Jasmine Tea Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior in Rats via the Gut-Brain Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Depressive Rat Model
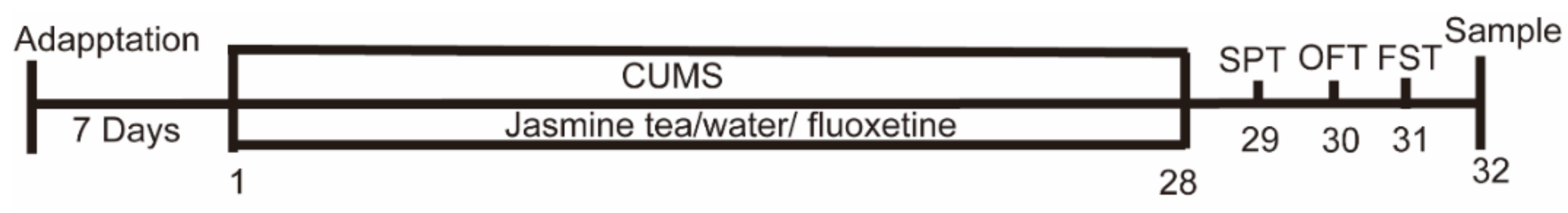
2.3. Experimental Design
2.4. Behavioral Test
2.4.1. SPT (Sugar Preference Test)
2.4.2. OFT (Open Field Test)
2.4.3. FST (Forced Swimming Test)
2.5. Tissue Collection
2.6. Biochemical Analysis
2.7. Histological Analysis and Morphometry
2.8. Intestinal Microbial Diversity Analysis
2.8.1. Illumina MiSeq Sequencing
2.8.2. Processing of Sequencing Data
2.9. Data Analysis
3. Results
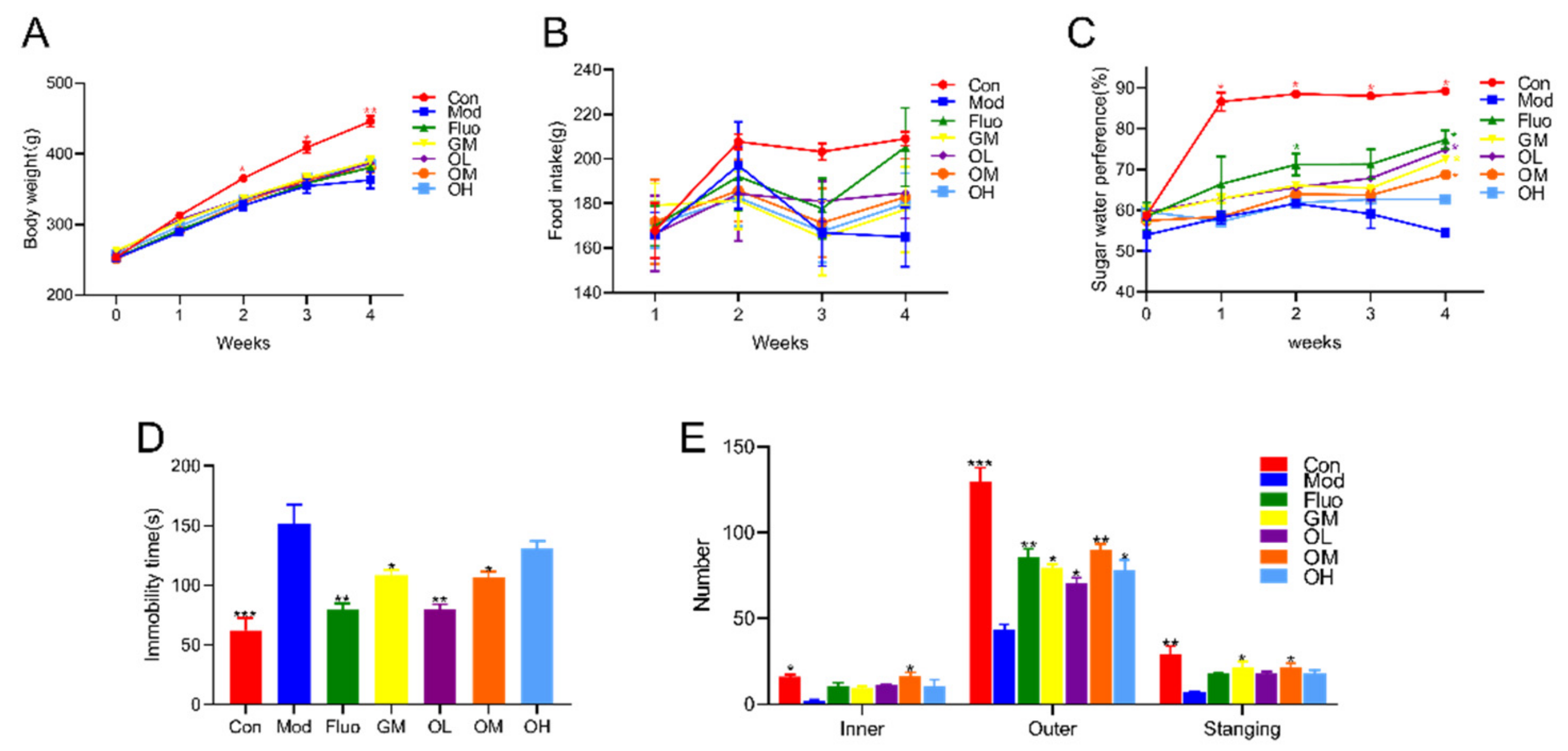
3.1. Jasmine Tea Reduced CUMS-Induced Depression and Ameliorated Depression-Like Behavior
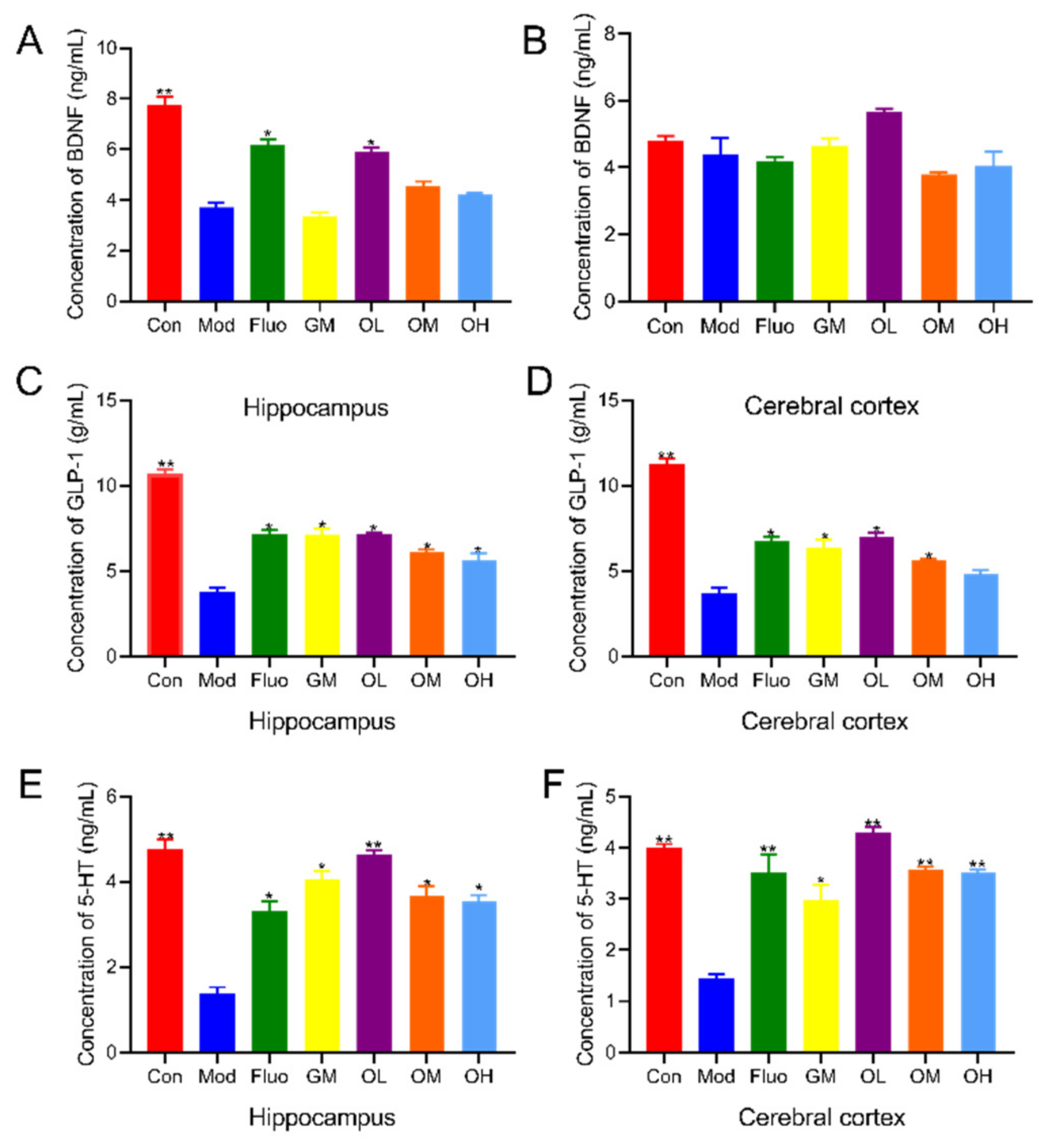
3.2. Jasmine Tea Increased Neurotransmitters in CUMS-Induced Depression
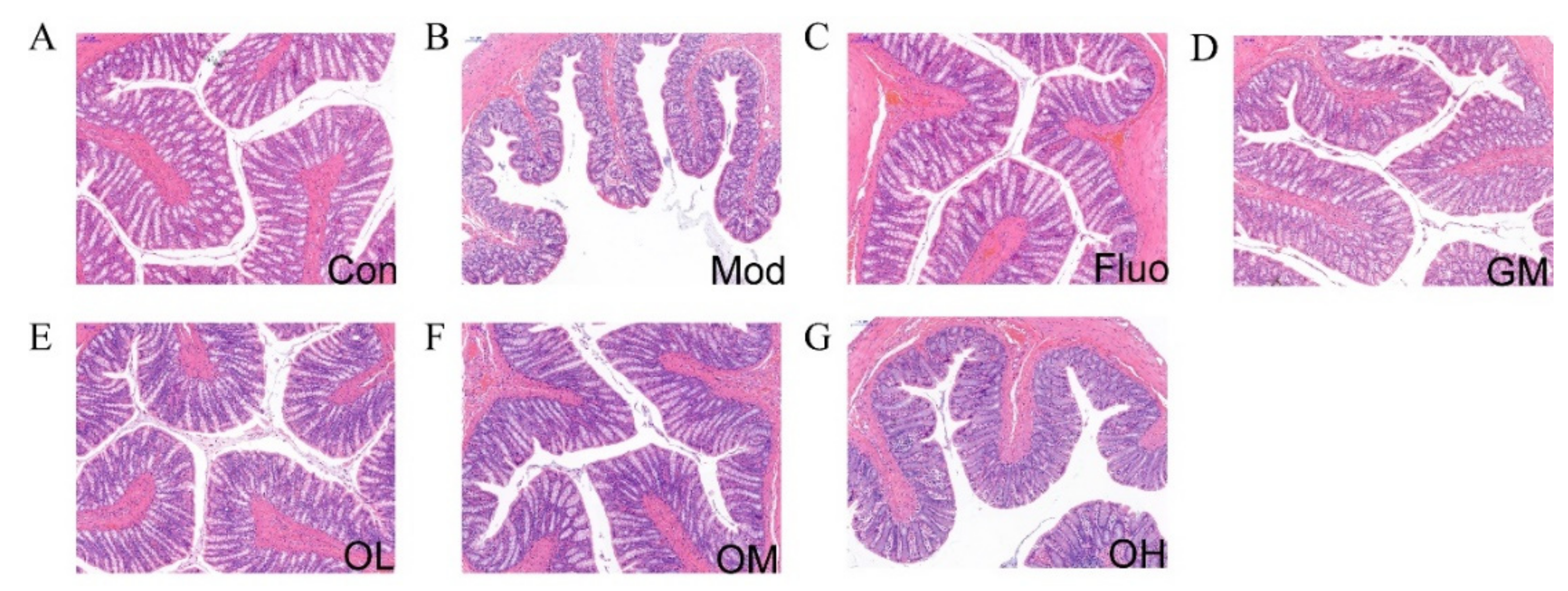
3.3. Jasmine Tea Restored the Structure of Colon in CUMS-Induced Depression
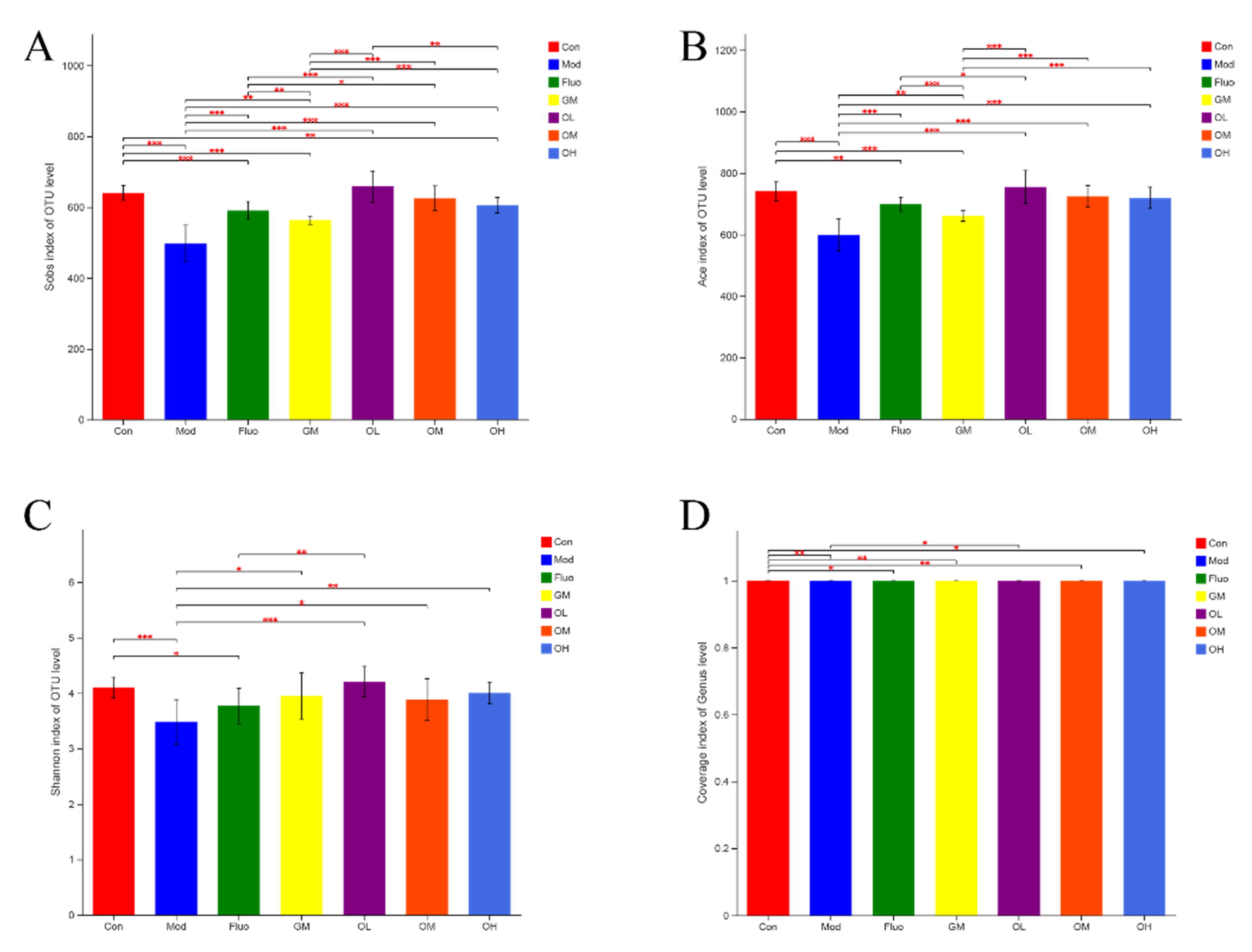
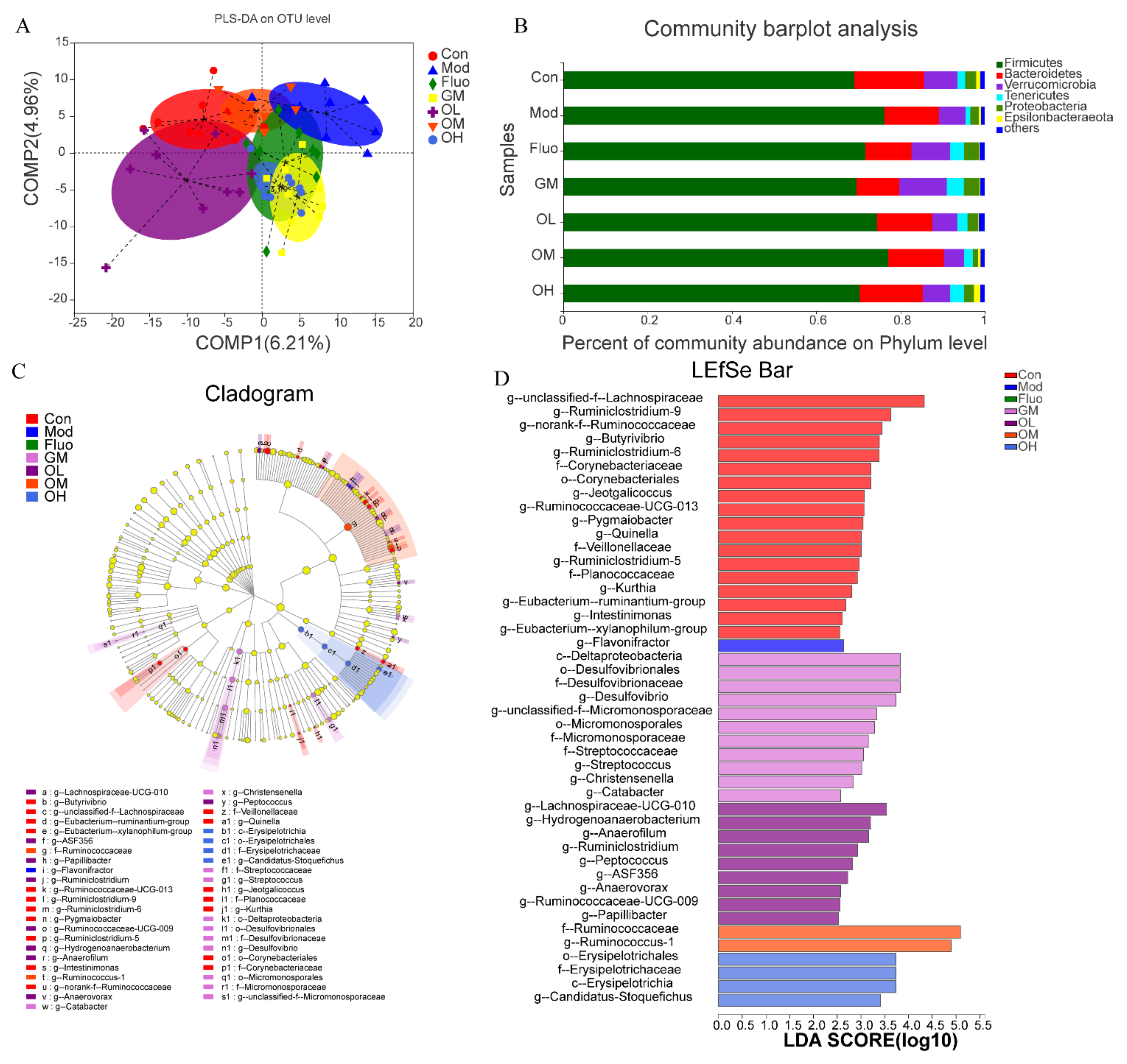
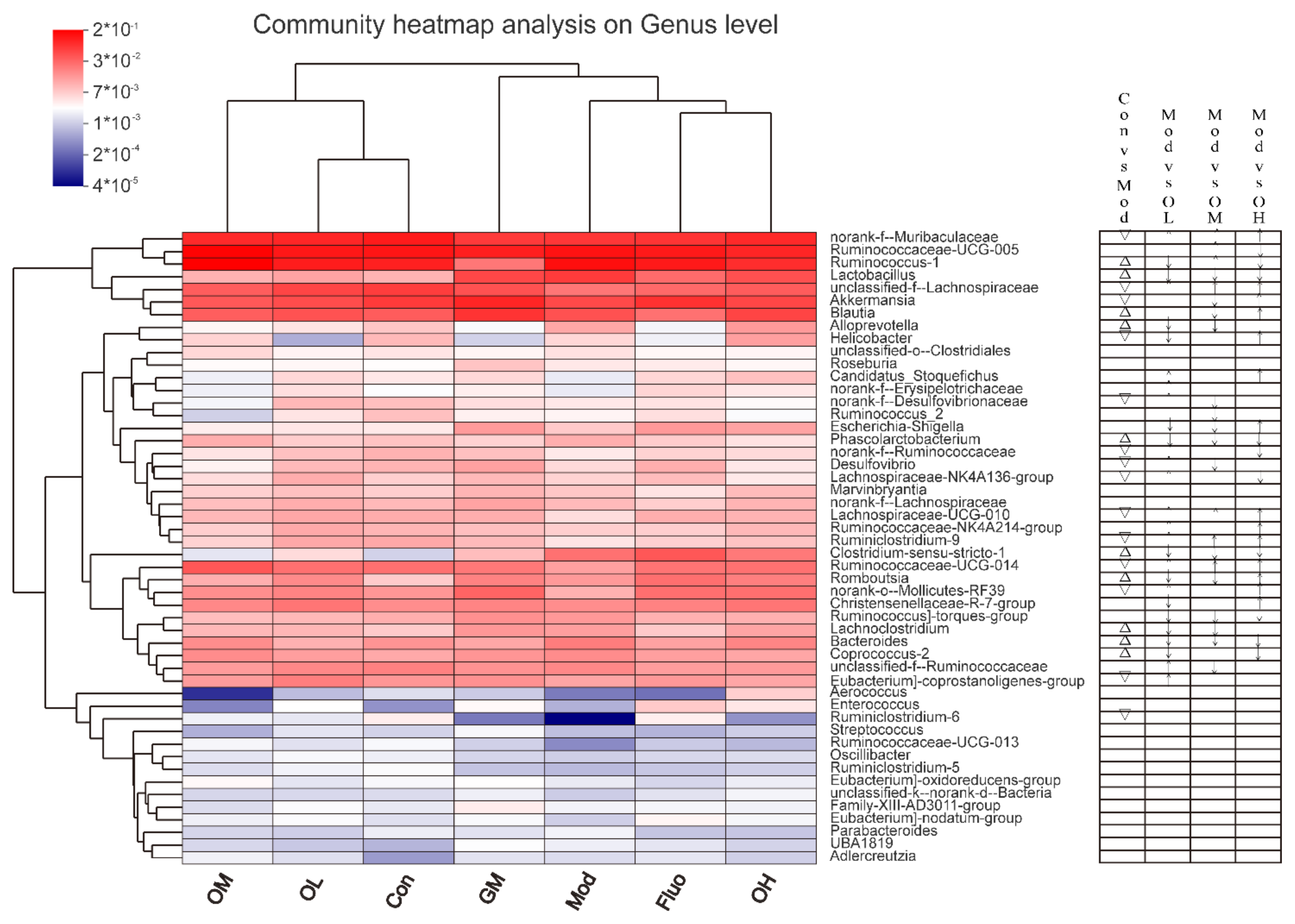
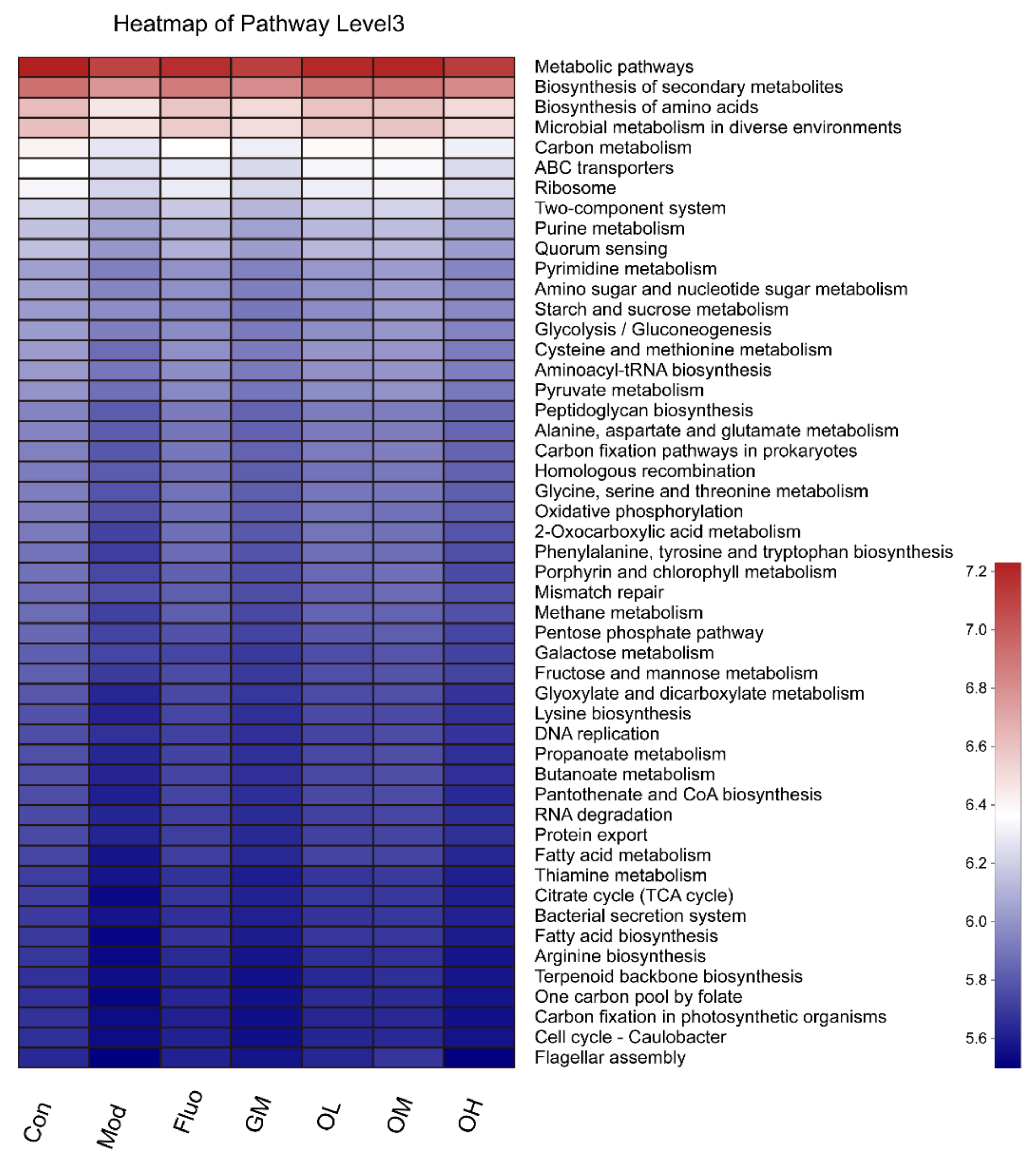
3.4. Jasmine Tea Ameliorated the Gut Microbiota Communities in Rats with CUMS-Induced Depression
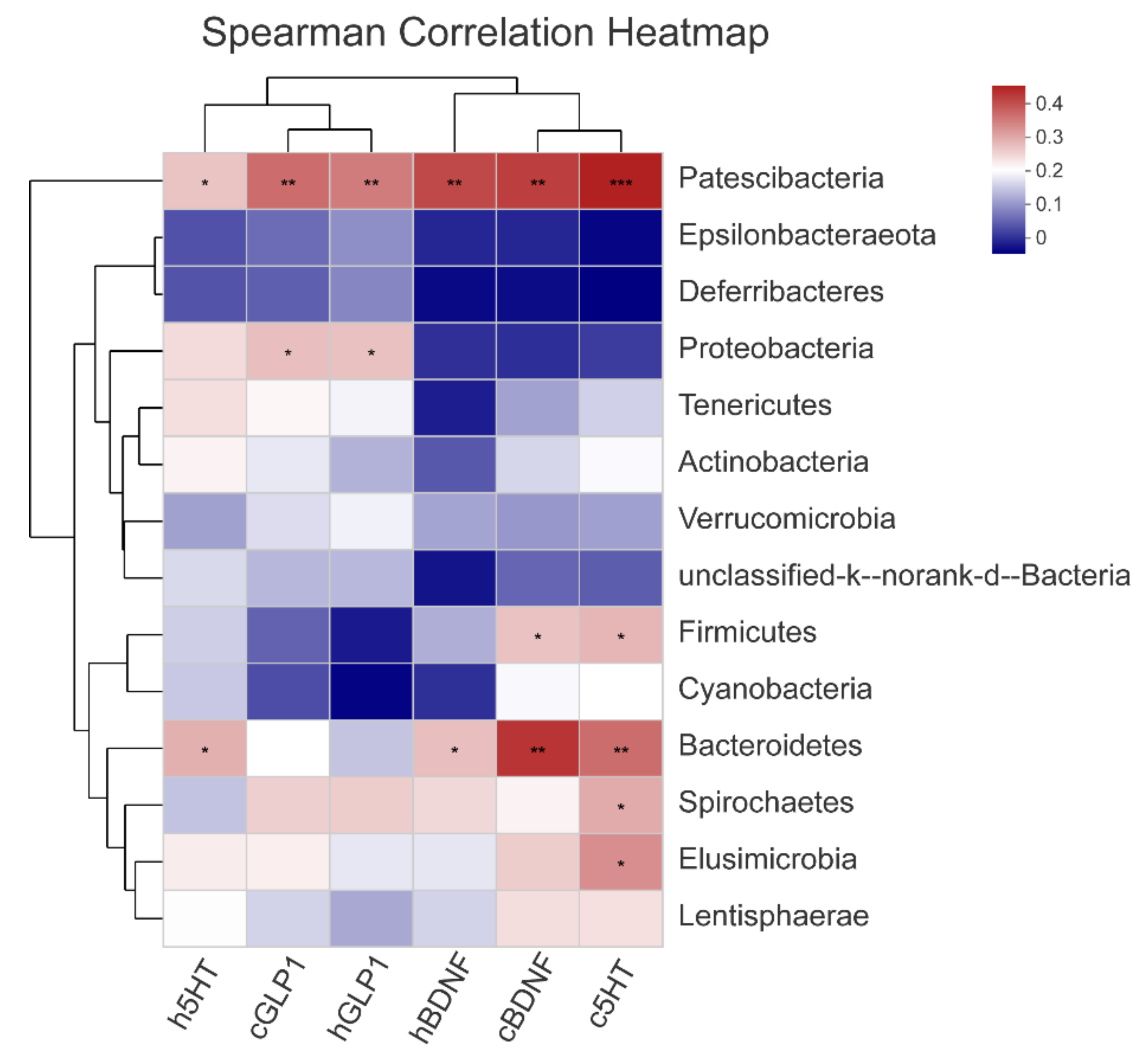
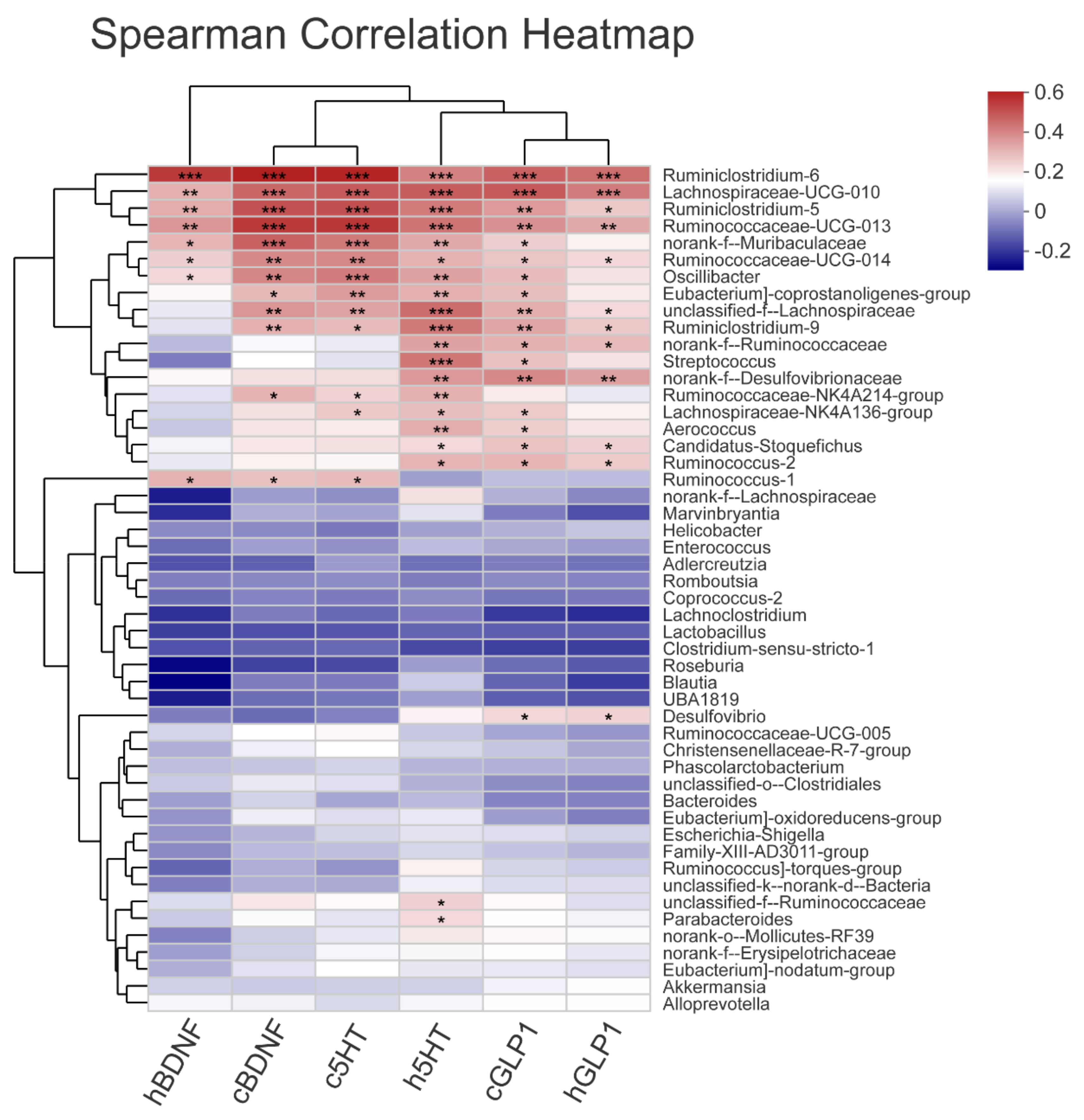
3.5. The Gut Microbiota Orchestrates CUMS-Induced Depression through Jasmine Tea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef]
- Gilliard, L.; Harriet, S.; Dinan, T.G.; John, F.C. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar]
- Hu, P.; Ma, L.; Wang, Y.G.; Ye, F.; Wang, C.; Zhou, W.H.; Zhao, X. Genistein, a dietary soy isoflavone, exerts antidepressant-like effects in mice: Involvement of ser-otonergic system. Neurochem. Int. 2017, 108, 426–435. [Google Scholar] [CrossRef]
- Sun, B.; Lv, Y.; Xu, H.; Qi, C.H.; Li, C.P.; Liu, P.F. Effects of Vortioxetine on depression model rats and expression of BDNF and Trk B in hippocampus. Exp. Ther. Med. 2020, 20, 2895–2902. [Google Scholar] [CrossRef]
- Elbassuoni, E.A.; Ahmed, R.F. Mechanism of the neuroprotective effect of GLP-1 in a rat model of Parkinson’s with pre-existing diabetes. Neurochem. Int. 2019, 131, 104583. [Google Scholar] [CrossRef]
- Glover, M.E.; Cohen, J.L.; Singer, J.R.; Sabbagh, M.N.; Rainville, J.R.; Hyland, M.T.; Morrow, C.D.; Weaver, C.T.; Hodes, G.E.; Kerman, I.A.; et al. Examining the Role of Microbiota in Emotional Behavior: Antibiotic Treatment Exacerbates Anxiety in High Anxiety-Prone Male Rats. Neuroscience 2021, 459, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, L.; Pettorruso, M.; Vellante, F.; Di Carlo, F.; Ceci, F.; Santovito, M.C.; Di Muzio, I.; Fornaro, M.; Ventriglio, A.; Tomasetti, C.; et al. Gut Microbiota and Bipolar Disorder: An Overview on a Novel Biomarker for Diagnosis and Treatment. Int. J. Mol. Sci. 2021, 22, 3723. [Google Scholar] [CrossRef] [PubMed]
- Rotroff, D.M.; Corum, D.G.; Motsinger-Reif, A.; Motsinger-Reif, A.; Fiehn, O.; Bottrel, N.; Drevets, W.C.; Singh, J.; Salvadore, G.; Kaddurah-Daouk, R. Metabolomic signatures of drug response phenotypes for ketamine and keta-mine in subjects with refractory major depressive disorder: New mechanistic insights for rapid acting antidepressants. Transl. Psychiatry 2016, 6, e894. [Google Scholar] [CrossRef] [Green Version]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Bear, T.; Dalziel, J.E.; Jane, C.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef] [Green Version]
- Park, A.J.; Collins, J.; Blennerhassett, P.A.; Ghia, J.E.; Verdu, E.F.; Bercik, P.; Collins, S.M. Altered colonic function and microbiota profile in a mouse model of chronic de-pression. Neurogastroenterol. Motil. 2013, 25, 733-e575. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, Y.-K. Understanding the Connection Between the Gut-Brain Axis and Stress/Anxiety Disorders. Curr. Psychiatry Rep. 2021, 23, 22. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; Aidy, S.E.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Gao, X.; Xia, G.; Chen, M.; Yin, J. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut 2021, 70, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Z.; Yu, Y.-J.; Adeli, K. Role of Gut Microbiota in Neuroendocrine Regulation of Carbohydrate and Lipid Metabolism via the Microbiota-Gut-Brain-Liver Axis. Microorg. 2020, 8, 527. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.; Liu, S.; Zhang, W.; Zhu, W.; Tao, Q.; Wang, H.; Yan, H. Impact of traditional Chinese medicine treatment on chronic unpredictable mild stress-induced depression-like behaviors: Intestinal microbiota and gut microbiome function. Food Funct. 2019, 10, 5886–5897. [Google Scholar] [CrossRef]
- Shen, J.-X.; Rana, M.M.; Liu, G.F.; Ling, T.J.; Gruber, M.Y.; Wei, S. Differential Contribution of Jasmine Floral Volatiles to the Aroma of Scented Green Tea. J. Food Qual. 2017, 2017, 5849501. [Google Scholar] [CrossRef] [Green Version]
- Inoue, N.; Kuroda, K.; Sugimoto, A.; Kakuda, T.; Fushiki, T. Autonomic nervous responses according to preference for the odor of jasmine tea. Biosci. Biotechnol. Biochem. 2003, 67, 1206–1214. [Google Scholar] [CrossRef] [Green Version]
- Sengar, N.; Joshi, A.; Prasad, S.K.; Hemalatha, S. Anti-inflammatory, analgesic and anti-pyretic activities of standardized root extract of Jasminum sambac. J. Ethnopharmacol. 2015, 160, 140–148. [Google Scholar] [CrossRef]
- Addae, J.I.; Pingal, R.; Walkins, K.; Cruickshank, R.; Youssef, F.F.; Nayak, S.B. Effects of Jasminum multiflorum leaf extract on rodent models of epilepsy, motor coordination and anxiety. Epilepsy Res. 2017, 131, 58–63. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Hu, X.Z.; Yang, C.W.; Xu, H.L.; Yao, Y.; Liu, J.M. Clostridium butyricum Attenuates Chronic Unpredictable Mild Stress-Induced Depres-sive-Like Behavior in Mice via the Gut-Brain Axis. J. Agric. Food Chem. 2018, 66, 8415–8421. [Google Scholar] [CrossRef]
- Mou, Z.; Huang, Q.; Chu, S.F.; Zhang, M.J.; Hu, J.F.; Chen, N.H.; Zhang, J.T. Antidepressive effects of ginsenoside Rg1 via regulation of HPA and HPG axis. Biomed. Pharmacother. 2017, 92, 962–971. [Google Scholar] [CrossRef]
- Liu, D.; Huang, J.; Luo, Y.; Wen, B.; Wu, W.; Zeng, H.; Liu, Z.H. Fuzhuan Brick Tea Attenuates High-Fat Diet-Induced Obesity and Associated Metabolic Disorders by Shaping Gut Microbiota. J. Agric. Food Chem. 2019, 67, 13589–13604. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhao, J.; Li, C.; Zhang, M.; Wei, L.; Zhang, X.; Kurskaya, O.; Bi, H.; Gao, T. Effect of combined chronic predictable and unpredictable stress on depression-like symptoms in mice. Ann. Transl. Med. 2020, 8, 942. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, D.O.; Zhang, L. Mechanisms Underlying the Anti-Depressive Effects of Regular Tea Consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, J.; Xie, R.; Lin, L.; Jiang, J.; Du, L.; Zeng, X.; Li, G.; Wang, C.; Qian, Y. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur. J. Neurosci. 2021, 53, 3598–3611. [Google Scholar] [CrossRef]
- Wang, Q.; Dwivedi, Y. Advances in novel molecular targets for antidepressants. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110041. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, Y.; Singh, S.; Singh, R.B. Gut microbiome-mediated epigenetic regulation of brain disorder and application of machine learning for multi-omics data analysis. Genome 2021, 64, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Xie, J.; Zeng, B.H.; Li, W.W.; Bai, S.J.; Zhou, C.; Chen, W.; Wei, H.; Xie, P. Absence of gut microbiota affects lipid metabolism in the prefrontal cortex of mice. Neurol. Res. 2019, 41, 1104–1112. [Google Scholar] [CrossRef]
- Zhang, M.; Li, A.; Yang, Q.; Li, J.; Wang, L.; Liu, X.; Huang, Y.; Liu, L. Beneficial Effect of Alkaloids from Sophora alopecuroides L. on CUMS-Induced Depression Model Mice via Modulating Gut Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 665159. [Google Scholar] [CrossRef]
- Ma, T.; Jin, H.; Kwok, L.Y.; Sun, Z.; Liong, M.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 2021, 14, 100294. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; Hung, Y.; et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef]
- Jun, L.; Shuilian, G.; Jiangfan, Y. Antidepressant effect of jasmine tea. J. Fujian Agric. For. Univ. 2014, 43, 139–145. [Google Scholar]
- Wang, X.; Zou, Z.; Shen, Q.; Huang, Z.; Chen, J.; Tang, J.; Xue, W.; Tao, W.; Wu, H.; Wang, D.; et al. Involvement of NMDA-AKT-mTOR Signaling in Rapid Antidepressant-Like Activity of Chaihu-jia-Longgu-Muli-tang on Olfactory Bulbectomized Mice. Front. Pharmacol. 2018, 9, 1537. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.A.; Fahim, A.T.; Sadik, N.A.H.; Ali, B.M. Resveratrol and dimethyl fumarate ameliorate depression-like behavior in a rat model of chronic unpredictable mild stress. Brain Res. 2018, 1701, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sheng, H.; Xu, Y.; Liu, Y.; Lu, J.; Ni, X. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: Relevant to proinflammatory cytokines and IDO activation. Behav. Brain Res. 2013, 242, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yang, Y.; Wu, Y.; Zhang, B.; Wu, H.; Wang, L.; Tang, H.; Chen, J. L-theanine ameliorate depressive-like behavior in a chronic unpredictable mild stress rat model via modulating the monoamine levels in limbic-cortical-striatal-pallidal-thalamic-circuit related brain regions. Phytother. Res. 2019, 33, 412–421. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, L.; Shen, C.L.; Wang, J.S. Green tea polyphenols boost gut-microbiota-dependent mitochondrial TCA and urea cycles in Sprague–Dawley rats. J. Nutr. Biochem. 2020, 81, 108395. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef]
- Hao, W.Z.; Li, X.J.; Zhang, P.W.; Chen, J.X. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. 2019, 284, 112691. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, A.; Schumacher, F.; Becker, K.A.; Wilker, B.; Soddemann, M.; Boldrin, F.; Muller, C.P.; Edwards, M.J.; Goodman, M.; Caldwell, C.C. Antidepressants act by inducing autophagy controlled by sphingomyelin-ceramide. Mol. Psychiatry 2018, 23, 2324–2346. [Google Scholar] [CrossRef] [Green Version]
- Alauzet, C.; Cunat, L.; Wack, M.; Lanfumey, L.; Legrand-Frossi, C.; Lozniewski, A.; Agrinier, N.; Cailliez-Grimal, C.; Frippiat, J.P. Impact of a Model Used to Simulate Chronic Socio-Environmental Stressors Encountered during Spaceflight on Murine Intestinal Microbiota. Int. J. Mol. Sci. 2020, 21, 7863. [Google Scholar] [CrossRef]
- Winter, G.; Hart, R.A.; Charlesworth, R.P.G.; Sharpley, C.F. Gut microbiome and depression: What we know and what we need to know. Rev. Neurosci. 2018, 29, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Wang, Y.; Qu, P.; Li, S.; Yu, Z.; Qin, X.; Liu, X. A combination of cecum microbiome and metabolome in CUMS depressed rats reveals the antide-pressant mechanism of traditional Chinese medicines: A case study of Xiaoyaosan. J. Ethnopharmacol. 2021, 276, 114167. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, J.; Yin, M.; Liu, Y.; You, Y.; Zhan, J.; Huang, W. Grape Extract Activates Brown Adipose Tissue Through Pathway Involving the Regulation of Gut Microbiota and Bile Acid. Mol. Nutr. Food Res. 2020, 64, 2000149. [Google Scholar] [CrossRef]
- Wang, J.; Lai, S.; Li, G.; Zhou, T.; Wang, B.; Cao, F.; Chen, T.; Zhang, X.; Chen, Y. Microglial activation contributes to depressive-like behavior in dopamine D3 receptor knockout mice. Brain Behav. Immun. 2020, 83, 226–238. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Wang, F.; Yu, X.; Ling, Z.; Li, H.; Zhang, H.; Jin, J.; Chen, W.; Pang, M.; et al. Neuroprotective Effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed Res. Int. 2015, 2015, 412946. [Google Scholar] [CrossRef] [Green Version]
- Abildgaard, A.; Kern, T.; Pedersen, O.; Hansen, T.; Lund, S.; Wegener, G. A diet-induced gut microbiota component and related plasma metabolites are associated with depressive-like behaviour in rats. Eur. Neuropsychopharmacol. 2020, 43, 10–21. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Gui, X.; Shi, X.; Bao, Z.; Han, H.; Li, M.D. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 2020, 25, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Getachew, B.; Aubee, J.I.; Schottenfeld, R.S.; Csoka, A.B.; Thompson, K.M.; Tizabi, Y. Ketamine interactions with gut-microbiota in rats: Relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 2018, 18, 222. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Bäckhed, F. From Association to Causality: The Role of the Gut Microbiota and Its Functional Products on Host Metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Huang, J.; Xiong, Y.; Zhang, X.; Lin, Y.; Liu, Z. Jasmine Tea Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior in Rats via the Gut-Brain Axis. Nutrients 2022, 14, 99. https://doi.org/10.3390/nu14010099
Zhang Y, Huang J, Xiong Y, Zhang X, Lin Y, Liu Z. Jasmine Tea Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior in Rats via the Gut-Brain Axis. Nutrients. 2022; 14(1):99. https://doi.org/10.3390/nu14010099
Chicago/Turabian StyleZhang, Yangbo, Jianan Huang, Yifan Xiong, Xiangna Zhang, Yong Lin, and Zhonghua Liu. 2022. "Jasmine Tea Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior in Rats via the Gut-Brain Axis" Nutrients 14, no. 1: 99. https://doi.org/10.3390/nu14010099
APA StyleZhang, Y., Huang, J., Xiong, Y., Zhang, X., Lin, Y., & Liu, Z. (2022). Jasmine Tea Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior in Rats via the Gut-Brain Axis. Nutrients, 14(1), 99. https://doi.org/10.3390/nu14010099






