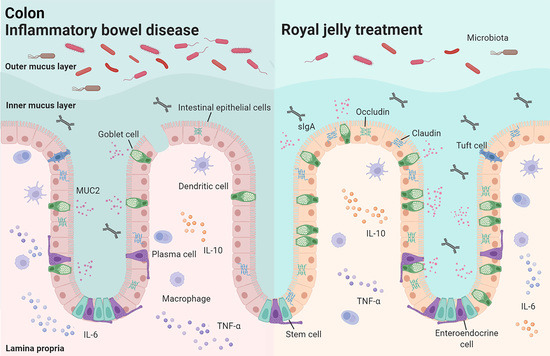
Royal Jelly Protected against Dextran-Sulfate-Sodium-Induced Colitis by Improving the Colonic Mucosal Barrier and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Royal Jelly Samples and Physicochemical Analysis
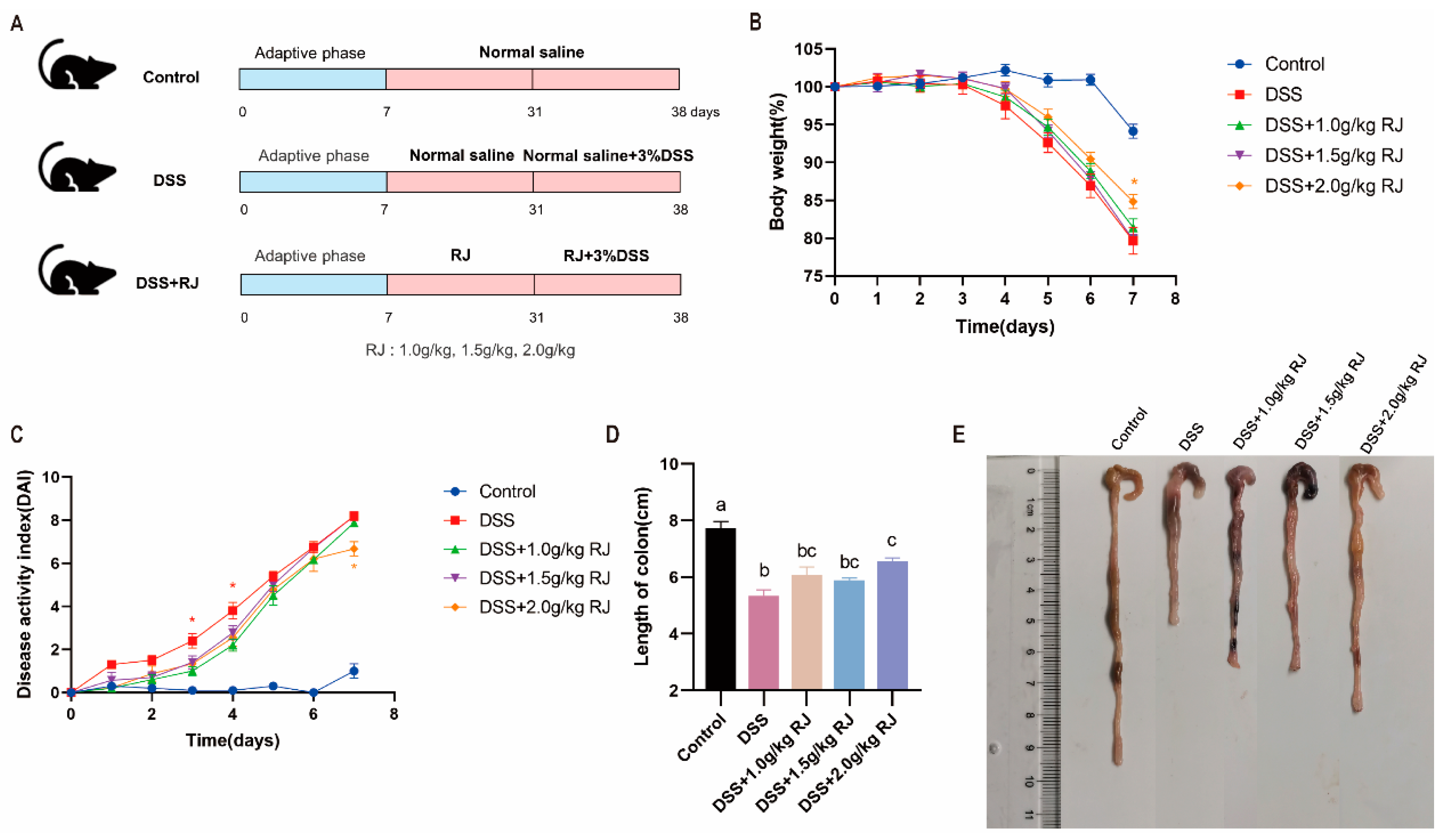
2.3. Disease Activity Index
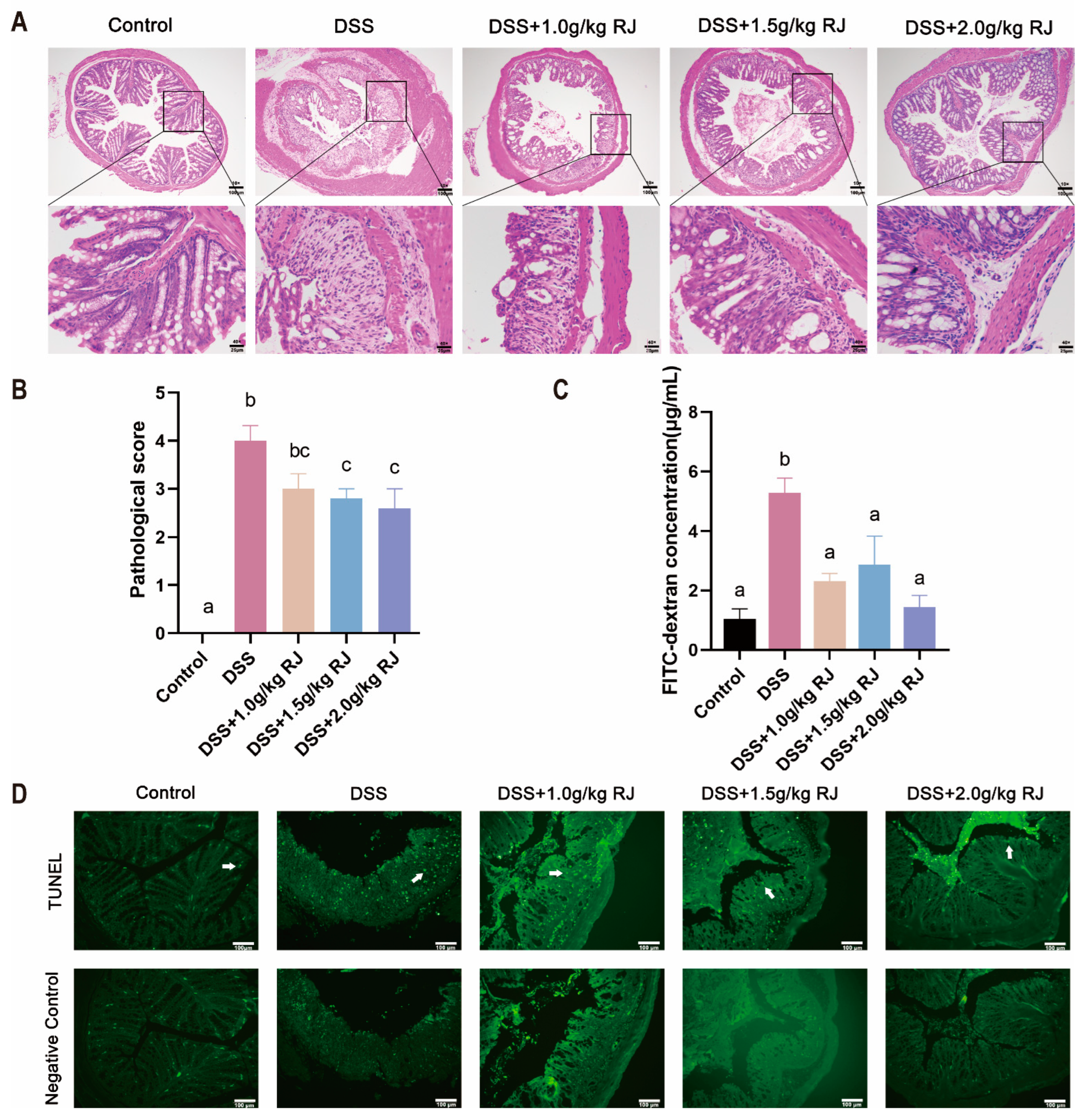
2.4. Histologic Analysis and Periodic acid-Schiff Staining
2.5. FITC-Labeled Dextran Intestinal Permeability Assays
2.6. Enzyme-Linked Immunosorbent Assay
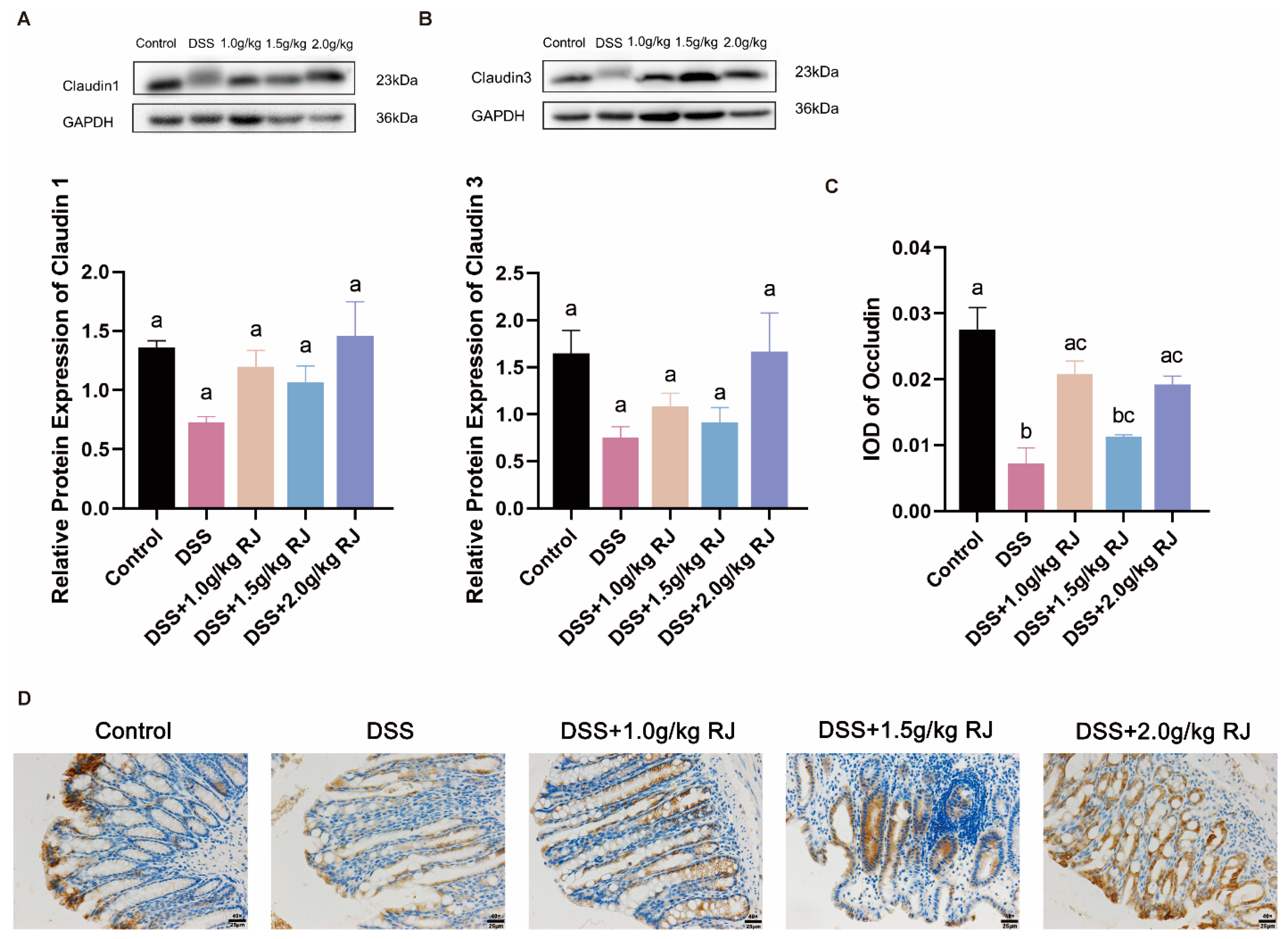
2.7. Western Blotting
2.8. Immunohistochemistry
2.9. TUNEL
2.10. Microbiota Analysis
2.11. Statistical Analyses
3. Results
3.1. Analysis of Physicochemical Parameters of Royal Jelly
3.2. Royal Jelly Alleviated Acute Colitis Induced by DSS in Mice
3.3. Royal Jelly Reduced Colon Damage in Acute Colitis Induced by DSS in Mice
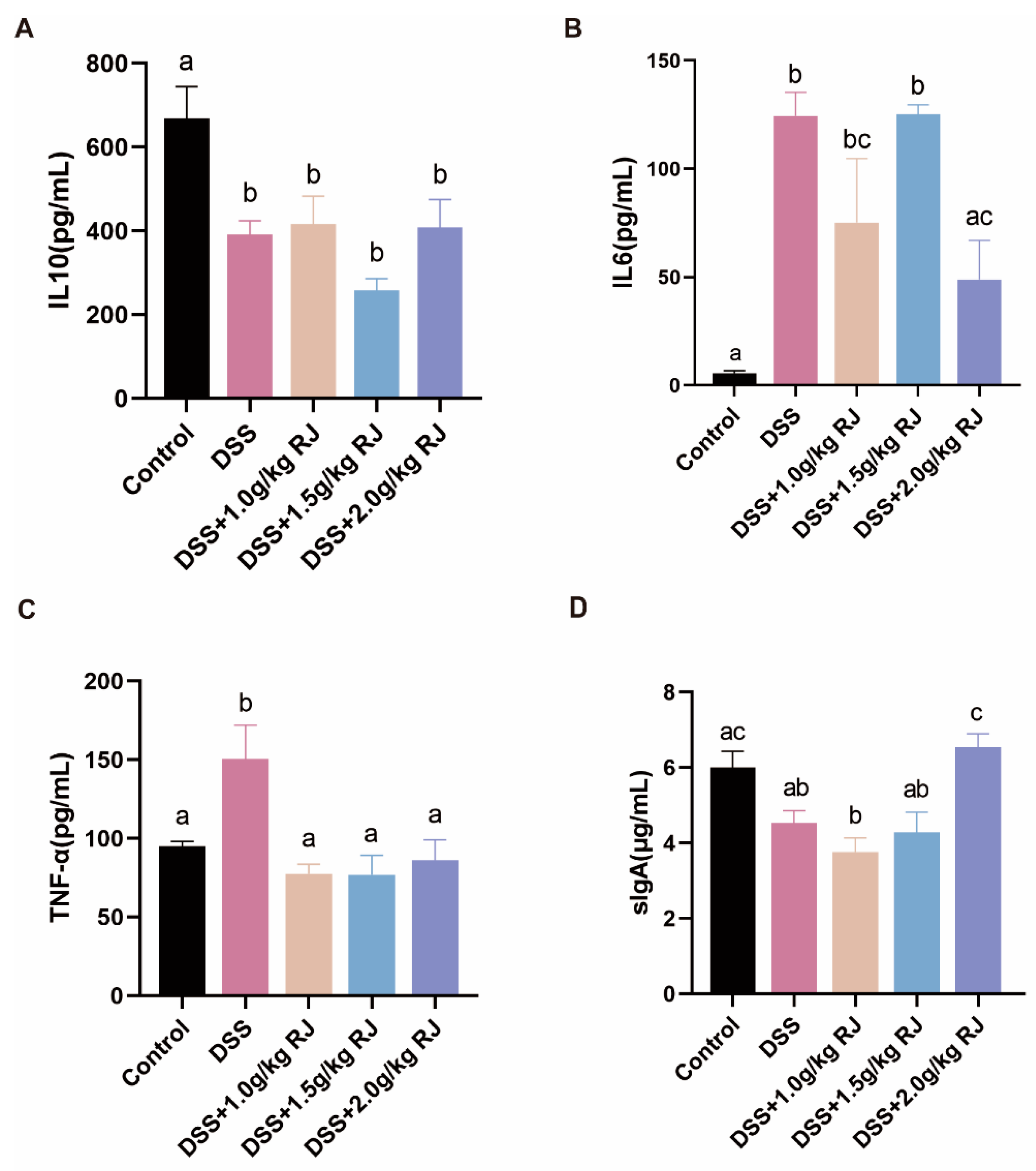
3.4. Royal Jelly Improved Intestinal Permeability and Inflammation in Mice with DSS-Induced Acute Colitis
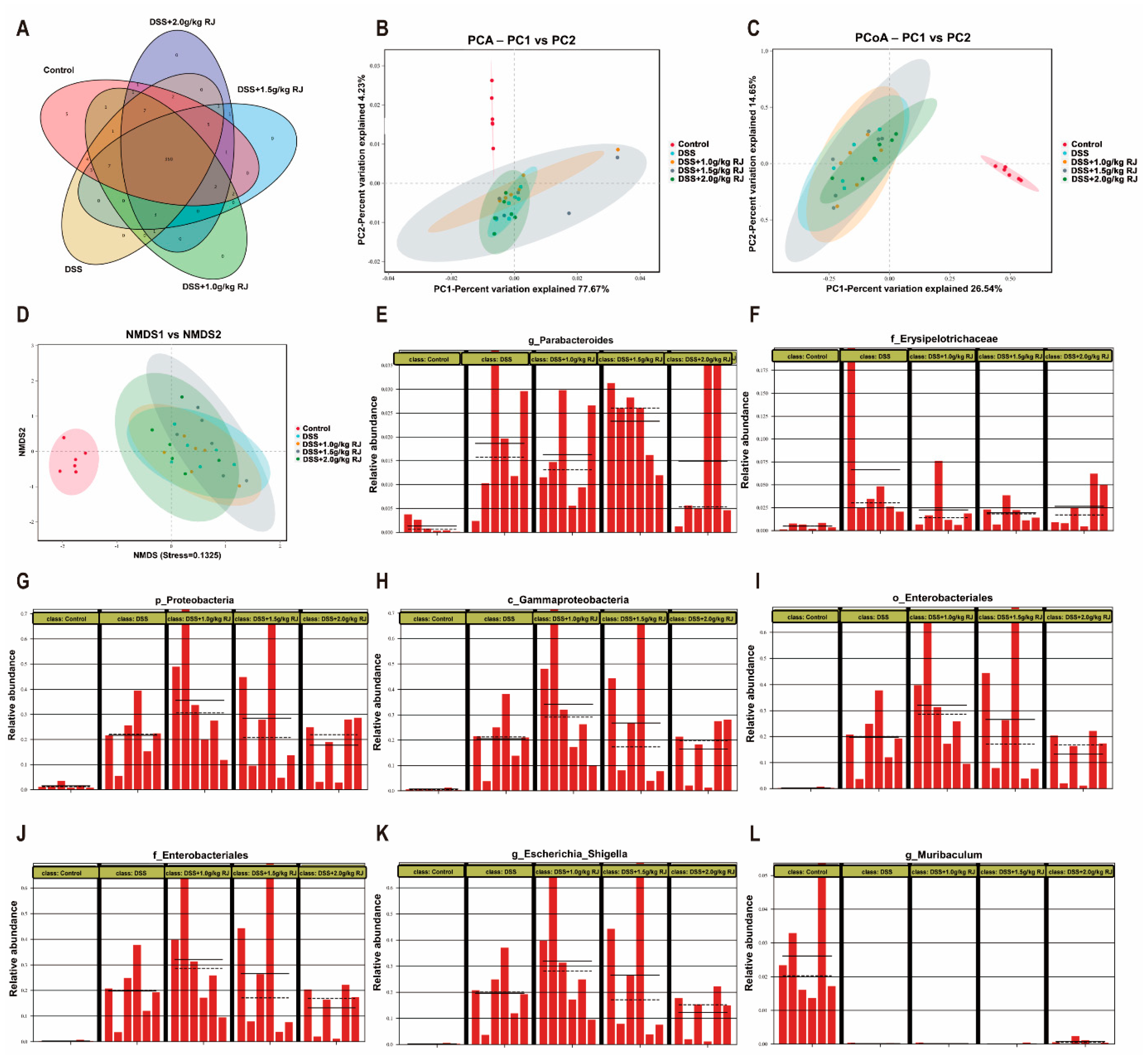
3.5. Gut Microbiota Profiling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkhetkan, W.; Thitiorul, S.; Jansom, C.; Ratanavalachai, T. Molecular and cytogenetic effects of Thai royal jelly: Modulation through c-MYC, h-TERT, NRF2, HO-1, BCL2, BAX and cyclins in human lymphocytes in vitro. Mutagenesis 2017, 32, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Altaye, S.Z.; Meng, L.; Lu, Y.; Li, J. The Emerging Proteomic Research Facilitates in-Depth Understanding of the Biology of Honeybees. Int. J. Mol. Sci. 2019, 20, 4252. [Google Scholar] [CrossRef]
- Cao, L.F.; Zheng, H.Q.; Pirk, C.W.W.; Hu, F.L.; Xu, Z.W. High Royal Jelly-Producing Honeybees (Apis mellifera ligustica) (Hymenoptera: Apidae) in China. J. Econ. Entomol. 2016, 109, 510–514. [Google Scholar] [CrossRef]
- Yang, W.; Tian, Y.; Han, M.; Miao, X. Longevity extension of worker honey bees (Apis mellifera) by royal jelly: Optimal dose and active ingredient. PeerJ 2017, 5, e3118. [Google Scholar] [CrossRef]
- Shorter, J.R.; Geisz, M.; Özsoy, E.; Magwire, M.M.; Carbone, M.A.; Mackay, T.F.C. The Effects of Royal Jelly on Fitness Traits and Gene Expression in Drosophila melanogaster. PLoS ONE 2015, 10, e0134612. [Google Scholar] [CrossRef]
- Kayashima, Y.; Yamanashi, K.; Sato, A.; Kumazawa, S.; Yamakawa-Kobayashi, K. Freeze-dried royal jelly maintains its developmental and physiological bioactivity in Drosophila melanogaster. Biosci. Biotechnol. Biochem. 2012, 76, 2107–2111. [Google Scholar] [CrossRef][Green Version]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Miyauchi-Wakuda, S.; Kagota, S.; Maruyama-Fumoto, K.; Wakuda, H.; Yamada, S.; Shinozuka, K. Effect of Royal Jelly on Mouse Isolated Ileum and Gastrointestinal Motility. J. Med. Food 2019, 22, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Kohno, K.; Inoue, S.-i.; Koya-Miyata, S.; Okamoto, I.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int. Immunopharmacol. 2003, 3, 1313–1324. [Google Scholar] [CrossRef]
- Kai, H.; Motomura, Y.; Saito, S.; Hashimoto, K.; Tatefuji, T.; Takamune, N.; Misumi, S. Royal jelly enhances antigen-specific mucosal IgA response. Food Sci. Nutr. 2013, 1, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Kosek, M.; Bern, C.; Guerrant, R.L. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 2003, 81, 197–204. [Google Scholar] [PubMed]
- Reiner, R.C.; Wiens, K.E.; Deshpande, A.; Baumann, M.M.; Lindstedt, P.A.; Blacker, B.F.; Troeger, C.E.; Earl, L.; Munro, S.B.; Abate, D.; et al. Mapping geographical inequalities in childhood diarrhoeal morbidity and mortality in low-income and middle-income countries, 2000–2017: Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 1779–1801. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.M.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Mowat, C.; Cole, A.; Windsor, A.; Ahmad, T.; Arnott, I.; Driscoll, R.; Mitton, S.; Orchard, T.; Rutter, M.; Younge, L.; et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011, 60, 571–607. [Google Scholar] [CrossRef]
- Mulder, D.J.; Noble, A.J.; Justinich, C.J.; Duffin, J.M. A tale of two diseases: The history of inflammatory bowel disease. J. Crohns. Colitis. 2014, 8, 341–348. [Google Scholar] [CrossRef]
- Karaca, T.; Bayiroglu, F.; Yoruk, M.; Kaya, M.S.; Uslu, S.; Comba, B.; Mis, L. Effect of royal jelly on experimental colitis Induced by acetic acid and alteration of mast cell distribution in the colon of rats. Eur. J. Histochem. 2010, 54, e35. [Google Scholar] [CrossRef]
- Karaca, T.; Şimşek, N.; Uslu, S.; Kalkan, Y.; Can, I.; Kara, A.; Yörük, M. The effect of royal jelly on CD3(+), CD5(+), CD45(+) T-cell and CD68(+) cell distribution in the colon of rats with acetic acid-induced colitis. Allergol. Immunopath. 2012, 40, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Karaca, T.; Uz, Y.H.; Demirtas, S.; Karaboga, I.; Can, G. Protective effect of royal jelly in 2,4,6 trinitrobenzene sulfonic acid-induced colitis in rats. Iran. J. Basic. Med. Sci. 2015, 18, 370–379. [Google Scholar] [PubMed]
- Manzo, L.P.; de-Faria, F.M.; Dunder, R.J.; Rabelo-Socca, E.A.; Consonni, S.R.; de Almeida, A.C.A.; Souza-Brito, A.R.M.; Luiz-Ferreira, A. Royal Jelly and its dual role in TNBS colitis in mice. Sci. World J. 2015, 2015, 956235. [Google Scholar] [CrossRef] [PubMed]
- Jones-Hall, Y.L.; Nakatsu, C.H. The Intersection of TNF, IBD and the Microbiome. Gut Microbes 2016, 7, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Kim, B.Y.; Park, M.J.; Deng, Y.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Antibacterial activity of major royal jelly proteins of the honeybee (Apis mellifera) royal jelly. J. Asia-Pac. Entomol. 2019, 22, 737–741. [Google Scholar] [CrossRef]
- Cao, Q.; Gao, X.; Lin, Y.; Yue, C.; Wang, Y.; Quan, F.; Zhang, Z.; Liu, X.; Lu, Y.; Zhan, Y.; et al. Thymopentin ameliorates dextran sulfate sodium-induced colitis by triggering the production of IL-22 in both innate and adaptive lymphocytes. Theranostics 2019, 9, 7490–7505. [Google Scholar] [CrossRef]
- Loher, F.; Schmall, K.; Freytag, P.; Landauer, N.; Hallwachs, R.; Bauer, C.; Siegmund, B.; Rieder, F.; Lehr, H.-A.; Dauer, M.; et al. The specific type-4 phosphodiesterase inhibitor mesopram alleviates experimental colitis in mice. J. Pharmacol. Exp. Ther. 2003, 305, 549–556. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, L.; Feng, M.; Fang, Y.; Hu, H.; Han, B.; Meng, L.; Li, J. Metabolic profiling unravels the effects of enhanced output and harvesting time on royal jelly quality. Food Res. Int. 2021, 139, 109974. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Sugiyama, T.; Takahashi, K.; Mori, H. Royal Jelly Acid, 10-Hydroxy-trans-2-Decenoic Acid, as a Modulator of the Innate Immune Responses. Endocr. Metab. Immune. 2012, 12, 368–376. [Google Scholar] [CrossRef]
- Antinelli, J.F.; Zeggane, S.; Davico, R.; Rognone, C.; Faucon, J.P.; Lizzani, L. Evaluation of (E)-10-hydroxydec-2-enoic acid as a freshness parameter for royal jelly. Food Chem. 2003, 80, 85–89. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; You, M.; Tian, W.; Le Leu, R.K.; Topping, D.L.; Conlon, M.A.; Wu, L.; Hu, F. Dietary Propolis Ameliorates Dextran Sulfate Sodium-Induced Colitis and Modulates the Gut Microbiota in Rats Fed a Western Diet. Nutrients 2017, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, D.; Cadwell, K. Intrinsic Defense Mechanisms of the Intestinal Epithelium. Cell Host Microbe 2016, 19, 434–441. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E.V. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Moughan, P.J. Intestinal barrier dysfunction: Implications for chronic inflammatory conditions of the bowel. Nutr. Res. Rev. 2016, 29, 40–59. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Andoh, A. Physiological Role of Gut Microbiota for Maintaining Human Health. Digestion 2016, 93, 176–181. [Google Scholar] [CrossRef]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]

| Variable | Unit | Royal Jelly |
|---|---|---|
| Water | g/100 g | 62.4 |
| Protein | g/100 g | 14.2 |
| 10-HDA | g/100 g | 1.51 |
| Total sugar | g/100 g | 11.1 |
| Amylum | g/100 g | Not detected |
| Ash | g/100 g | 0.4 |
| Acidity | mL/100 g | 33.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Ma, B.; Wang, Z.; Chen, Y.; Tian, W.; Dong, Y. Royal Jelly Protected against Dextran-Sulfate-Sodium-Induced Colitis by Improving the Colonic Mucosal Barrier and Gut Microbiota. Nutrients 2022, 14, 2069. https://doi.org/10.3390/nu14102069
Guo J, Ma B, Wang Z, Chen Y, Tian W, Dong Y. Royal Jelly Protected against Dextran-Sulfate-Sodium-Induced Colitis by Improving the Colonic Mucosal Barrier and Gut Microbiota. Nutrients. 2022; 14(10):2069. https://doi.org/10.3390/nu14102069
Chicago/Turabian StyleGuo, Jianying, Baochen Ma, Zixu Wang, Yaoxing Chen, Wenli Tian, and Yulan Dong. 2022. "Royal Jelly Protected against Dextran-Sulfate-Sodium-Induced Colitis by Improving the Colonic Mucosal Barrier and Gut Microbiota" Nutrients 14, no. 10: 2069. https://doi.org/10.3390/nu14102069
APA StyleGuo, J., Ma, B., Wang, Z., Chen, Y., Tian, W., & Dong, Y. (2022). Royal Jelly Protected against Dextran-Sulfate-Sodium-Induced Colitis by Improving the Colonic Mucosal Barrier and Gut Microbiota. Nutrients, 14(10), 2069. https://doi.org/10.3390/nu14102069