A Novel Herbal Extract Blend Product Prevents Particulate Matters-Induced Inflammation by Improving Gut Microbiota and Maintaining the Integrity of the Intestinal Barrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Composition Analysis of PM2.5
2.2. The Herbal Extract Blend Fresh Clear (FC)
2.3. Cell Culture and Cytotoxicity Test
2.4. Cell Experiment
2.5. Animal Experiment
2.6. Inflammatory Factors and Tight Junction Proteins Detection of Animal Tissues
2.7. Histological Analysis
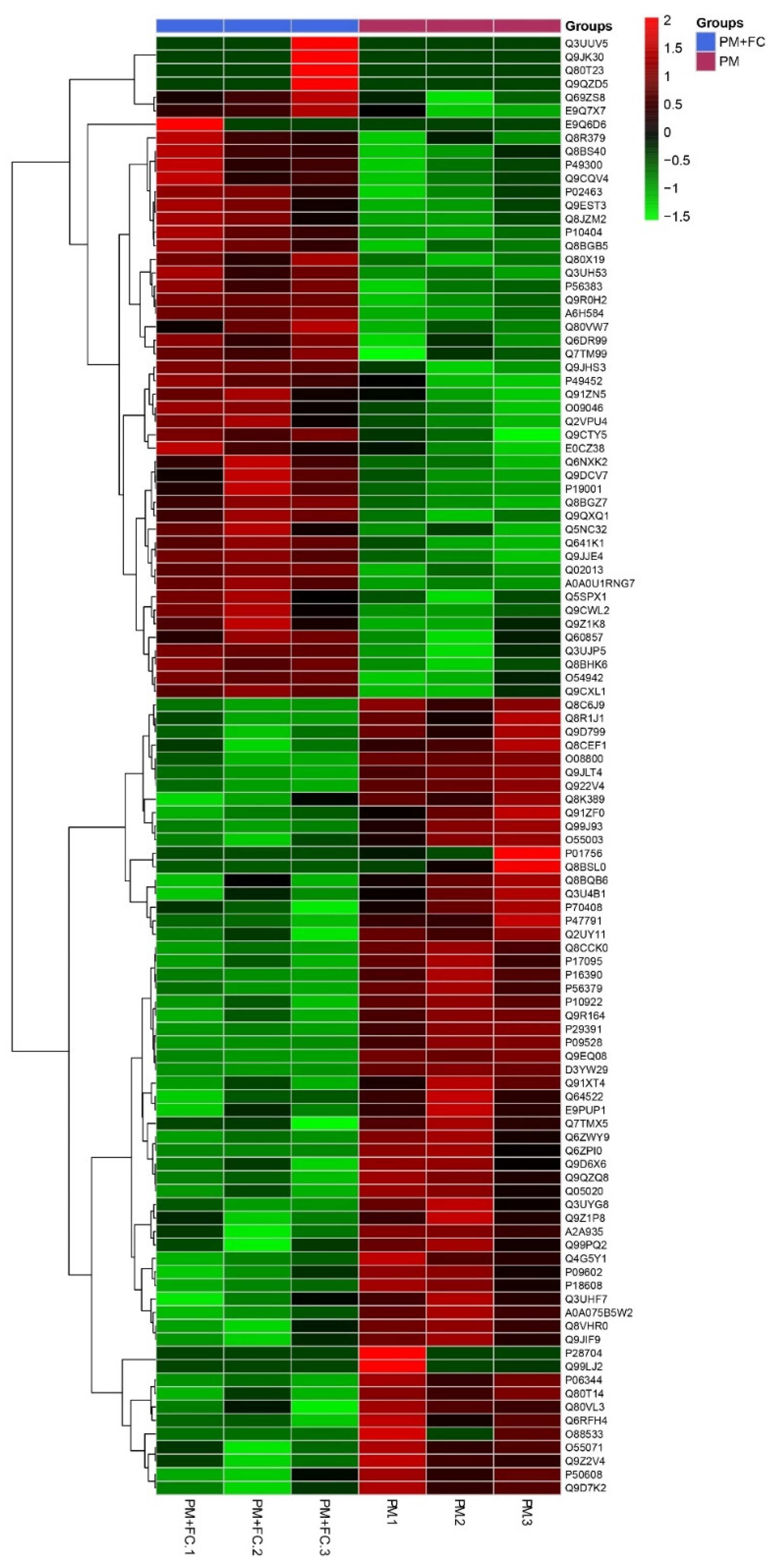
2.8. Proteomics Analysis
2.9. Fecal Microbiota Analysis
2.10. Statistical Analysis
3. Results
3.1. The Components of PM2.5
3.2. Cell Proliferation Treated by Herbal Extract Blend (FC)
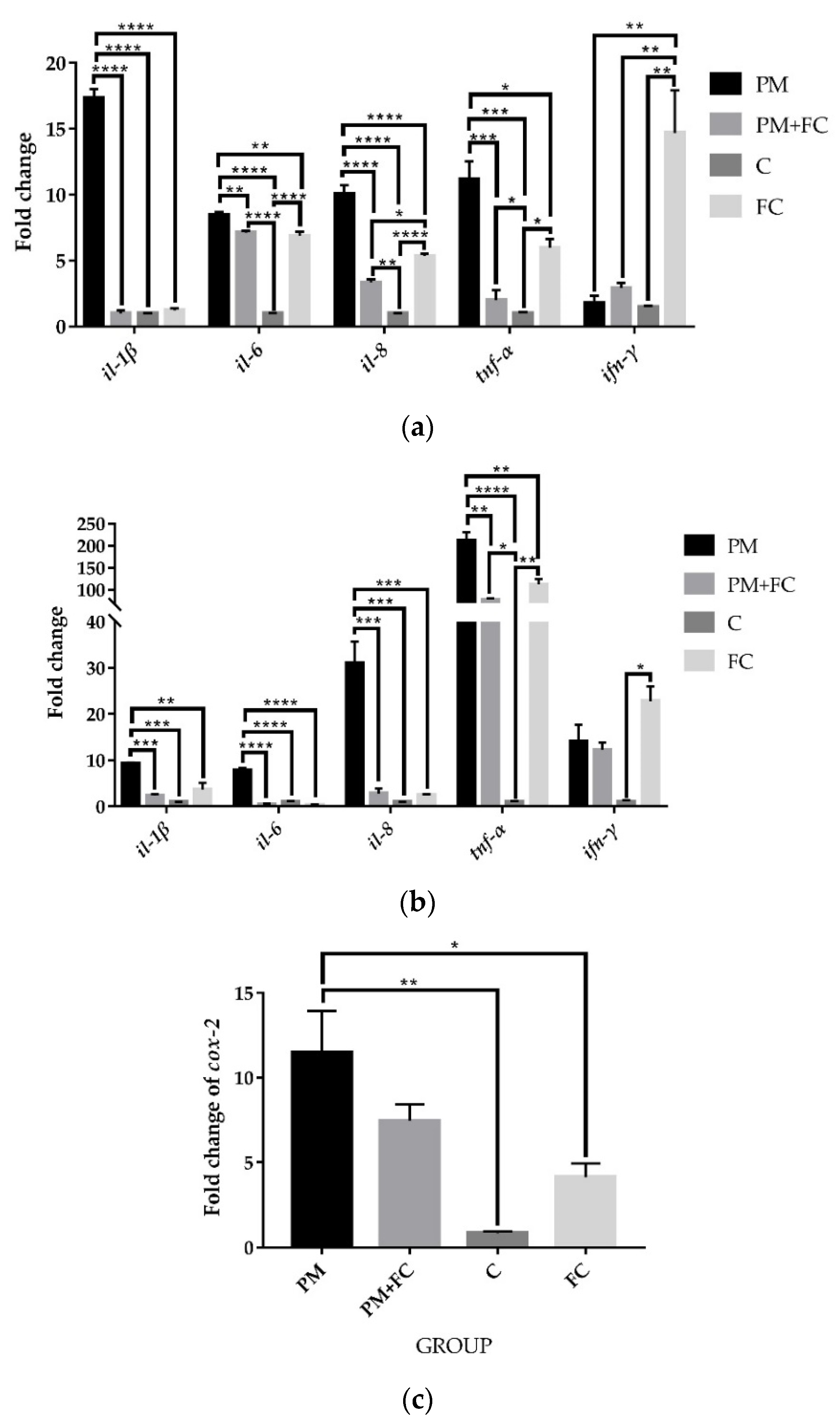
3.3. Herbal Extract Blend (FC) Downregulates the Expression of Inflammatory Cytokines in Cells Induced by PM2.5
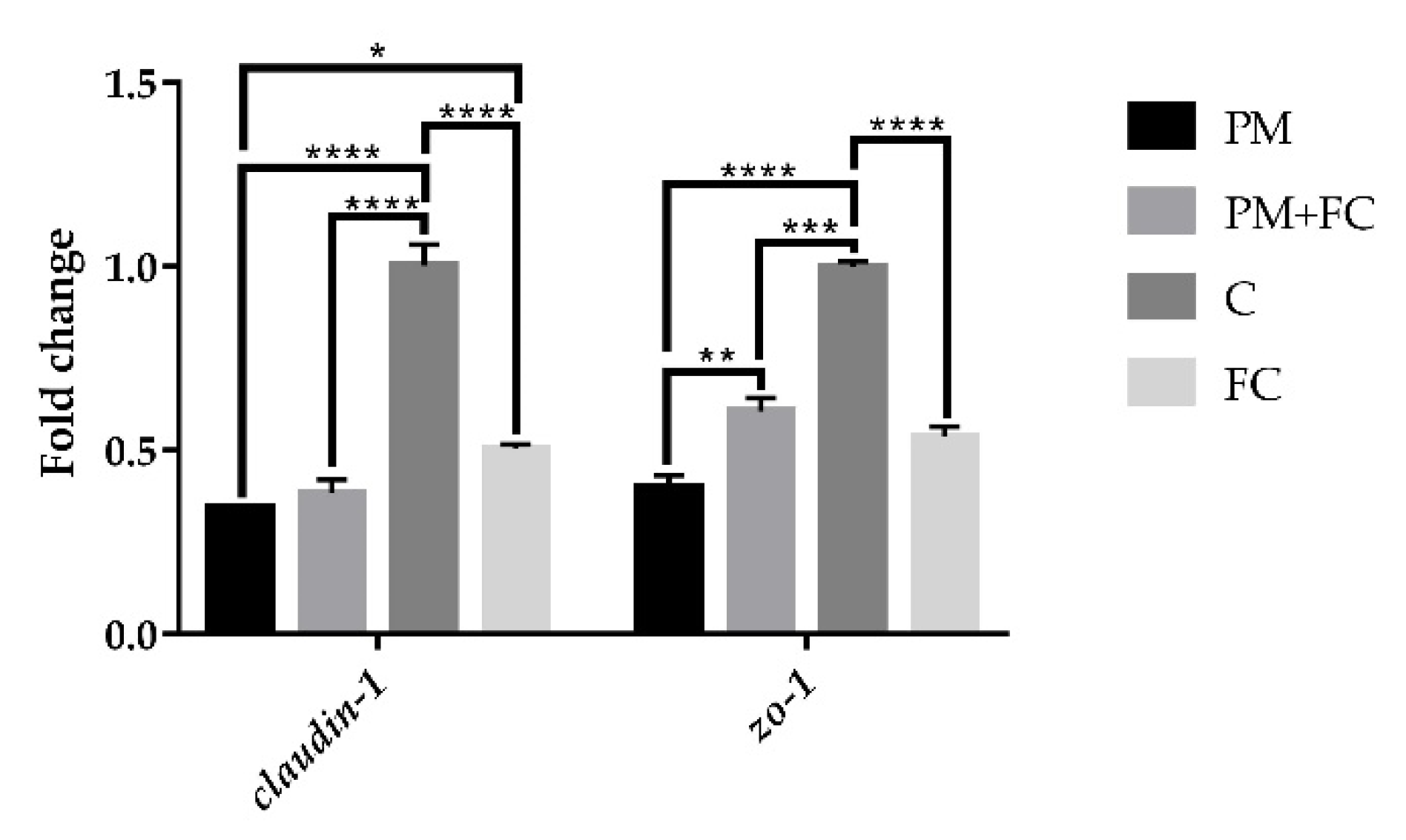
3.4. Tight Junction Protein Expression in A549 Cells
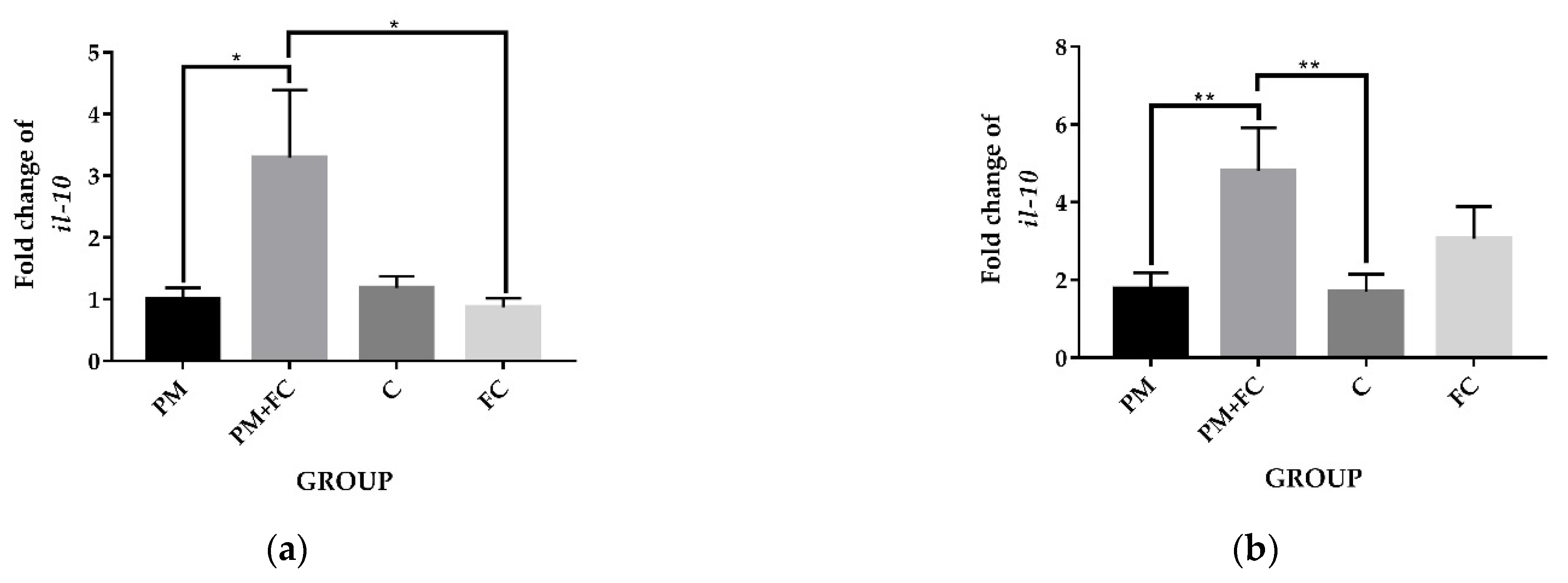
3.5. The Anti-Inflammatory Effects of Herbal Extract Blend (FC) in Animal Tissues
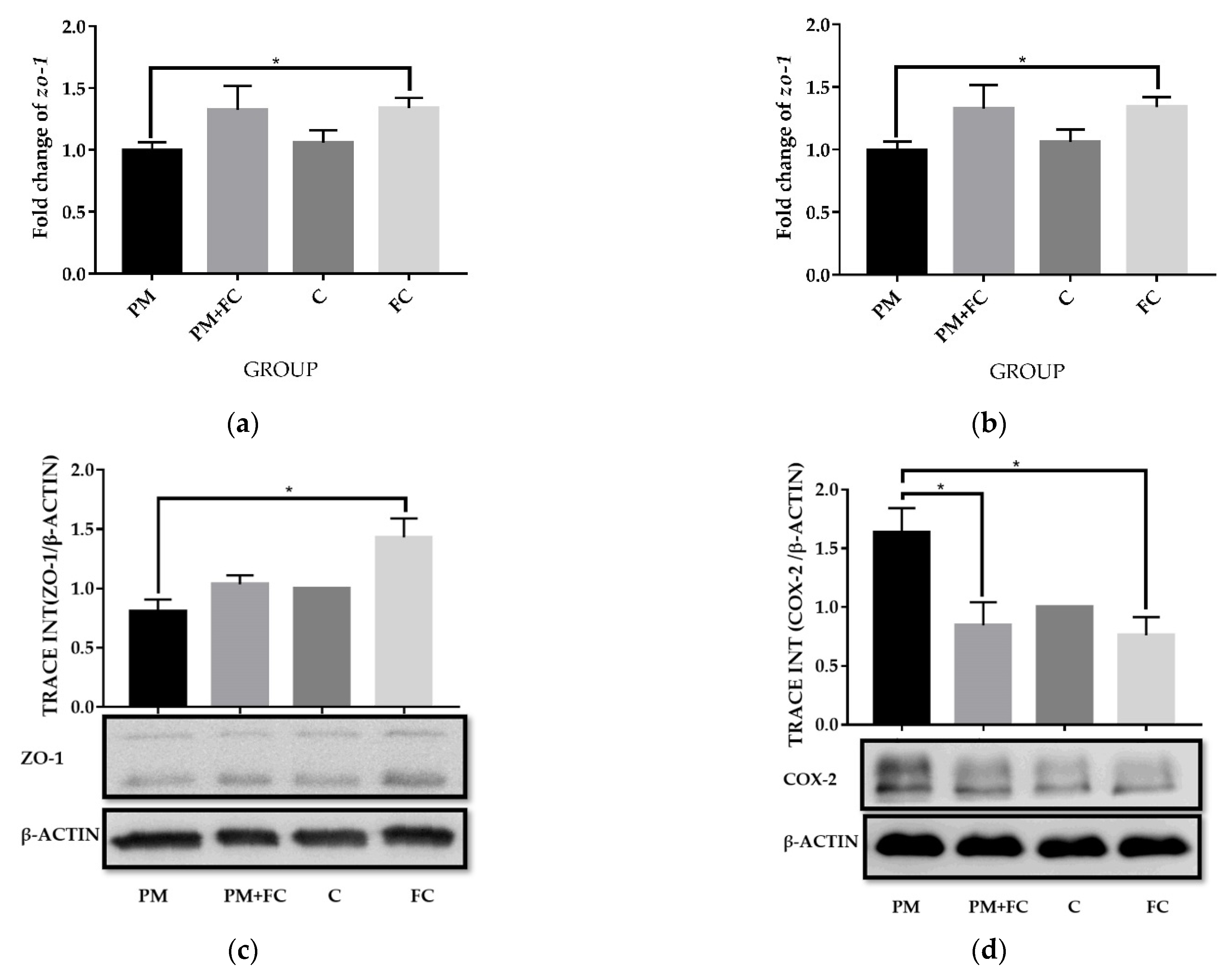
3.6. Modulation of Herbal Extract Blend (FC) on ZO-1 and COX-2 Expression in Animal Tissues
3.7. Effects of Herbal Extract Blend (FC) on Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsu, C.-Y.; Chiang, H.-C.; Lin, S.-L.; Chen, M.-J.; Lin, T.-Y.; Chen, Y.-C. Elemental characterization and source apportionment of PM 10 and PM 2.5 in the western coastal area of central Taiwan. Sci. Total Environ. 2016, 541, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yin, P.; Meng, X.; Liu, C.; Wang, L.; Xu, X.; Ross, J.A.; Tse, L.A.; Zhao, Z.; Kan, H.; et al. Fine Particulate Air Pollution and Daily Mortality. A Nationwide Analysis in 272 Chinese Cities. Am. J. Respir. Crit. Care Med. 2017, 196, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Gripenback, S.; Lundgren, L.; Eklund, A.; Liden, C.; Skare, L.; Tornling, G.; Grunewald, J. Accumulation of eosinophils and T-lymphocytes in the lungs after exposure to pinewood dust. Eur. Respir. J. 2005, 25, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.Y.; Kaplan, G.G.; Madsen, K.L. Air pollution effects on the gut microbiota: A link between exposure and inflammatory disease. Gut Microbes 2014, 5, 215–219. [Google Scholar] [CrossRef]
- He, M.; Ichinose, T.; Yoshida, S.; Nishikawa, M.; Mori, I.; Yanagisawa, R.; Takano, H.; Inoue, K.; Sun, G.; Shibamoto, T. Urban particulate matter in Beijing, China, enhances allergen-induced murine lung eosinophilia. Inhal. Toxicol. 2010, 22, 709–718. [Google Scholar] [CrossRef]
- Park, E.J.; Roh, J.; Kim, Y.; Park, K.; Kim, D.S.; Yu, S.D. PM 2.5 collected in a residential area induced Th1-type inflammatory responses with oxidative stress in mice. Environ. Res. 2011, 111, 348–355. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Brito, J.M.; Toledo, A.C.; Nakagawa, N.K.; Piccin, V.S.; Junqueira, M.S.; Negri, E.M.; Carvalho, A.L.; Oliveira, A.P.; Lima, W.T.; et al. Subchronic effects of nasally instilled diesel exhaust particulates on the nasal and airway epithelia in mice. Inhal. Toxicol. 2010, 22, 610–617. [Google Scholar] [CrossRef]
- Fu, H.; Liu, X.; Li, W.; Zu, Y.; Zhou, F.; Shou, Q.; Ding, Z. PM2.5 Exposure Induces Inflammatory Response in Macrophages via the TLR4/COX-2/NF-kappaB Pathway. Inflammation 2020, 43, 1948–1958. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Engen, P.A.; Yazici, C.; Soberanes, S.; Hamanaka, R.B.; Nigdelioglu, R.; Meliton, A.Y.; Ghio, A.J.; et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 2018, 240, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.A.; Engen, P.A.; Soberanes, S.; Urich, D.; Forsyth, C.B.; Nigdelioglu, R.; Chiarella, S.E.; Radigan, K.A.; Gonzalez, A.; Jakate, S.; et al. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part. Fibre Toxicol. 2011, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odenwald, M.A.; Choi, W.; Buckley, A.; Shashikanth, N.; Joseph, N.E.; Wang, Y.; Warren, M.H.; Buschmann, M.M.; Pavlyuk, R.; Hildebrand, J.; et al. ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J. Cell Sci. 2017, 130, 243–259. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Zhang, L.; Hu, L.; Liu, S.; Xiong, A.; Wang, J.; Xiong, Y.; Li, G. PM2.5 Aggravated OVA-Induced Epithelial Tight Junction Disruption Through Fas Associated via Death Domain-Dependent Apoptosis in Asthmatic Mice. J. Asthma Allergy 2021, 14, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; McGinley, E.L.; Binion, D.G.; Saeian, K. Ambient air pollution correlates with hospitalizations for inflammatory bowel disease: An ecologic analysis. Inflamm. Bowel Dis. 2011, 17, 1138–1145. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Chen, M.; Huang, X.; Xie, X.; Li, W.; Cao, Q.; Kan, H.; Xu, Y.; Ying, Z. Exposure to concentrated ambient PM2.5 alters the composition of gut microbiota in a murine model. Part. Fibre Toxicol. 2018, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Huxley, E.J.; Viroslav, J.; Gray, W.R.; Pierce, A.K. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am. J. Med. 1978, 64, 564–568. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Liu, L.; Wang, Q.; Zeng, J.; Chen, C. PM2.5 exposure perturbs lung microbiome and its metabolic profile in mice. Sci. Total Environ. 2020, 721, 137432. [Google Scholar] [CrossRef]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sze, M.A.; Hogg, J.C.; Sin, D.D. Bacterial microbiome of lungs in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipuma, J.J. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010, 23, 299–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Q.; Jiang, F.; Yin, R.; Wang, J.; Xia, W.; Dong, G.; Ma, W.; Yang, Y.; Xu, L.; Hu, J. Interplay between the lung microbiome and lung cancer. Cancer Lett. 2018, 415, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Nikzad-Langerodi, R.; Ortmann, S.; Pferschy-Wenzig, E.M.; Bochkov, V.; Zhao, Y.M.; Miao, J.H.; Saukel, J.; Ladurner, A.; Heiss, E.H.; Dirsch, V.M.; et al. Assessment of anti-inflammatory properties of extracts from Honeysuckle (Lonicera sp. L., Caprifoliaceae) by ATR-FTIR spectroscopy. Talanta 2017, 175, 264–272. [Google Scholar] [CrossRef]
- Li, Y.; Zou, L.; Li, T.; Lai, D.; Wu, Y.; Qin, S. Mogroside V inhibits LPS-induced COX-2 expression/ROS production and overexpression of HO-1 by blocking phosphorylation of AKT1 in RAW264.7 cells. Acta Biochim. Biophys. Sin. 2019, 51, 365–374. [Google Scholar] [CrossRef]
- Kong, D.; Li, Y.; Bai, M.; Deng, Y.; Liang, G.; Wu, H. A comparative study of the dynamic accumulation of polyphenol components and the changes in their antioxidant activities in diploid and tetraploid Lonicera japonica. Plant Physiol. Biochem. 2017, 112, 87–96. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, S.Y.; Deng, L.D.; Feng, L.P.; Huang, L.Z.; Yu, R.R. Antioxidant effect of mogrosides against oxidative stress induced by palmitic acid in mouse insulinoma NIT-1 cells. Braz. J. Med. Biol. Res. 2013, 46, 949–955. [Google Scholar] [CrossRef]
- Riedl, M.A.; Saxon, A.; Diaz-Sanchez, D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin. Immunol. 2009, 130, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Park, K.I.; Lee, D.H.; Kang, S.R.; Nagappan, A.; Kim, J.A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Hah, Y.S.; et al. Polyphenolic extract isolated from Korean Lonicera japonica Thunb. induce G2/M cell cycle arrest and apoptosis in HepG2 cells: Involvements of PI3K/Akt and MAPKs. Food Chem. Toxicol. 2012, 50, 2407–2416. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Murata, Y.; Sugiura, M.; Nishino, H.; Tokuda, H.; Matsumoto, K.; Kasai, R.; Yamasaki, K. Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides, from Momordica grosvenori. Cancer Lett. 2003, 198, 37–42. [Google Scholar] [CrossRef]
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Choi, Y.A.; Park, H.J.; Lee, J.Y.; Kim, D.K.; Choi, S.C.; Kim, T.H.; Nah, Y.H.; Yun, K.J.; Choi, S.J.; et al. Inhibition of trypsin-induced mast cell activation by water fraction of Lonicera japonica. Arch. Pharm. Res. 2004, 27, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Meyer, M.; Bauer, R.N.; Zhou, H.; Zhang, H.; Jones, S.; Robinette, C.; Noah, T.L.; Jaspers, I. Effect of Broccoli Sprouts and Live Attenuated Influenza Virus on Peripheral Blood Natural Killer Cells: A Randomized, Double-Blind Study. PLoS ONE 2016, 11, e0147742. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Liu, Z.; Liu, C.; Wu, M.; Su, H.; Ma, X.; Zang, Y.; Wang, J.; Zhao, Y.; Xiao, X. Spectrum-Effect Relationships Between Chemical Fingerprints and Antibacterial Effects of Lonicerae Japonicae Flos and Lonicerae Flos Base on UPLC and Microcalorimetry. Front. Pharmacol. 2016, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Bae, H.; Lee, G.; Hong, B.G.; Yoo, H.H.; Lim, S.J.; Lee, K.; Kim, J.; Ryu, B.; Lee, B.J.; et al. Prophylactic effects of Lonicera japonica extract on dextran sulphate sodium-induced colitis in a mouse model by the inhibition of the Th1/Th17 response. Br. J. Nutr. 2013, 109, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Paturi, G.; Mandimika, T.; Butts, C.A.; Zhu, S.; Roy, N.C.; McNabb, W.C.; Ansell, J. Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a(−/−) mice, a model of inflammatory bowel diseases. Nutrition 2012, 28, 324–330. [Google Scholar] [CrossRef]
- Tae, J.; Han, S.-W.; Yoo, J.-Y.; Kim, J.-A.; Kang, O.-H.; Baek, O.-S.; Lim, J.-P.; Kim, D.-K.; Kim, Y.-H.; Bae, K.-H.; et al. Anti-inflammatory effect of Lonicera japonica in proteinase-activated receptor 2-mediated paw edema. Clin. Chim. Acta 2003, 330, 165–171. [Google Scholar] [CrossRef]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Song, J.L.; Qian, B.; Pan, C.; Lv, F.; Wang, H.; Gao, Y.; Zhou, Y. Protective activity of mogroside V against ovalbumin-induced experimental allergic asthma in Kunming mice. J. Food Biochem. 2019, 43, e12973. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Liu, Y.; Guo, D.; He, W.; Zhao, L.; Xia, S. PM2.5-induced pulmonary inflammation via activating of the NLRP3/caspase-1 signaling pathway. Environ. Toxicol. 2021, 36, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.J.; Li, J.; Zhou, Q.L.; Pan, W.; Li, Y.Q.; Zhang, Y.; Wang, J.; Jiao, Z. Rosiglitazone inhibits PM2.5-induced cytotoxicity in human lung epithelial A549 cells. Ann. Transl. Med. 2018, 6, 152. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Y.; Hou, T.; Liao, J.; Zhang, C.; Sun, C.; Wang, G. PM2.5 induces EMT and promotes CSC properties by activating Notch pathway in vivo and vitro. Ecotoxicol. Environ. Saf. 2019, 178, 159–167. [Google Scholar] [CrossRef]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Rehman, S.U.; Choe, K.; Yoo, H.H. Review on a Traditional Herbal Medicine, Eurycoma longifolia Jack (Tongkat Ali): Its Traditional Uses, Chemistry, Evidence-Based Pharmacology and Toxicology. Molecules 2016, 21, 331. [Google Scholar] [CrossRef] [Green Version]
- Maleki-Saghooni, N.; Karimi, F.Z.; Behboodi Moghadam, Z.; Mirzaii Najmabadi, K. The effectiveness and safety of Iranian herbal medicines for treatment of premenstrual syndrome: A systematic review. Avicenna J. Phytomed. 2018, 8, 96–113. [Google Scholar]
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. Br. J. Pharmacol. 2020, 177, 1227–1240. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, H.; Li, Y.; Lynch, B.; Jia, X. Broccoli seed extract: Genotoxicity and subchronic toxicity studies. Regul. Toxicol. Pharmacol. 2015, 73, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Chartoumpekis, D.V.; Ziros, P.G.; Chen, J.G.; Groopman, J.D.; Kensler, T.W.; Sykiotis, G.P. Broccoli sprout beverage is safe for thyroid hormonal and autoimmune status: Results of a 12-week randomized trial. Food Chem. Toxicol. 2019, 126, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhu, Z.H.; Zhang, L.; Wang, Q.; Xu, M.M.; Lu, C.; Zhu, Y.; Zeng, J.; Duan, J.A.; Zhao, M. Anti-inflammatory property and functional substances of Lonicerae Japonicae Caulis. J. Ethnopharmacol. 2021, 267, 113502. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yin, Z.; Feng, T.; Zhang, M.; Zhou, Z.; Zhou, Y. An integrated network pharmacology and RNA-Seq approach for exploring the preventive effect of Lonicerae japonicae flos on LPS-induced acute lung injury. J. Ethnopharmacol. 2021, 264, 113364. [Google Scholar] [CrossRef]
- Tzeng, T.F.; Liou, S.S.; Chang, C.J.; Liu, I.M. The ethanol extract of Lonicera japonica (Japanese honeysuckle) attenuates diabetic nephropathy by inhibiting p-38 MAPK activity in streptozotocin-induced diabetic rats. Planta Med. 2014, 80, 121–129. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Liu, J.; Qiao, C.; Xue, N.; Lv, H.; Li, S. Mogroside V Alleviates Lipopolysaccharide-Induced Neuroinflammation via Inhibition of TLR4-MyD88 and Activation of AKT/AMPK-Nrf2 Signaling Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 5521519. [Google Scholar] [CrossRef]
- Shi, D.; Zheng, M.; Wang, Y.; Liu, C.; Chen, S. Protective effects and mechanisms of mogroside V on LPS-induced acute lung injury in mice. Pharm. Biol. 2014, 52, 729–734. [Google Scholar] [CrossRef]
- Schepici, G.; Bramanti, P.; Mazzon, E. Efficacy of Sulforaphane in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8637. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.A.; Jakaria, M.; Thangapandiyan, S.; Ahmad, J.; Rahman, M.A.; Mathew, B.; Abdel-Daim, M.M.; Aleya, L. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ. 2020, 707, 135624. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Suzuki, K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef]
- Morita, I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002, 68–69, 165–175. [Google Scholar] [CrossRef]
- Carey, M.A.; Germolec, D.R.; Bradbury, J.A.; Gooch, R.A.; Moorman, M.P.; Flake, G.P.; Langenbach, R.; Zeldin, D.C. Accentuated T helper type 2 airway response after allergen challenge in cyclooxygenase-1−/− but not cyclooxygenase-2−/− mice. Am. J. Respir. Crit. Care Med. 2003, 167, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Bose, S.; Kim, G.C.; Hong, S.U.; Kim, J.H.; Kim, J.E.; Kim, H. Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota. PLoS ONE 2014, 9, e86117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulagina, E.V.; Efimov, B.A.; Maximov, P.Y.; Kafarskaia, L.I.; Chaplin, A.V.; Shkoporov, A.N. Species composition of Bacteroidales order bacteria in the feces of healthy people of various ages. Biosci. Biotechnol. Biochem. 2012, 76, 169–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Cocconi, D.; van Sinderen, D.; Ventura, M. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol. Ecol. 2017, 93, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ottman, N.A. Host Immunostimulation and Substrate Utilization of the Gut Symbiont Akkermansia muciniphila; Wageningen University and Research: Wageningen, The Netherlands, 2015. [Google Scholar]
- Zhang, L.; Li, J.; Young, L.H.; Caplan, M.J. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc. Natl. Acad. Sci. USA 2006, 103, 17272–17277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, B.; Cantley, L.C. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl. Acad. Sci. USA 2007, 104, 819–822. [Google Scholar] [CrossRef] [Green Version]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Hasani, A.; Ebrahimzadeh, S.; Hemmati, F.; Khabbaz, A.; Hasani, A.; Gholizadeh, P. The role of Akkermansia muciniphila in obesity, diabetes and atherosclerosis. J. Med. Microbiol. 2021, 70, 001435. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Çicek, S.S. Structure-Dependent Activity of Plant-Derived Sweeteners. Molecules 2020, 25, 1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soejarto, D.D.; Addo, E.M.; Kinghorn, A.D. Highly sweet compounds of plant origin: From ethnobotanical observations to wide utilization. J. Ethnopharmacol. 2019, 243, 112056. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]








| Gene | Species | Sequences (5′-3′) | |
|---|---|---|---|
| Gapdh | human | forward | CTGACTTCAACAGCGACACC |
| reverse | TGCTGTAGCCAAATTCGTTGT | ||
| il-1β | human | forward | GAAATGCCACCTTTTGACAGTG |
| reverse | TGGATGCTCATCAGGACAT | ||
| il-6 | human | forward | CCGGAGAGGAGACTTCACAG |
| reverse | CAGAATTGCCATTGCACA | ||
| il-8 | human | forward | GACCACACTGCGCCAACAC |
| reverse | CTTCTCCACAACCCTCTGCAC | ||
| tnf-α | human | forward | GAGGCCAAGCCCTGGTATG |
| reverse | CGGGCCGATTGATCTCAGC | ||
| ifn-γ | human | forward | TCGGTAACTGACTTGAATGTCCA |
| reverse | TCGCTTCCCTGTTTTAGCTGC | ||
| claudin-1 | human | forward | TCTGGCTATTTTAGTTGCCACAG |
| reverse | AGAGAGCCTGACCAAATTCGT | ||
| zo-1 | human | forward | CCCCACTCTGAAAATGAGGA |
| reverse | GGGAACAACATACAGTGACGC | ||
| cox-2 | human | forward | GATACTCAGGCAGAGATGATCTACCC |
| reverse | AGACCAGGCACCAGACCAAAGA | ||
| β-actin | mouse | forward | AGTGTGACGTTGACATCCGT |
| reverse | TGCTAGGAGCCAGAGCAGTA | ||
| il-10 | mouse | forward | CTTACTGACTGGCATGAGGATCA |
| reverse | GCAGCTCTAGGAGCATGTGG | ||
| tnf-α | mouse | forward | CCTCCAGAAAAGACACCA |
| reverse | ACAAGCAGGAATGAGAAGAG | ||
| zo-1 | mouse | forward | TGAACGCTCTCATAAGCTTCGTAA |
| reverse | ACCGTACCAACCATCATTCATTG | ||
| cox-2 | mouse | forward | GAAGTCTTTGGTCTGGTGCCTG |
| reverse | GTCTGCTGGTTTGGAATAGTTGC |
| Parameters | Concentration (mg/kg) | Concentration (μg/m³) | Reference Value (Annual Average) (μg/m³) |
|---|---|---|---|
| Copper | 43.7 | 1.71 × 10−3 | |
| Nickel | 13.3 | 5.21 × 10−4 | |
| Zinc | 615 | 2.41 × 10−2 | |
| Vanadium | 0.417 | 1.63 × 10−5 | |
| Stibium | 3 | 1.17 × 10−4 | |
| Iron | 4607 | 1.80 × 10−1 | |
| Sulfide | 1.02 | 3.99 × 10−5 | 60 (sulfur dioxide) |
| Naphthalin | 0 | 0 | |
| Acenaphthylene | 0 | 0 | |
| Acenaphthene | 0 | 0 | |
| Fluorene | 0 | 0 | |
| Phenanthrene | 0 | 0 | |
| Anthracene | 0 | 0 | |
| Fluoranthene | 0.013 | 5.09 × 10−7 | |
| Pyrene | 0.011 | 4.31 × 10−7 | |
| Benzo(a)anthracene | 0.007 | 2.74 × 10−7 | |
| Chrysene | 0.01 | 3.92 × 10−7 | |
| Benzo(b)fluoranthene | 0.022 | 8.61 × 10−7 | |
| Benzo(k)fluoranthene | 0.011 | 4.31 × 10−7 | |
| Benzo(a)pyrene | 0.006 | 2.35 × 10−7 | 0.001 |
| Indene(1,2,3-cd)pyrene | 0.017 | 6.66 × 10−7 | |
| Dibenzo(a,h)anthracene | 0.01 | 3.92 × 10−7 | |
| Benzo(ghi)perylene | 0.02 | 7.83 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Deng, L.; Bartlett, M.; Ren, Y.; Lu, J.; Chen, Q.; Pan, Y.; Wang, H.; Guo, X.; Liu, C. A Novel Herbal Extract Blend Product Prevents Particulate Matters-Induced Inflammation by Improving Gut Microbiota and Maintaining the Integrity of the Intestinal Barrier. Nutrients 2022, 14, 2010. https://doi.org/10.3390/nu14102010
Jin L, Deng L, Bartlett M, Ren Y, Lu J, Chen Q, Pan Y, Wang H, Guo X, Liu C. A Novel Herbal Extract Blend Product Prevents Particulate Matters-Induced Inflammation by Improving Gut Microbiota and Maintaining the Integrity of the Intestinal Barrier. Nutrients. 2022; 14(10):2010. https://doi.org/10.3390/nu14102010
Chicago/Turabian StyleJin, Lilan, Lu Deng, Mark Bartlett, Yiping Ren, Jihong Lu, Qian Chen, Yixiao Pan, Hai Wang, Xiaokui Guo, and Chang Liu. 2022. "A Novel Herbal Extract Blend Product Prevents Particulate Matters-Induced Inflammation by Improving Gut Microbiota and Maintaining the Integrity of the Intestinal Barrier" Nutrients 14, no. 10: 2010. https://doi.org/10.3390/nu14102010
APA StyleJin, L., Deng, L., Bartlett, M., Ren, Y., Lu, J., Chen, Q., Pan, Y., Wang, H., Guo, X., & Liu, C. (2022). A Novel Herbal Extract Blend Product Prevents Particulate Matters-Induced Inflammation by Improving Gut Microbiota and Maintaining the Integrity of the Intestinal Barrier. Nutrients, 14(10), 2010. https://doi.org/10.3390/nu14102010






