procalcitonin test (PCT) | low < 0.042, normal 0.042–0.102, high > 0.102 |
| 15 | Vitamin B12 | low < 211, normal 211–963, high > 963 |
| 16 | Vitamin D-T | low < 20, normal 20–32, high > 32 |
| Items Intake | Mean | DRI | Difference | T-Test | p-Value |
|---|---|---|---|---|---|
| Calories (kcal) | 1769.00 | 2000.00 | −231.00 | −1.917 * | 0.016 |
| Protein (g) | 40.72 | 46.00 | −5.28 | −2.29 * | 0.026 |
| Carbohydrates (g) | 165.03 | 130.00 | 35.03 | 4.04 ** | 0.001 |
| Dietary fiber (g) | 10.35 | 28.00 | −17.65 | −15.69 ** | 0.000 |
| Total fat (g) | 47.41 | 65.00 | −17.59 | −5.24 ** | 0.003 |
| Saturated fat (g) | 15.84 | 20.00 | −4.16 | −4.05 ** | 0.007 |
| Unsaturated fat (g) | 32.36 | 45.00 | −12.64 | −5.11 ** | 0.005 |
| Cholesterol (mg) | 156.80 | 300.00 | −143.20 | −10.34 ** | 0.000 |
| Vit A µg (RE) | 191.80 | 700.00 | −508.20 | −6.07 ** | 0.010 |
| Vit B1 (mg) | 0.62 | 1.10 | −0.48 | −2.64 * | 0.011 |
| Vit B2 (mg) | 0.59 | 1.10 | −0.51 | −6.43 ** | 0.000 |
| Niacin (mg) | 7.01 | 14.00 | −6.99 | −11.15 ** | 0.008 |
| Vit B6 (mg) | 0.54 | 1.30 | −0.76 | −8.55 ** | 0.000 |
| Vit B12 (mg) | 0.96 | 2.40 | −1.44 | −3.22 ** | 0.002 |
| Vit E (mg) | 1.78 | 15.00 | −13.22 | −84.36 ** | 0.000 |
| Folate (mg) | 101.10 | 400.00 | −298.90 | −37.05 ** | 0.005 |
| Calcium (mg) | 438.49 | 1000.00 | −561.51 | −14.31 ** | 0.001 |
| Copper (mg) | 0.50 | 900.00 | −899.50 | −5134.28 ** | 0.000 |
| Iron (mg) | 5.91 | 18.00 | −12.09 | −25.22 ** | 0.003 |
| Phosphorus (mg) | 289.84 | 700.00 | −410.16 | −18.56 ** | 0.001 |
| Selenium (mg) | 28.13 | 55.00 | −26.87 | −10.47 ** | 0.000 |
| Zinc (mg) | 2.28 | 11 | −8.72 | −41.97 ** | 0.004 |
| Variables | LOW | NORMAL | HIGH | |||||
|---|---|---|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | Frequency | Percent | Chi-Square | p-Value | |
| WBC (103/μL) | 4 | 7.8 | 46 | 90.2 | 1 | 2.0 | 74.471 ** | 0.002 |
| RBC (106/μL) | 2 | 3.9 | 49 | 96.1 | __ | __ | 43.342 ** | 0.005 |
| HGP (g/dL) | 23 | 45.1 | 28 | 54.9 | __ | __ | 0.490 | 0.484 |
| HCT (%) | 19 | 37.3 | 32 | 62.7 | __ | __ | 3.314 | 0.069 |
| MCV (fl) | 20 | 39.2 | 31 | 60.8 | __ | __ | 2.373 | 0.123 |
| MCH (pg) | 21 | 41.2 | 30 | 58.8 | __ | __ | 1.588 | 0.208 |
| MCHC (g/dL) | 19 | 37.3 | 32 | 62.7 | __ | __ | 3.314 * | 0.043 |
| PLT (103/μL) | __ | __ | 41 | 80.4 | 10 | 19.6 | 18.843 ** | 0.004 |
| RDW-SD (fL) | 1 | 2.0 | 50 | 98.0 | __ | __ | 47.007 ** | 0.001 |
| RDW-CV (%) | 1 | 2.0 | 36 | 70.6 | 14 | 27.5 | 36.824 ** | 0.003 |
| PDW (fL) | 49 | 96.1 | 2 | 3.9 | __ | __ | 43.314 ** | 0.001 |
| MPV (fL) | __ | __ | 50 | 98.0 | 1 | 2.0 | 47.078 ** | 0.006 |
| P-LCR (%) | 2 | 3.9 | 47 | 92.2 | 2 | 3.9 | 79.412 ** | 0.001 |
| PCT (%) | __ | __ | __ | __ | 51 | 100.0 | __ | __ |
| B12 (pg/mL) | 0 | 0.0 | 51 | 100.0 | __ | __ | __ | __ |
| VITD-T (ng/mL) | 28 | 54.9 | 19 | 37.3 | 4 | 7.8 | 17.294 ** | 0.0001 |
| Anthropometric | Frequency | Percentage |
|---|---|---|
| Body mass index (BMI) | ||
| Underweight | 5.0 | 9.8 |
| Normal | 26.0 | 51.0 |
| Overweight | 14.0 | 27.5 |
| Obesity I | 2.0 | 3.9 |
| Obesity II | 4.0 | 7.8 |
| Obesity III | 0.0 | 0.0 |
| Total | 51 | 100.0 |
| Waist-to-hip ratio (WHR) | ||
| Low | 26 | 51.0 |
| Normal | 3 | 5.9 |
| High | 22 | 43.1 |
| Total | 51 | 100.0 |
| Body fat (BF) | ||
| Decreased | 22 | 43.1 |
| Normal | 19 | 37.3 |
| High | 3 | 5.9 |
| Very high | 7 | 13.7 |
| Total | 51 | 100.0 |
| Visceral fat (VF) | ||
| Decreased | 31 | 60.8 |
| Normal | 13 | 25.5 |
| High | 7 | 13.7 |
| Very high | ̶ | ̶ |
| Total | 51 | 100.0 |
| Waist-to-height ratio (WHtR) | ||
| Extremely slim | 3 | 5.9 |
| Slim | 17 | 33.3 |
| Normal | 13 | 25.5 |
| Overweight | 3 | 5.9 |
| Too overweight | 15 | 29.4 |
| Total | 51 | 100.0 |
| Independent Variable/ Dependent Variable | WHR | WHtR | BMI | BF | VF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| rho | (β, SE) | rho | (β, SE) | Rho | (β, SE) | rho | (β, SE) | r | (β, SE) | |
| Age | 0.597 ** | (2.870 **, 0.271) | 0.449 ** | (3.836 **, 0.201) | 0.459 ** | (0.907 **, 0.177) | 0.498 ** | (0.620 **, 0.239) | 0.351 ** | (1.316 **, 0.205) |
| Type of residence | 0.022 ** | (0.282 **, 00.169) | 0.427 ** | (1.028 **, 0.182) | 0.311 * | (0.335 *, 0.097) | 0.139 | (0.088, 0.019) | 0.002 | (0.003, 0.007) |
| Occupation | 0.669 ** | (0.670 **, 0.724) | 0.779 ** | (7.151 **, 0.668) | 0.909 ** | (2.081 **, 0.825) | 0.898 ** | (1.212 **, 0.807) | 0.768 ** | (2.371 **, 0.589) |
| Marital status | 0.347 * | (1.679 *, 0.103) | 0.261 | (0.014, 0.069) | 0.332 * | (0.026 *, 0.072) | 0.376 * | (0.019 *, 0.112) | 0.421 ** | (0.056 **, 0.179) |
| Education level | −0.106 * | (−1.098 *, 0.128) | −0.356 * | (0.284 *, 0.205) | −0.237 * | (−0.042 *, 0.113) | −0.040 * | (−0.015 *, 0.120) | −0.017 * | (−0.022 *, 0.107) |
| Independent Variable/ Dependent Variable | BMI | BF | VF | |||
|---|---|---|---|---|---|---|
| p-Value | (β, SE) | p-Value | (β, SE) | p-Value | (β, SE) | |
| Have you ever been concerned about the emergence of a health issue related to celiac disease? | 0.000 | 0.987 **, 1.027 | 0.029 | 1.747 *, 1.641 | 0.050 | 0.015 *, 0.798 |
| Have your daily activities been restricted due to celiac disease? | 0.377 | 0.116, 1.123 | 0.884 | 0.264, 1.794 | 0.522 | 0.564, 0.873 |
| Have you felt that others kept you away from attending social activities? | 0.918 | 1.705, 1.146 | 0.231 | 2.234, 1.832 | 0.779 | 0.252, 0.892 |
| Did you feel depressed and anxious? | 0.046 | −0.149 *, 0.907 | 0.061 | −0.642 *, 1.450 | 0.240 | −0.842, 0.706 |
| Have you been bothered by your weight? | 0.870 | −0.802, 0.806 | 0.381 | −1.143, 1.288 | 0.471 | −0.456, 0.627 |
| Did you avoid social activities? | 0.026 | −1.118 *, 1.030 | 0.013 | −2.749 *, 1.646 | 0.678 | −0.335, 0.801 |
| Have you been annoyed or frustrated about the cost of alternative gluten-free foods? | 0.285 | 1.752, 1.290 | 0.027 | 3.825 *, 2.063 | 0.019 | 1.408 *, 1.004 |
| Have you faced financial difficulties as a result of your coeliac disease (e.g., the cost of a gluten-free diet or inability to work)? | 0.038 | −0.037 *, 1.303 | 0.081 | −0.507*, 2.083 | 0.315 | −1.033, 1.013 |
| Have you had trouble finding appropriate food? | 0.978 | −1.112, 1.028 | 0.481 | −1.169, 1.643 | 0.912 | −0.089, 0.799 |
| Have you ever had to skip meals or snacks due to a lack of suitable food? | 0.286 | 0.272, 0.926 | 0.301 | 1.553, 1.480 | 0.489 | 0.503, 0.720 |
| Have you experienced fatigue or a lack of energy that you believe is caused by celiac disease? | 0.771 | 1.123, 1.119 | 0.290 | 1.919, 1.788 | 0.628 | 0.618, 0.870 |
| Have you been worried that you might accidentally eat or drink products containing gluten? | 0.022 | 0.559 *, 1.181 | 0.002 | 0.832 **, 1.888 | 0.042 | 0.449 *, 0.919 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhosain, A.I.; Alshammari, G.M.; Almoteri, B.L.; Mohammed, M.A.; Binobead, M.A.; Yahya, M.A. Long-Term Effect of Gluten-Free Diets on Nutritional Status, Body Composition, and Associated Factors in Adult Saudi Females with Celiac Disease. Nutrients 2022, 14, 2090. https://doi.org/10.3390/nu14102090
Alhosain AI, Alshammari GM, Almoteri BL, Mohammed MA, Binobead MA, Yahya MA. Long-Term Effect of Gluten-Free Diets on Nutritional Status, Body Composition, and Associated Factors in Adult Saudi Females with Celiac Disease. Nutrients. 2022; 14(10):2090. https://doi.org/10.3390/nu14102090
Chicago/Turabian StyleAlhosain, Aeshah Ibrahim, Ghedeir M. Alshammari, Barakat Lafi Almoteri, Mohammed A. Mohammed, Manal Abdulaziz Binobead, and Mohammed Abdo Yahya. 2022. "Long-Term Effect of Gluten-Free Diets on Nutritional Status, Body Composition, and Associated Factors in Adult Saudi Females with Celiac Disease" Nutrients 14, no. 10: 2090. https://doi.org/10.3390/nu14102090
APA StyleAlhosain, A. I., Alshammari, G. M., Almoteri, B. L., Mohammed, M. A., Binobead, M. A., & Yahya, M. A. (2022). Long-Term Effect of Gluten-Free Diets on Nutritional Status, Body Composition, and Associated Factors in Adult Saudi Females with Celiac Disease. Nutrients, 14(10), 2090. https://doi.org/10.3390/nu14102090






