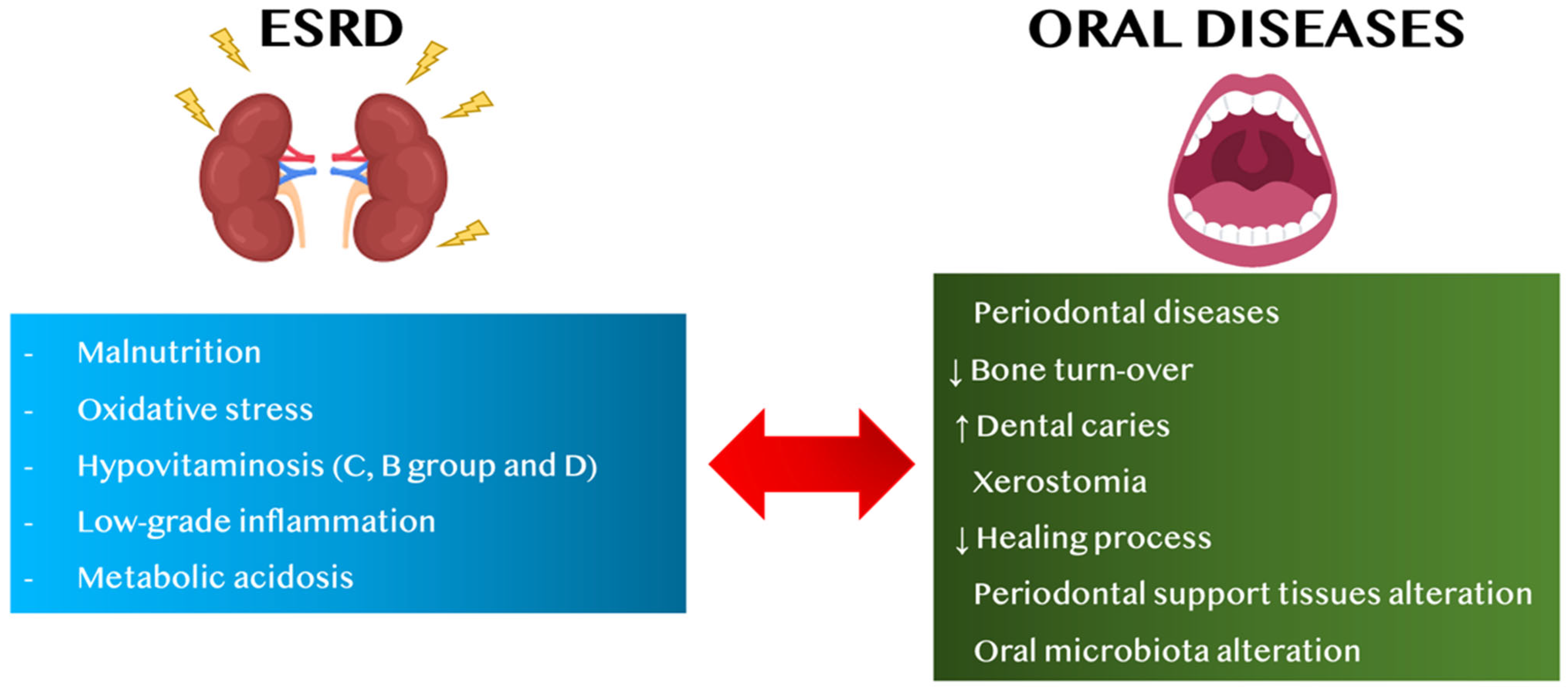
The Impact of Chronic Kidney Disease on Nutritional Status and Its Possible Relation with Oral Diseases
Abstract
1. Introduction
- to assess alterations of nutritional status and vitamin dysregulation in HD patients regarding oral diseases and vice versa;
- to evaluate the role of metabolic acidosis and low-grade inflammation (LGI) in CKD patients on oral diseases and vice versa.
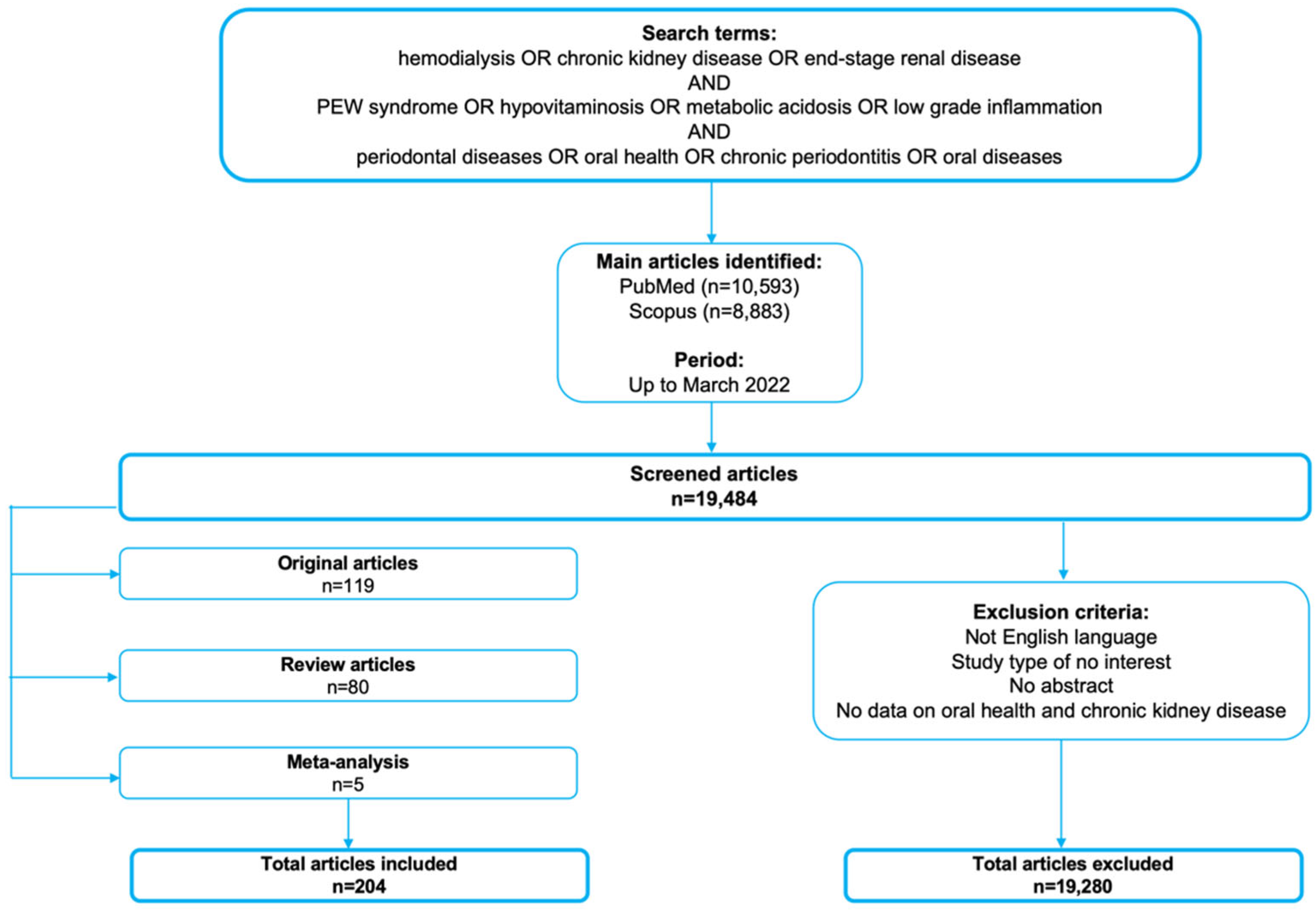
2. Search Methods
3. Alterations of Nutritional Status in Hemodialysis Patients
- (i)
- inadequate caloric and protein intake (protein-energy wasting, PEW) and typical CKD-related comorbidities (like metabolic acidosis, uremic gastritis, anorexia, depression, etc.);
- (ii)
- chronic systemic inflammatory state and complications typical of HD treatment (such as hypercatabolic state, loss of protein and amino acids during the dialytic session, etc.) [28].
4. Nutritional Therapy in Hemodialysis Patients
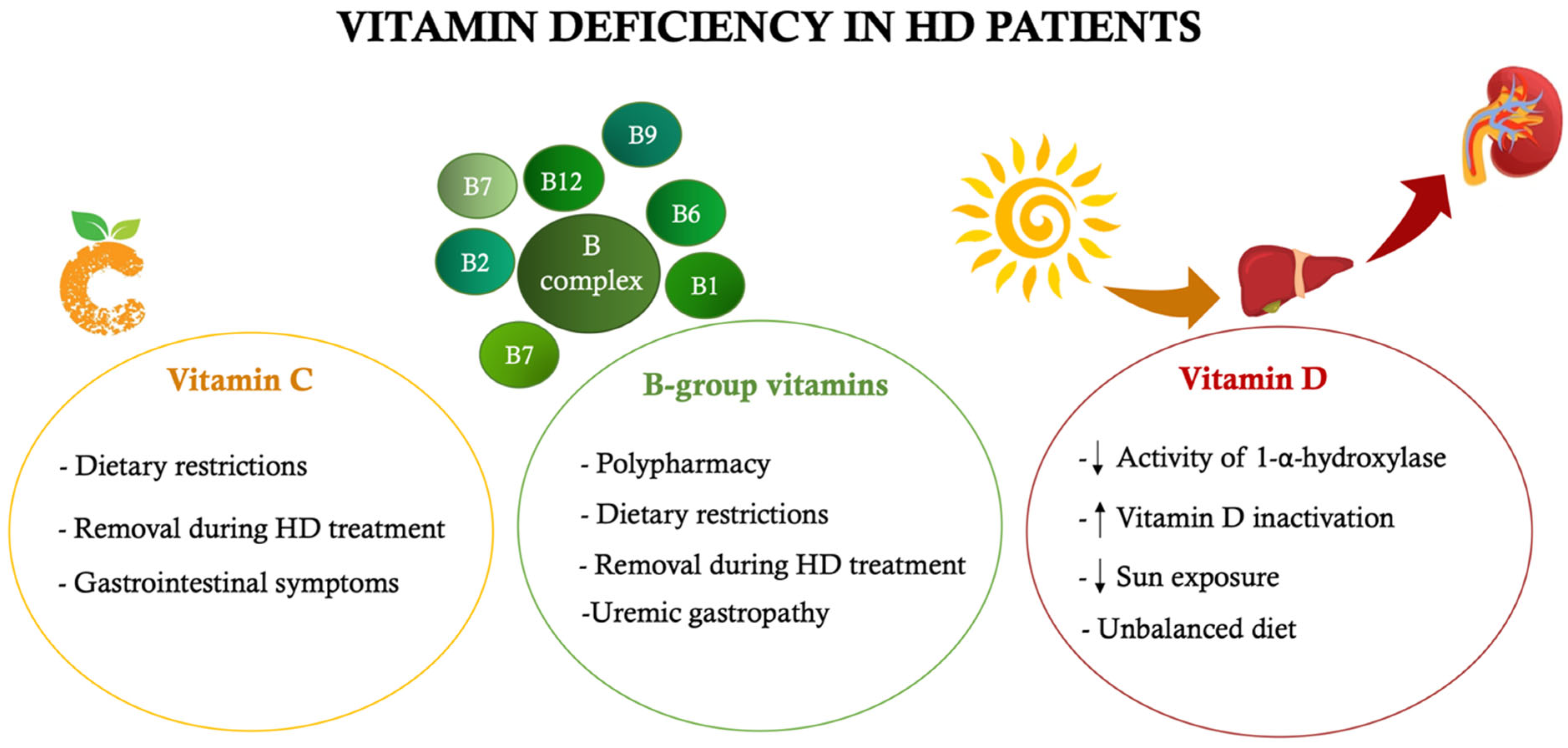
5. Alterations of Vitamins Levels in CKD Patients and Their Influence on Oral Diseases
5.1. The Role of Vitamin C
5.2. The Role of B-Group Vitamins
5.3. The Role of Vitamin D
6. The Role of Metabolic Acidosis on Onset of Oral Diseases
7. The Relation between Low-Grade Chronic Inflammation and Oral Diseases
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| AH | Hypertension |
| AhR | Aryl hydrocarbon receptor |
| AMP | Antimicrobial peptides |
| BOP | Bleeding index on probing |
| CAL | Clinical attachment level |
| CKD | Chronic kidney disease |
| CKD-MBD | CKD-mineral bone disorder |
| CV | Cardiovascular |
| DHA | Docosahexaenoic acid |
| EFP | European federation of periodontology |
| e-GFR | Estimated-GFR |
| EPA | Eicosapentaenoic acid |
| ESRD | End-stage renal disease |
| FGF-23 | Fibroblast growth factor-23 |
| GFR | Glomerular filtration rate |
| GI | Gingival index |
| GR | Gingival recession |
| HBD-2 | Human β-defensin 2 |
| Hcy | Homocysteine |
| HD | Hemodialysis |
| HGE | Human gingival epithelium |
| HPL | Human periodontal ligament |
| hs-CRP | Highly sensitive C-reactive protein |
| i.b.w. | Ideal body weight |
| IL | Interleukin |
| ISRNM | International Society of Renal Nutrition and Metabolism |
| LGI | Low-grade inflammation |
| LPS | Lipopolysaccharide |
| MIA | Malnutrition, inflammation and atherosclerosis |
| MKP-1 | Mitogen-activated protein kinase phosphatase 1 |
| MMP | Matrix metalloproteinase |
| NADH | Nicotinamide adenine dinucleotide |
| NF-kB | Nuclear factor kappa B |
| NHANES | National Health and Nutrition Examination Survey |
| NO | Nitric oxide |
| ORCA | European organisation for caries research |
| OS | Oxidative stress |
| PD | Periodontal diseases |
| PEW | Protein-energy wasting |
| PI | Plaque index |
| PPD | Probing pocket depth |
| ROS | Reactive oxygen species |
| SRP | Scaling and root planing |
| TGF | Transforming growth factor |
| TNF-α | Tumor necrosis factor-α |
| TRH | Thyrotropin-releasing hormone |
| VDR | Vitamin D receptor |
| VDRE | Vitamin D response element |
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Mochizuki, Y.; Miyata, Y.; Obata, Y.; Mitsunari, K.; Matsuo, T.; Ohba, K.; Mukae, H.; Yoshimura, A.; Nishino, T.; et al. Pathological Characteristics of Periodontal Disease in Patients with Chronic Kidney Disease and Kidney Transplantation. Int. J. Mol. Sci. 2019, 20, 3413. [Google Scholar] [CrossRef] [PubMed]
- Serni, L.; Caroti, L.; Barbato, L.; Nieri, M.; Serni, S.; Cirami, C.L.; Cairo, F. Association between chronic kidney disease and periodontitis. A systematic review and metanalysis. Oral Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Park, J.B. Tooth loss and risk of end-stage renal disease: A nationwide cohort study. J. Periodontol. 2021, 92, 371–377. [Google Scholar] [CrossRef]
- Limeres, J.; Garcez, J.F.; Marinho, J.S.; Loureiro, A.; Diniz, M.; Diz, P. Early tooth loss in end-stage renal disease patients on haemodialysis. Oral Dis. 2016, 22, 530–535. [Google Scholar] [CrossRef]
- Iwasaki, M.; Borgnakke, W.S.; Awano, S.; Yoshida, A.; Hamasaki, T.; Teratani, G.; Kataoka, S.; Kakuta, S.; Soh, I.; Ansai, T.; et al. Periodontitis and health-related quality of life in hemodialysis patients. Clin. Exp. Dent. Res. 2017, 3, 13–18. [Google Scholar] [CrossRef]
- Fisher, M.A.; Taylor, G.W. A prediction model for chronic kidney disease includes periodontal disease. J. Periodontol. 2009, 80, 16–23. [Google Scholar] [CrossRef]
- Chambrone, L.; Foz, A.M.; Guglielmetti, M.R.; Pannuti, C.M.; Artese, H.P.; Feres, M.; Romito, G.A. Periodontitis and chronic kidney disease: A systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J. Clin. Periodontol. 2013, 40, 443–456. [Google Scholar] [CrossRef]
- Ioannidou, E.; Swede, H. Disparities in periodontitis prevalence among chronic kidney disease patients. J. Dent. Res. 2011, 90, 730–734. [Google Scholar] [CrossRef]
- Ioannidou, E.; Hall, Y.; Swede, H.; Himmelfarb, J. Periodontitis associated with chronic kidney disease among Mexican Americans. J. Public Health Dent. 2013, 73, 112–119. [Google Scholar] [CrossRef]
- Iwasaki, M.; Taylor, G.W.; Nesse, W.; Vissink, A.; Yoshihara, A.; Miyazaki, H. Periodontal disease and decreased kidney function in Japanese elderly. Am. J. Kidney Dis. 2012, 59, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Yu, T.M.; Ke, T.Y.; Wu, M.J.; Chuang, Y.W.; Li, C.Y.; Chiu, C.W.; Lin, C.L.; Liang, W.M.; Chou, T.C.; et al. Intensive Periodontal Treatment Reduces Risks of Hospitalization for Cardiovascular Disease and All-Cause Mortality in the Hemodialysis Population. J. Clin. Med. 2018, 7, 344. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.M.; Braosi, A.P.; Luczyszyn, S.M.; Olandoski, M.; Kotanko, P.; Craig, R.G.; Trevilatto, P.C.; Pecoits-Filho, R. Association among oral health parameters, periodontitis, and its treatment and mortality in patients undergoing hemodialysis. J. Periodontol. 2014, 85, e169–e178. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.P.; Chiang, C.K.; Peng, Y.S.; Hsu, S.P.; Lin, C.Y.; Lai, C.F.; Hung, K.Y. Relationship between periodontal disease and mortality in patients treated with maintenance hemodialysis. Am. J. Kidney Dis. 2011, 57, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Mikami, R.; Gohda, T.; Gotoh, H.; Aoyama, N.; Matsuura, T.; Kido, D.; Takeda, K.; Izumi, Y.; Sasaki, Y.; et al. Poor oral hygiene and dental caries predict high mortality rate in hemodialysis: A 3-year cohort study. Sci. Rep. 2020, 10, 21872. [Google Scholar] [CrossRef]
- Ricardo, A.C.; Athavale, A.; Chen, J.; Hampole, H.; Garside, D.; Marucha, P.; Lash, J.P. Periodontal disease, chronic kidney disease and mortality: Results from the third National Health and Nutrition Examination Survey. BMC Nephrol. 2015, 16, 97. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Wilson Jones, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2021, 13, 2534. [Google Scholar] [CrossRef]
- Bastos Jdo, A.; Andrade, L.C.; Ferreira, A.P.; Barroso Ede, A.; Daibert Pde, C.; Barreto, P.L.; Vilela, E.M.; Marcaccini, A.M.; Colugnati, F.A.; Bastos, M.G. Serum levels of vitamin D and chronic periodontitis in patients with chronic kidney disease. Braz. J. Nephrol. 2013, 35, 20–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ausavarungnirun, R.; Wisetsin, S.; Rongkiettechakorn, N.; Chaichalermsak, S.; Udompol, U.; Rattanasompattikul, M. Association of dental and periodontal disease with chronic kidney disease in patients of a single, tertiary care centre in Thailand. BMJ Open 2016, 6, e011836. [Google Scholar] [CrossRef]
- Raimann, J.G.; Levin, N.W.; Craig, R.G.; Sirover, W.; Kotanko, P.; Handelman, G. Is vitamin C intake too low in dialysis patients? Semin. Dial. 2013, 26, 1–5. [Google Scholar] [CrossRef]
- Clase, C.M.; Ki, V.; Holden, R.M. Water-soluble vitamins in people with low glomerular filtration rate or on dialysis: A review. Semin. Dial. 2013, 26, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Vidiri, M.F.; Marrone, G.; Moriconi, E.; Bocedi, A.; Capria, A.; Rovella, V.; Ricci, G.; De Lorenzo, A.; Di Daniele, N. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. 2016, 2, 16026. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Rovella, V.; Marrone, G.; Cattani, G.; Zingaretti, V.; Limongi, D.; D’Agostini, C.; Sorge, R.; Casasco, M.; Di Daniele, N.; et al. Hemodialysis biomarkers: Total advanced glycation end products (AGEs) against oxidized human serum albumin (HSAox). Acta Diabetol. 2019, 56, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, O.; Lappin, S.L. End-Stage Renal Disease; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lacquaniti, A.; Bolignano, D.; Campo, S.; Perrone, C.; Donato, V.; Fazio, M.R.; Buemi, A.; Sturiale, A.; Buemi, M. Malnutrition in the elderly patient on dialysis. Ren. Fail. 2009, 31, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.R.; Alvestrand, A.; Danielsson, A.; Divino-Filho, J.C.; Gutierrez, A.; Lindholm, B.; Bergstrom, J. Factors predicting malnutrition in hemodialysis patients: A cross-sectional study. Kidney Int. 1998, 53, 773–782. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sahu, K.M. Nutrition in dialysis patients. J. Indian Med. Assoc. 2001, 99, 206–208, 210–211, 213. [Google Scholar]
- Stenvinkel, P.; Heimburger, O.; Lindholm, B.; Kaysen, G.A.; Bergstrom, J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol. Dial. Transplant. 2000, 15, 953–960. [Google Scholar] [CrossRef]
- Bossola, M.; Muscaritoli, M.; Tazza, L.; Panocchia, N.; Liberatori, M.; Giungi, S.; Tortorelli, A.; Rossi Fanelli, F.; Luciani, G. Variables associated with reduced dietary intake in hemodialysis patients. J. Ren. Nutr. 2005, 15, 244–252. [Google Scholar] [CrossRef]
- Di Daniele, N.; Di Renzo, L.; Noce, A.; Iacopino, L.; Ferraro, P.M.; Rizzo, M.; Sarlo, F.; Domino, E.; De Lorenzo, A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014, 27, 529–536. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Kalantar-Zadeh, K. Kidney cachexia or protein-energy wasting in chronic kidney disease: Facts and numbers. J. Cachexia Sarcopenia Muscle 2019, 10, 479–484. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef]
- Anderstam, B.; Mamoun, A.H.; Sodersten, P.; Bergstrom, J. Middle-sized molecule fractions isolated from uremic ultrafiltrate and normal urine inhibit ingestive behavior in the rat. J. Am. Soc. Nephrol. 1996, 7, 2453–2460. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, B.; Lange, M.; Sulowicz, W.; Gafter, U.; Lai, K.N.; Wiedemann, J.; Christiansen, J.S.; El Nahas, M.; Group, A.S. Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J. Am. Soc. Nephrol. 2007, 18, 2161–2171. [Google Scholar] [CrossRef]
- Stenvinkel, P. Can treating persistent inflammation limit protein energy wasting? Semin. Dial. 2013, 26, 16–19. [Google Scholar] [CrossRef]
- Rocco, M.V.; Paranandi, L.; Burrowes, J.D.; Cockram, D.B.; Dwyer, J.T.; Kusek, J.W.; Leung, J.; Makoff, R.; Maroni, B.; Poole, D. Nutritional status in the HEMO Study cohort at baseline. Am. J. Kidney Dis. 2002, 39, 245–256. [Google Scholar] [CrossRef]
- Mitch, W.E.; Medina, R.; Grieber, S.; May, R.C.; England, B.K.; Price, S.R.; Bailey, J.L.; Goldberg, A.L. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J. Clin. Investig. 1994, 93, 2127–2133. [Google Scholar] [CrossRef]
- Bailey, J.L.; Wang, X.; England, B.K.; Price, S.R.; Ding, X.; Mitch, W.E. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J. Clin. Investig. 1996, 97, 1447–1453. [Google Scholar] [CrossRef]
- Stenvinkel, P. Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Purif. 2001, 19, 53–61. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Moio, M.R.; Fois, A.; Sofronie, A.; Gendrot, L.; Cabiddu, G.; D’Alessandro, C.; Cupisti, A. The Diet and Haemodialysis Dyad: Three Eras, Four Open Questions and Four Paradoxes. A Narrative Review, Towards a Personalized, Patient-Centered Approach. Nutrients 2017, 9, 372. [Google Scholar] [CrossRef]
- Maraj, M.; Kusnierz-Cabala, B.; Dumnicka, P.; Gala-Bladzinska, A.; Gawlik, K.; Pawlica-Gosiewska, D.; Zabek-Adamska, A.; Mazur-Laskowska, M.; Ceranowicz, P.; Kuzniewski, M. Malnutrition, Inflammation, Atherosclerosis Syndrome (MIA) and Diet Recommendations among End-Stage Renal Disease Patients Treated with Maintenance Hemodialysis. Nutrients 2018, 10, 69. [Google Scholar] [CrossRef]
- Noce, A.; Romani, A.; Bernini, R. Dietary Intake and Chronic Disease Prevention. Nutrients 2021, 13, 1358. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Metabolic Acidosis of CKD: An Update. Am. J. Kidney Dis. 2016, 67, 307–317. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Mehrotra, R.; Fouque, D.; Kopple, J.D. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin. Dial. 2004, 17, 455–465. [Google Scholar] [CrossRef]
- Khoueiry, G.; Waked, A.; Goldman, M.; El-Charabaty, E.; Dunne, E.; Smith, M.; Kleiner, M.; Lafferty, J.; Kalantar-Zadeh, K.; El-Sayegh, S. Dietary intake in hemodialysis patients does not reflect a heart healthy diet. J. Ren. Nutr. 2011, 21, 438–447. [Google Scholar] [CrossRef]
- Di Daniele, N.; Condo, S.; Ferrannini, M.; Bertoli, M.; Rovella, V.; Di Renzo, L.; De Lorenzo, A. Brown tumour in a patient with secondary hyperparathyroidism resistant to medical therapy: Case report on successful treatment after subtotal parathyroidectomy. Int. J. Endocrinol. 2009, 2009, 827652. [Google Scholar] [CrossRef]
- Noce, A.; Canale, M.P.; Capria, A.; Rovella, V.; Tesauro, M.; Splendiani, G.; Annicchiarico-Petruzzelli, M.; Manzuoli, M.; Simonetti, G.; Di Daniele, N. Coronary artery calcifications predict long term cardiovascular events in non diabetic Caucasian hemodialysis patients. Aging 2015, 7, 269–279. [Google Scholar] [CrossRef]
- Schlieper, G.; Aretz, A.; Verberckmoes, S.C.; Kruger, T.; Behets, G.J.; Ghadimi, R.; Weirich, T.E.; Rohrmann, D.; Langer, S.; Tordoir, J.H.; et al. Ultrastructural analysis of vascular calcifications in uremia. J. Am. Soc. Nephrol. 2010, 21, 689–696. [Google Scholar] [CrossRef]
- Moe, S.M.; Chen, N.X. Pathophysiology of vascular calcification in chronic kidney disease. Circ. Res. 2004, 95, 560–567. [Google Scholar] [CrossRef]
- Choi, S.R.; Lee, Y.K.; Cho, A.J.; Park, H.C.; Han, C.H.; Choi, M.J.; Koo, J.R.; Yoon, J.W.; Noh, J.W. Malnutrition, inflammation, progression of vascular calcification and survival: Inter-relationships in hemodialysis patients. PLoS ONE 2019, 14, e0216415. [Google Scholar] [CrossRef]
- Raj, D.S.; Sun, Y.; Tzamaloukas, A.H. Hypercatabolism in dialysis patients. Curr. Opin. Nephrol. Hypertens. 2008, 17, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Flakoll, P.J.; Parker, R.A.; Hakim, R.M. Amino acid and albumin losses during hemodialysis. Kidney Int. 1994, 46, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.S.; Oladipo, A.; Lim, V.S. Amino Acid and protein kinetics in renal failure: An integrated approach. Semin. Nephrol. 2006, 26, 158–166. [Google Scholar] [CrossRef]
- Pieroni, L.; Levi Mortera, S.; Greco, V.; Sirolli, V.; Ronci, M.; Felaco, P.; Fucci, G.; De Fulviis, S.; Massoud, R.; Condo, S.; et al. Biocompatibility assessment of haemodialysis membrane materials by proteomic investigations. Mol. Biosyst. 2015, 11, 1633–1643. [Google Scholar] [CrossRef]
- Jofre, R.; Rodriguez-Benitez, P.; Lopez-Gomez, J.M.; Perez-Garcia, R. Inflammatory syndrome in patients on hemodialysis. J. Am. Soc. Nephrol. 2006, 17, S274–S280. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Nordfors, L.; Heimburger, O.; Lindholm, B.; Anderstam, B.; Marchlewska, A.; Stenvinkel, P. Soluble leptin receptors and serum leptin in end-stage renal disease: Relationship with inflammation and body composition. Eur. J. Clin. Investig. 2002, 32, 811–817. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Tortorici, A.R.; Chen, J.L.; Kamgar, M.; Lau, W.L.; Moradi, H.; Rhee, C.M.; Streja, E.; Kovesdy, C.P. Dietary restrictions in dialysis patients: Is there anything left to eat? Semin. Dial. 2015, 28, 159–168. [Google Scholar] [CrossRef]
- Tediosi, F.; Bertolini, G.; Parazzini, F.; Mecca, G.; Garattini, L. Cost analysis of dialysis modalities in Italy. Health Serv. Manag. Res. 2001, 14, 9–17. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Nutrition in CKD Guideline Work Group. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Narasaki, Y.; Okuda, Y.; Kalantar, S.S.; You, A.S.; Novoa, A.; Nguyen, T.; Streja, E.; Nakata, T.; Colman, S.; Kalantar-Zadeh, K.; et al. Dietary Potassium Intake and Mortality in a Prospective Hemodialysis Cohort. J. Ren. Nutr. 2021, 31, 411–420. [Google Scholar] [CrossRef]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Cupisti, A.; Santoro, A.; Cozzolino, M. Phosphate balance in ESRD: Diet, dialysis and binders against the low evident masked pool. J. Nephrol. 2015, 28, 415–429. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.C. The fallacy of the calcium-phosphorus product. Kidney Int. 2007, 72, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Taskapan, H.; Esbaei, K.; Jassal, S.V.; Bargman, J.M.; Oreopoulos, D.G. K/DOQI guideline requirements for calcium, phosphate, calcium phosphate product, and parathyroid hormone control in dialysis patients: Can we achieve them? Int. Urol. Nephrol. 2006, 38, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Kuroo, M. New Developments in CKD-MBD. Why is phosphate overload harmful? Clin. Calcium 2014, 24, 1785–1792. [Google Scholar]
- Christopoulou, E.C.; Filippatos, T.D.; Megapanou, E.; Elisaf, M.S.; Liamis, G. Phosphate imbalance in patients with heart failure. Heart Fail. Rev. 2017, 22, 349–356. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Piccoli, G.B.; Cupisti, A. The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015, 16, 9. [Google Scholar] [CrossRef]
- Noori, N.; Sims, J.J.; Kopple, J.D.; Shah, A.; Colman, S.; Shinaberger, C.S.; Bross, R.; Mehrotra, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. Organic and inorganic dietary phosphorus and its management in chronic kidney disease. Iran. J. Kidney Dis. 2010, 4, 89–100. [Google Scholar]
- Jones, W.L. Demineralization of a wide variety of foods for the renal patient. J. Ren. Nutr. 2001, 11, 90–96. [Google Scholar] [CrossRef]
- Cobb, M.; Pacitti, D. The Importance of Sodium Restrictions in Chronic Kidney Disease. J. Ren. Nutr. 2018, 28, e37–e40. [Google Scholar] [CrossRef]
- Smyth, A.; O’Donnell, M.J.; Yusuf, S.; Clase, C.M.; Teo, K.K.; Canavan, M.; Reddan, D.N.; Mann, J.F. Sodium intake and renal outcomes: A systematic review. Am. J. Hypertens. 2014, 27, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, S.; Provenzano, M.; Gagliardi, I.; Michael, A.; Liberti, M.E.; De Nicola, L.; Conte, G.; Garofalo, C.; Andreucci, M. Sodium Intake and Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 4744. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.; Lopes, G.B.; Cunha, T.O.; Protasio, B.M.; Pisoni, R.L.; James, S.A.; Lopes, A.A. Coping with fluid restriction and the quality of life in hemodialysis patients with very low or no daily urine output. Int. J. Artif. Organs 2014, 37, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Grantham, J.J.; Wetmore, J.B. The medicinal use of water in renal disease. Kidney Int. 2013, 84, 45–53. [Google Scholar] [CrossRef]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef]
- Mitch, W.E. Malnutrition: A frequent misdiagnosis for hemodialysis patients. J. Clin. Investig. 2002, 110, 437–439. [Google Scholar] [CrossRef]
- Cupisti, A.; D’Alessandro, C.; Valeri, A.; Capitanini, A.; Meola, M.; Betti, G.; Barsotti, G. Food intake and nutritional status in stable hemodialysis patients. Ren. Fail. 2010, 32, 47–54. [Google Scholar] [CrossRef]
- Fleury, B. Sleep apnea syndrome in the elderly. Sleep 1992, 15, S39–S41. [Google Scholar] [CrossRef][Green Version]
- Kalantar-Zadeh, K.; Kopple, J.D.; Deepak, S.; Block, D.; Block, G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J. Ren. Nutr. 2002, 12, 17–31. [Google Scholar] [CrossRef]
- Sullivan, J.F.; Eisenstein, A.B. Ascorbic acid depletion during hemodialysis. JAMA 1972, 220, 1697–1699. [Google Scholar] [CrossRef]
- Said, H.M.; Vaziri, N.D.; Oveisi, F.; Hussienzadha, S. Effect of chronic renal failure on intestinal transport of biotin in the rat. J. Lab. Clin. Med. 1992, 120, 471–475. [Google Scholar] [PubMed]
- Khamiseh, G.; Vaziri, N.D.; Oveisi, F.; Ahmadnia, M.R.; Ahmadnia, L. Vitamin D absorption, plasma concentration and urinary excretion of 25-hydroxyvitamin D in nephrotic syndrome. Proc. Soc. Exp. Biol. Med. 1991, 196, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 5, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Yadav, N.R.; Banik, K.B. Ascorbic Acid-mediated Reactions in Organic Synthesis. Curr. Organocatal. 2020, 7, 212–241. [Google Scholar] [CrossRef]
- Valdes, F. Vitamin C. Actas Dermo-Sifiliográficas 2006, 97, 557–568. [Google Scholar] [CrossRef]
- Gossweiler, A.G.; Martinez-Mier, E.A. Chapter 6: Vitamins and Oral Health. Monogr. Oral Sci. 2020, 28, 59–67. [Google Scholar] [CrossRef]
- Kazmierczak-Baranska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Moores, J. Vitamin C: A wound healing perspective. Br. J. Community Nurs. 2013, 18 (Suppl. S12), S6–S11. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Yoshida-Hiroi, M.; Sotiriou, S.; Levine, M.; Hartwig, H.G.; Nussbaum, R.L.; Eisenhofer, G. Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2). FASEB J. 2003, 17, 1–13. [Google Scholar] [CrossRef]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care 2015, 19, 418. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R., III. Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 95, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Ginter, E. Cholesterol: Vitamin C controls its transformation to bile acids. Science 1973, 179, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Szarka, A.; Lorincz, T. The role of ascorbate in protein folding. Protoplasma 2014, 251, 489–497. [Google Scholar] [CrossRef]
- Liu, D.; Gu, Y.; Pang, Q.; Han, Q.; Li, A.; Wu, W.; Zhang, X.; Shi, Q.; Zhu, L.; Yu, H.; et al. Vitamin C inhibits lipid deposition through GSK-3beta/mTOR signaling in the liver of zebrafish. Fish Physiol. Biochem. 2020, 46, 383–394. [Google Scholar] [CrossRef]
- Lynch, S.R.; Cook, J.D. Interaction of vitamin C and iron. Ann. N. Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef]
- Sharma, S.C.; Wilson, C.W. The celluar interaction of ascorbic acid with histamine, cyclic nucleotides and prostaglandins in the immediate hypersensitivity reaction. Int. J. Vitam. Nutr. Res. 1980, 50, 163–170. [Google Scholar]
- Sonni, F.; Clark, A.C.; Prenzler, P.D.; Riponi, C.; Scollary, G.R. Antioxidant action of glutathione and the ascorbic acid/glutathione pair in a model white wine. J. Agric. Food Chem. 2011, 59, 3940–3949. [Google Scholar] [CrossRef]
- Harris, S.S.; D’Ercole, A.J. Neonatal hyperparathyroidism: The natural course in the absence of surgical intervention. Pediatrics 1989, 83, 53–56. [Google Scholar] [CrossRef]
- Kittisakmontri, K.; Swangtrakul, N.; Padungmaneesub, W.; Charoenkwan, P. Gingival Bleeding and Bloody Dialysate: A Case Report of Scurvy in a Child With End-Stage Renal Disease Receiving Peritoneal Dialysis. J. Ren. Nutr. 2016, 26, 407–411. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, J.; Cheng, X.; Bai, W.; Guo, W.; Wu, L.; Zuo, L. Association between vitamin C deficiency and dialysis modalities. Nephrology 2012, 17, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Chaghouri, P.; Maalouf, N.; Peters, S.L.; Nowak, P.J.; Peczek, K.; Zasowska-Nowak, A.; Nowicki, M. Two Faces of Vitamin C in Hemodialysis Patients: Relation to Oxidative Stress and Inflammation. Nutrients 2021, 13, 791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, L.; Cheng, X.; Dong, J.; Geng, Q.; Zuo, L. Low levels of vitamin C in dialysis patients is associated with decreased prealbumin and increased C-reactive protein. BMC Nephrol. 2011, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Panatta, E.; Lena, A.; Castiglia, D.; Di Daniele, N.; Melino, G.; Candi, E. FOXM1 regulates proliferation, senescence and oxidative stress in keratinocytes and cancer cells. Aging 2016, 8, 1384–1397. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Granata, S.; Zaza, G.; Simone, S.; Villani, G.; Latorre, D.; Pontrelli, P.; Carella, M.; Schena, F.P.; Grandaliano, G.; Pertosa, G. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genom. 2009, 10, 388. [Google Scholar] [CrossRef]
- Coaccioli, S.; Standoli, M.L.; Biondi, R.; Panaccione, A.; Landucci, P.; Del Giorno, R.; Paladini, A.; Standoli, M.; Puxeddu, A. Open comparison study of oxidative stress markers between patients with chronic renal failure in conservative therapy and patients in haemodialysis. Clin. Ter. 2010, 161, 435–439. [Google Scholar]
- Varan, H.I.; Dursun, B.; Dursun, E.; Ozben, T.; Suleymanlar, G. Acute effects of hemodialysis on oxidative stress parameters in chronic uremic patients: Comparison of two dialysis membranes. Int. J. Nephrol. Renov. Dis. 2010, 3, 39–45. [Google Scholar] [CrossRef]
- Horl, W.H.; Steinhauer, H.B.; Schollmeyer, P. Plasma levels of granulocyte elastase during hemodialysis: Effects of different dialyzer membranes. Kidney Int. 1985, 28, 791–796. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Shin, M.S.; Kim, E.J.; Ahn, Y.B.; Kim, H.D. The association of dietary vitamin C intake with periodontitis among Korean adults: Results from KNHANES. PLoS ONE 2017, 12, e0177074. [Google Scholar] [CrossRef] [PubMed]
- Amarasena, N.; Ogawa, H.; Yoshihara, A.; Hanada, N.; Miyazaki, H. Serum vitamin C-periodontal relationship in community-dwelling elderly Japanese. J. Clin. Periodontol. 2005, 32, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Magiorkinis, E.; Beloukas, A.; Diamantis, A. Scurvy: Past, present and future. Eur. J. Intern. Med. 2011, 22, 147–152. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006, 50, 85–94. [Google Scholar] [CrossRef]
- Kuzmanova, D.; Jansen, I.D.; Schoenmaker, T.; Nazmi, K.; Teeuw, W.J.; Bizzarro, S.; Loos, B.G.; van der Velden, U. Vitamin C in plasma and leucocytes in relation to periodontitis. J. Clin. Periodontol. 2012, 39, 905–912. [Google Scholar] [CrossRef]
- Bourne, G.H. The effect of vitamin C on the healing of wounds. Proc. Nutr. Soc. 1946, 4, 204–210. [Google Scholar] [CrossRef]
- Esteves, A.; Teixeira da Silva, F.; Carvalho, J.; Felgueiras, P.; Laranjeira, P. Scurvy, Starvation, and Flea Infestation—A Case Report From 21st Century Europe. Cureus 2021, 13, e13158. [Google Scholar] [CrossRef]
- Basilicata, M.; Di Lauro, M.; Campolattano, V.; Marrone, G.; Celotto, R.; Mitterhofer, A.P.; Bollero, P.; Di Daniele, N.; Noce, A. Natural Bioactive Compounds in the Management of Oral Diseases in Nephropathic Patients. Int. J. Environ. Res. Public Health 2022, 19, 1665. [Google Scholar] [CrossRef]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zeng, W.; Song, S.; Zhang, F.; He, W.; Liang, W.; Niu, Z. Vitamin C induces periodontal ligament progenitor cell differentiation via activation of ERK pathway mediated by PELP1. Protein Cell 2013, 4, 620–627. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staudte, H.; Sigusch, B.W.; Glockmann, E. Grapefruit consumption improves vitamin C status in periodontitis patients. Br. Dent. J. 2005, 199, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Blignaut, J.B.; Grobler, S.R. High fruit consumption and the periodontal status of farm workers. Clin. Prev. Dent. 1992, 14, 25–28. [Google Scholar] [PubMed]
- Yussif, N.M.; Abdul Aziz, M.A.; Abdel Rahman, A.R. Evaluation of the Anti-Inflammatory Effect of Locally Delivered Vitamin C in the Treatment of Persistent Gingival Inflammation: Clinical and Histopathological Study. J. Nutr. Metab. 2016, 2016, 2978741. [Google Scholar] [CrossRef]
- Raghavendra, U.; Rao, A.; Kashyap, S.; Souza, J.; Kumar, V.; Kalal, B.; Souza, N. Vitamin C supplementation as an adjunct to nonsurgical therapy in the treatment of chronic periodontitis: A clinical and biochemical study. J. Int. Oral Health 2018, 10, 256–261. [Google Scholar] [CrossRef]
- Kulkarni, V.; Bhatavadekar, N.B.; Uttamani, J.R. The effect of nutrition on periodontal disease: A systematic review. J. Calif. Dent. Assoc. 2014, 42, 302–311. [Google Scholar]
- Randaccio, L.; Geremia, S.; Demitri, N.; Wuerges, J. Vitamin B12: Unique metalorganic compounds and the most complex vitamins. Molecules 2010, 15, 3228–3259. [Google Scholar] [CrossRef]
- Kang, S.S.; Wong, P.W.; Malinow, M.R. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu. Rev. Nutr. 1992, 12, 279–298. [Google Scholar] [CrossRef]
- Fairfield, K.M.; Fletcher, R.H. Vitamins for chronic disease prevention in adults: Scientific review. JAMA 2002, 287, 3116–3126. [Google Scholar] [CrossRef]
- Fletcher, R.H.; Fairfield, K.M. Vitamins for chronic disease prevention in adults: Clinical applications. JAMA 2002, 287, 3127–3129. [Google Scholar] [CrossRef] [PubMed]
- Soohoo, M.; Ahmadi, S.F.; Qader, H.; Streja, E.; Obi, Y.; Moradi, H.; Rhee, C.M.; Kim, T.H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Association of serum vitamin B12 and folate with mortality in incident hemodialysis patients. Nephrol. Dial. Transplant. 2017, 32, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Dandge, V.A.; Variya, D. Study of vitamin B12 deficiency in chronic kidney disease. Int. J. Adv. Med. 2020, 7, 303–307. [Google Scholar] [CrossRef][Green Version]
- Noce, A.; Tarantino, A.; Tsague Djoutsop, C.; Vasili, E.; De Lorenzo, A.; Di Daniele, N. Gut Microbioma Population: An Indicator Really Sensible to Any Change in Age, Diet, Metabolic Syndrome, and Life-Style. Mediat. Inflamm. 2014, 2014, e901308. [Google Scholar]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef]
- Al Mutairi, F. Hyperhomocysteinemia: Clinical Insights. J. Cent. Nerv. Syst. Dis. 2020, 12. [Google Scholar] [CrossRef]
- Dessi, M.; Di Giovamberardino, G.; Pieri, M.; Noce, A.; Zenobi, R.; Di Daniele, N.; Pastore, A. Influence of dialysis techniques and alternate vitamin supplementation on homocysteine levels in patients with known MTHFR genotypes. Clin. Exp. Nephrol. 2015, 19, 140–145. [Google Scholar] [CrossRef]
- Pastore, A.; Noce, A.; Di Giovamberardino, G.; De Stefano, A.; Calla, C.; Zenobi, R.; Dessi, M.; Di Daniele, N. Homocysteine, cysteine, folate and vitamin B(1)(2) status in type 2 diabetic patients with chronic kidney disease. J. Nephrol. 2015, 28, 571–576. [Google Scholar] [CrossRef]
- Pastore, A.; De Angelis, S.; Casciani, S.; Ruggia, R.; Di Giovamberardino, G.; Noce, A.; Splendiani, G.; Cortese, C.; Federici, G.; Dessi, M. Effects of folic acid before and after vitamin B12 on plasma homocysteine concentrations in hemodialysis patients with known MTHFR genotypes. Clin. Chem. 2006, 52, 145–148. [Google Scholar] [CrossRef]
- Capelli, I.; Cianciolo, G.; Gasperoni, L.; Zappulo, F.; Tondolo, F.; Cappuccilli, M.; La Manna, G. Folic Acid and Vitamin B12 Administration in CKD. Why Not? Nutrients 2019, 11, 383. [Google Scholar] [CrossRef]
- Langan, R.C.; Zawistoski, K.J. Update on vitamin B12 deficiency. Am. Fam. Physician 2011, 83, 1425–1430. [Google Scholar] [PubMed]
- Vogel, R.I.; Fink, R.A.; Schneider, L.C.; Frank, O.; Baker, H. The effect of folic acid on gingival health. J. Periodontol. 1976, 47, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Polizzi, A.; Palazzo, G.; Indelicato, F.; Isola, G. Dietary Factors Affecting the Prevalence and Impact of Periodontal Disease. Clin. Cosmet. Investig. Dent. 2021, 13, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Keceli, H.G.; Ercan, N.; Karsiyaka Hendek, M.; Kisa, U.; Mesut, B.; Olgun, E. The effect of the systemic folic acid intake as an adjunct to scaling and root planing on clinical parameters and homocysteine and C-reactive protein levels in gingival crevicular fluid of periodontitis patients: A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2020, 47, 602–613. [Google Scholar] [CrossRef]
- Esaki, M.; Morita, M.; Akhter, R.; Akino, K.; Honda, O. Relationship between folic acid intake and gingival health in non-smoking adults in Japan. Oral Dis. 2010, 16, 96–101. [Google Scholar] [CrossRef]
- Yu, Y.H.; Kuo, H.K.; Lai, Y.L. The association between serum folate levels and periodontal disease in older adults: Data from the National Health and Nutrition Examination Survey 2001/02. J. Am. Geriatr. Soc. 2007, 55, 108–113. [Google Scholar] [CrossRef]
- LARN, Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana. Available online: https://sinu.it/2019/07/09/vitamine-fabbisogno-medio-ar/ (accessed on 5 April 2022).
- Zong, G.; Holtfreter, B.; Scott, A.E.; Volzke, H.; Petersmann, A.; Dietrich, T.; Newson, R.S.; Kocher, T. Serum vitamin B12 is inversely associated with periodontal progression and risk of tooth loss: A prospective cohort study. J. Clin. Periodontol. 2016, 43, 2–9. [Google Scholar] [CrossRef]
- Bashutski, J.D.; Eber, R.M.; Kinney, J.S.; Benavides, E.; Maitra, S.; Braun, T.M.; Giannobile, W.V.; McCauley, L.K. The impact of vitamin D status on periodontal surgery outcomes. J. Dent. Res. 2011, 90, 1007–1012. [Google Scholar] [CrossRef]
- Neiva, R.F.; Al-Shammari, K.; Nociti, F.H., Jr.; Soehren, S.; Wang, H.L. Effects of vitamin-B complex supplementation on periodontal wound healing. J. Periodontol. 2005, 76, 1084–1091. [Google Scholar] [CrossRef]
- Barroso, A.B.; Lima, V.; Guzzo, G.C.; Moraes, R.A.; Vasconcellos, M.C.; Bezerra, M.M.; Viana, F.A.; Bezerra, R.C.; Santana, G.S.; Frota-Bezerra, F.A.; et al. Efficacy and safety of combined piroxicam, dexamethasone, orphenadrine, and cyanocobalamin treatment in mandibular molar surgery. Braz. J. Med. Biol. Res. 2006, 39, 1241–1247. [Google Scholar] [CrossRef]
- Dessi, M.; Noce, A.; Bertucci, P.; Noce, G.; Rizza, S.; De Stefano, A.; Manca di Villahermosa, S.; Bernardini, S.; De Lorenzo, A.; Di Daniele, N. Plasma and erythrocyte membrane phospholipids and fatty acids in Italian general population and hemodialysis patients. Lipids Health Dis. 2014, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, H.; Aboelsaad, N.; Eliwa, M.; Darweesh, M.; Alshahat, M.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin. J. Periodontol. 2010, 81, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Elkhouli, A.M. The efficacy of host response modulation therapy (omega-3 plus low-dose aspirin) as an adjunctive treatment of chronic periodontitis (clinical and biochemical study). J. Periodontal Res. 2011, 46, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Lagana, A.S.; Rapisarda, A.M.; La Ferrera, G.M.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan Diet Health Benefits in Metabolic Syndrome. Nutrients 2021, 13, 817. [Google Scholar] [CrossRef]
- Varela-Lopez, A.; Navarro-Hortal, M.D.; Giampieri, F.; Bullon, P.; Battino, M.; Quiles, J.L. Nutraceuticals in Periodontal Health: A Systematic Review on the Role of Vitamins in Periodontal Health Maintenance. Molecules 2018, 23, 1226. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Ishimura, E.; Nishizawa, Y.; Inaba, M.; Matsumoto, N.; Emoto, M.; Kawagishi, T.; Shoji, S.; Okuno, S.; Kim, M.; Miki, T.; et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999, 55, 1019–1027. [Google Scholar] [CrossRef]
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahakar, D. Dietary antioxidants and their indispensable role in periodontal health. J. Food Drug Anal. 2016, 24, 239–246. [Google Scholar] [CrossRef]
- Rahman, N.; Walls, A. Chapter 12: Nutrient Deficiencies and Oral Health. Monogr. Oral Sci. 2020, 28, 114–124. [Google Scholar] [CrossRef]
- Martinon, P.; Fraticelli, L.; Giboreau, A.; Dussart, C.; Bourgeois, D.; Carrouel, F. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J. Clin. Med. 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S39–S51. [Google Scholar] [CrossRef] [PubMed]
- Kosnadi, L.; Rochmanadji, W.; Sumantri, A.G.; Trimulyo, T.; Anwar, M.R. Hypovolemic shock complicating nephrotic syndrome in a child. Paediatr. Indones. 1988, 28, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Dragonas, P.; El-Sioufi, I.; Bobetsis, Y.A.; Madianos, P.N. Association of Vitamin D With Periodontal Disease: A Narrative Review. Oral Health Prev. Dent. 2020, 18, 103–114. [Google Scholar] [CrossRef]
- Pinto, J.; Goergen, J.; Muniz, F.; Haas, A.N. Vitamin D levels and risk for periodontal disease: A systematic review. J. Periodontal Res. 2018, 53, 298–305. [Google Scholar] [CrossRef]
- Peric, M.; Cavalier, E.; Toma, S.; Lasserre, J.F. Serum vitamin D levels and chronic periodontitis in adult, Caucasian population-a systematic review. J. Periodontal Res. 2018, 53, 645–656. [Google Scholar] [CrossRef]
- Machado, V.; Lobo, S.; Proenca, L.; Mendes, J.J.; Botelho, J. Vitamin D and Periodontitis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2177. [Google Scholar] [CrossRef]
- Sinclair, P.R.; Bement, W.J.; Gorman, N.; Liem, H.H.; Wolkoff, A.W.; Muller-Eberhard, U. Effect of serum proteins on haem uptake and metabolism in primary cultures of liver cells. Biochem. J. 1988, 256, 159–165. [Google Scholar] [CrossRef]
- Isola, G.; Alibrandi, A.; Rapisarda, E.; Matarese, G.; Williams, R.C.; Leonardi, R. Association of vitamin D in patients with periodontitis: A cross-sectional study. J. Periodontal Res. 2020, 55, 602–612. [Google Scholar] [CrossRef]
- Dietrich, T.; Joshipura, K.J.; Dawson-Hughes, B.; Bischoff-Ferrari, H.A. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am. J. Clin. Nutr. 2004, 80, 108–113. [Google Scholar] [CrossRef]
- Park, J.; Ryu, S.Y.; Han, M.A.; Choi, S.W. The Association of Vitamin D With Estimated Glomerular Filtration Rate and Albuminuria: 5th Korean National Health and Nutritional Examination Survey 2011-2012. J. Ren. Nutr. 2016, 26, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Satirapoj, B.; Limwannata, P.; Chaiprasert, A.; Supasyndh, O.; Choovichian, P. Vitamin D insufficiency and deficiency with stages of chronic kidney disease in an Asian population. BMC Nephrol. 2013, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Chonchol, M.; Scragg, R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int. 2007, 71, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.; Lopez-Hilker, S.; Lewis-Finch, J.; Grooms, P.; Brown, A.; Martin, K.; Slatopolsky, E. Metabolic clearance rate and production rate of calcitriol in uremia. Kidney Int. 1989, 35, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K. Vitamins: Their Role in the Human Body by GFM Ball. Ann. Clin. Biochem. 2006, 43, 92. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proenca, L.; Delgado, A.S.; Mendes, J.J. Vitamin D Deficiency and Oral Health: A Comprehensive Review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef]
- Hu, X.; Niu, L.; Ma, C.; Huang, Y.; Yang, X.; Shi, Y.; Pan, C.; Liu, J.; Wang, H.; Li, Q.; et al. Calcitriol decreases live Porphyromonas gingivalis internalized into epithelial cells and monocytes by promoting autophagy. J. Periodontol. 2020, 91, 956–966. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Wang, Q. 1,25-dihydroxyvitamin D3 suppresses lipopolysaccharide-induced interleukin-6 production through aryl hydrocarbon receptor/nuclear factor-kappaB signaling in oral epithelial cells. BMC Oral Health 2019, 19, 236. [Google Scholar] [CrossRef]
- Nilsson, B.O. Mechanisms involved in regulation of periodontal ligament cell production of pro-inflammatory cytokines: Implications in periodontitis. J. Periodontal Res. 2021, 56, 249–255. [Google Scholar] [CrossRef]
- Abiko, Y.; Saitoh, M.; Nishimura, M.; Yamazaki, M.; Sawamura, D.; Kaku, T. Role of beta-defensins in oral epithelial health and disease. Med. Mol. Morphol. 2007, 40, 179–184. [Google Scholar] [CrossRef]
- Kapellas, K.; Singh, A.; Bertotti, M.; Nascimento, G.G.; Jamieson, L.M.; Perio-CKD Collaboration. Periodontal and chronic kidney disease association: A systematic review and meta-analysis. Nephrology 2019, 24, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Zehnder, D.; Bland, R.; Stewart, P.M. 1alpha-Hydroxylase and the action of vitamin D. J. Mol. Endocrinol. 2000, 25, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Costantini, E.; Sinjari, B.; Piscopo, F.; Porreca, A.; Reale, M.; Caputi, S.; Murmura, G. Evaluation of Salivary Cytokines and Vitamin D Levels in Periodontopathic Patients. Int. J. Mol. Sci. 2020, 21, 2669. [Google Scholar] [CrossRef] [PubMed]
- Bayirli, B.A.; Ozturk, A.; Avci, B. Serum vitamin D concentration is associated with antimicrobial peptide level in periodontal diseases. Arch. Oral Biol. 2020, 117, 104827. [Google Scholar] [CrossRef] [PubMed]
- Khammissa, R.A.G.; Ballyram, R.; Jadwat, Y.; Fourie, J.; Lemmer, J.; Feller, L. Vitamin D Deficiency as It Relates to Oral Immunity and Chronic Periodontitis. Int. J. Dent. 2018, 2018, 7315797. [Google Scholar] [CrossRef]
- Kaur, K.; Sculley, D.; Wallace, J.; Turner, A.; Ferraris, C.; Veysey, M.; Lucock, M.; Beckett, E. Micronutrients and bioactive compounds in oral inflammatory diseases. J. Nutr. Intermed. Metab. 2019, 18, 100105. [Google Scholar] [CrossRef]
- Meghil, M.M.; Hutchens, L.; Raed, A.; Multani, N.A.; Rajendran, M.; Zhu, H.; Looney, S.; Elashiry, M.; Arce, R.M.; Peacock, M.E.; et al. The influence of vitamin D supplementation on local and systemic inflammatory markers in periodontitis patients: A pilot study. Oral Dis. 2019, 25, 1403–1413. [Google Scholar] [CrossRef]
- Cagetti, M.G.; Wolf, T.G.; Tennert, C.; Camoni, N.; Lingstrom, P.; Campus, G. The Role of Vitamins in Oral Health. A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 938. [Google Scholar] [CrossRef]
- Gao, W.; Tang, H.; Wang, D.; Zhou, X.; Song, Y.; Wang, Z. Effect of short-term vitamin D supplementation after nonsurgical periodontal treatment: A randomized, double-masked, placebo-controlled clinical trial. J. Periodontal Res. 2020, 55, 354–362. [Google Scholar] [CrossRef]
- Raphael, K.L. Metabolic Acidosis and Subclinical Metabolic Acidosis in CKD. J. Am. Soc. Nephrol. 2018, 29, 376–382. [Google Scholar] [CrossRef]
- Van Slyke, D.D.; Linder, G.C.; Hiller, A.; Leiter, L.; McIntosh, J.F. The Excretion of Ammonia and Titratable Acid in Nephritis. J. Clin. Investig. 1926, 2, 255–288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- May, R.C.; Kelly, R.A.; Mitch, W.E. Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J. Clin. Investig. 1987, 79, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Lemann, J., Jr.; Litzow, J.R.; Lennon, E.J. The effects of chronic acid loads in normal man: Further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J. Clin. Investig. 1966, 45, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L.; Zhang, Y.; Ying, J.; Greene, T. Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology 2014, 19, 648–654. [Google Scholar] [CrossRef]
- Kraut, J.A. The role of metabolic acidosis in the pathogenesis of renal osteodystrophy. Adv. Chronic Kidney Dis. 1995, 2, 40–51. [Google Scholar] [CrossRef]
- Krieger, N.S.; Sessler, N.E.; Bushinsky, D.A. Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am. J. Physiol. Ren. Physiol. 1992, 262, F442–F448. [Google Scholar] [CrossRef]
- Pellegrino, E.D.; Biltz, R.M. The composition of human bone in uremia. Observations on the reservoir functions of bone and demonstration of a labile fraction of bone carbonate. Medicine 1965, 44, 397–418. [Google Scholar] [CrossRef]
- Cecoro, G.; Annunziata, M.; Iuorio, M.T.; Nastri, L.; Guida, L. Periodontitis, Low-Grade Inflammation and Systemic Health: A Scoping Review. Medicina 2020, 56, 272. [Google Scholar] [CrossRef]
- Vanni, G.; Pellicciaro, M.; Materazzo, M.; Dauri, M.; D’Angelillo, R.M.; Buonomo, C.; De Majo, A.; Pistolese, C.; Portarena, I.; Mauriello, A.; et al. Awake breast cancer surgery: Strategy in the beginning of COVID-19 emergency. Breast Cancer 2021, 28, 137–144. [Google Scholar] [CrossRef]
- Ebert, T.; Pawelzik, S.C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Back, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Josey, M.J.; Merchant, A.T. Low-grade Systemic Inflammation May Increase the Risk of Periodontitis. J. Evid. Based Dent. Pract. 2016, 16, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Sima, C.; Glogauer, M. Diabetes mellitus and periodontal diseases. Curr. Diabetes Rep. 2013, 13, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Pink, C.; Kocher, T.; Meisel, P.; Dorr, M.; Markus, M.R.; Jablonowski, L.; Grotevendt, A.; Nauck, M.; Holtfreter, B. Longitudinal effects of systemic inflammation markers on periodontitis. J. Clin. Periodontol. 2015, 42, 988–997. [Google Scholar] [CrossRef]
- Hickey, N.A.; Shalamanova, L.; Whitehead, K.A.; Dempsey-Hibbert, N.; van der Gast, C.; Taylor, R.L. Exploring the putative interactions between chronic kidney disease and chronic periodontitis. Crit. Rev. Microbiol. 2020, 46, 61–77. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costacurta, M.; Basilicata, M.; Marrone, G.; Di Lauro, M.; Campolattano, V.; Bollero, P.; Docimo, R.; Di Daniele, N.; Noce, A. The Impact of Chronic Kidney Disease on Nutritional Status and Its Possible Relation with Oral Diseases. Nutrients 2022, 14, 2002. https://doi.org/10.3390/nu14102002
Costacurta M, Basilicata M, Marrone G, Di Lauro M, Campolattano V, Bollero P, Docimo R, Di Daniele N, Noce A. The Impact of Chronic Kidney Disease on Nutritional Status and Its Possible Relation with Oral Diseases. Nutrients. 2022; 14(10):2002. https://doi.org/10.3390/nu14102002
Chicago/Turabian StyleCostacurta, Micaela, Michele Basilicata, Giulia Marrone, Manuela Di Lauro, Vincenzo Campolattano, Patrizio Bollero, Raffaella Docimo, Nicola Di Daniele, and Annalisa Noce. 2022. "The Impact of Chronic Kidney Disease on Nutritional Status and Its Possible Relation with Oral Diseases" Nutrients 14, no. 10: 2002. https://doi.org/10.3390/nu14102002
APA StyleCostacurta, M., Basilicata, M., Marrone, G., Di Lauro, M., Campolattano, V., Bollero, P., Docimo, R., Di Daniele, N., & Noce, A. (2022). The Impact of Chronic Kidney Disease on Nutritional Status and Its Possible Relation with Oral Diseases. Nutrients, 14(10), 2002. https://doi.org/10.3390/nu14102002







