Excess Folic Acid Supplementation before and during Pregnancy and Lactation Alters Behaviors and Brain Gene Expression in Female Mouse Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. FA Supplementation and Animals
2.2. Behavioral Analysis
2.2.1. Open Field Test
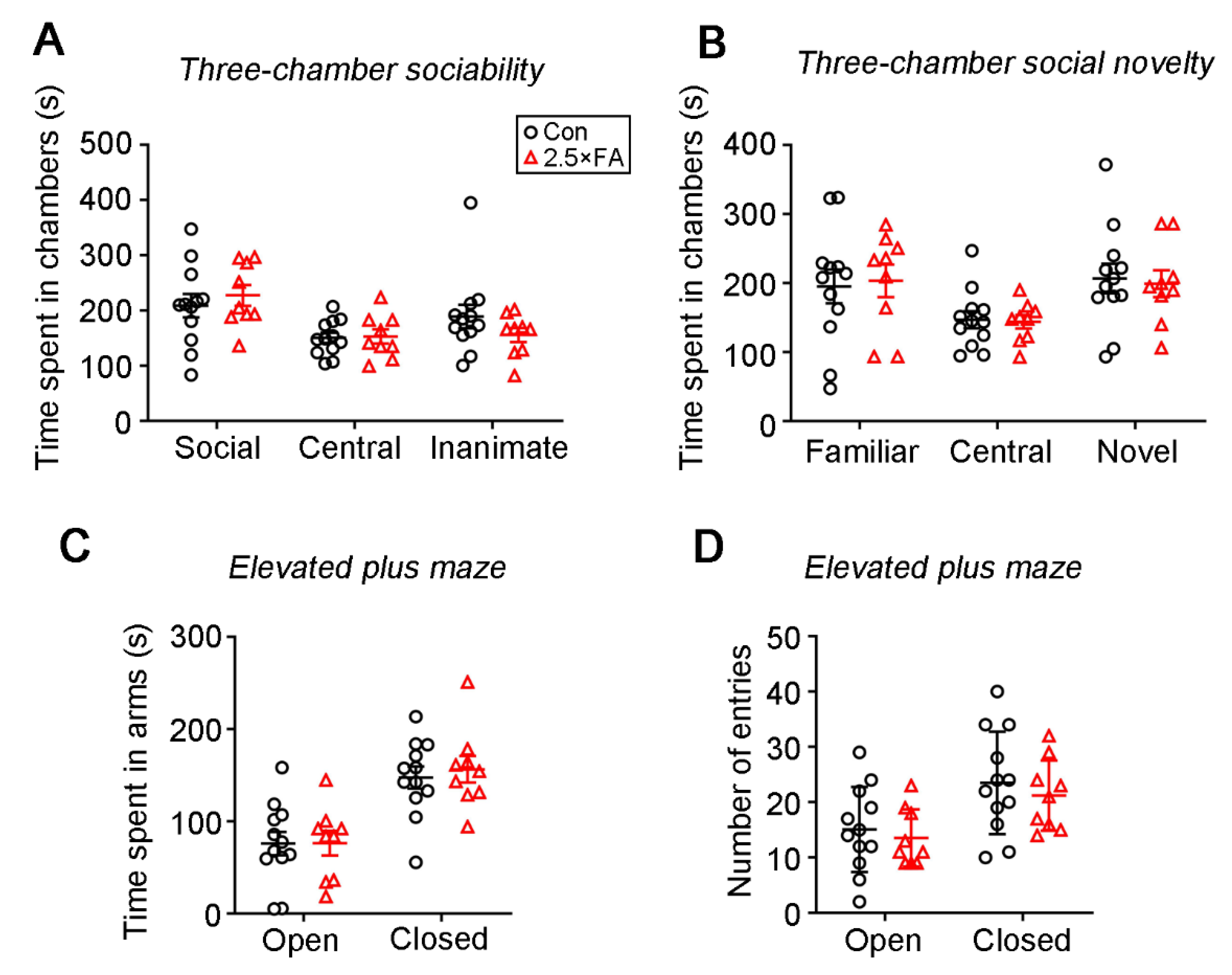
2.2.2. Three-Chamber Social Approach and Social Novelty Test
2.2.3. Elevated Plus-Maze
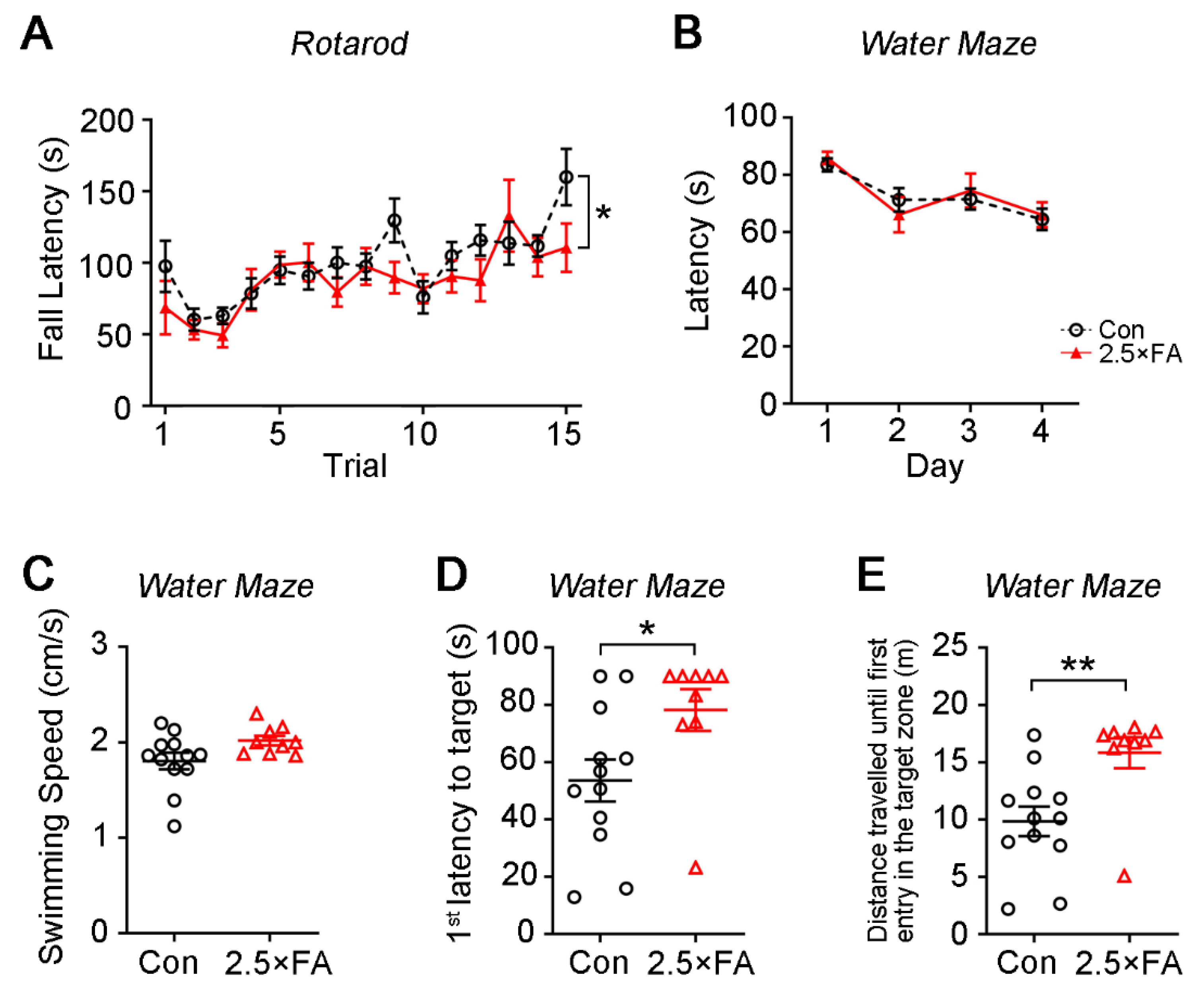
2.2.4. Rotarod
2.2.5. Morris Water Maze Task
2.3. Transcriptome Sequencing
2.4. qRT-PCR
2.5. Western Blot
2.6. Statistical Analysis
3. Results
3.1. Excess FA Supplementation throughout Pregnancy and Lactation Modifies Behaviors in the Adult Female Offspring
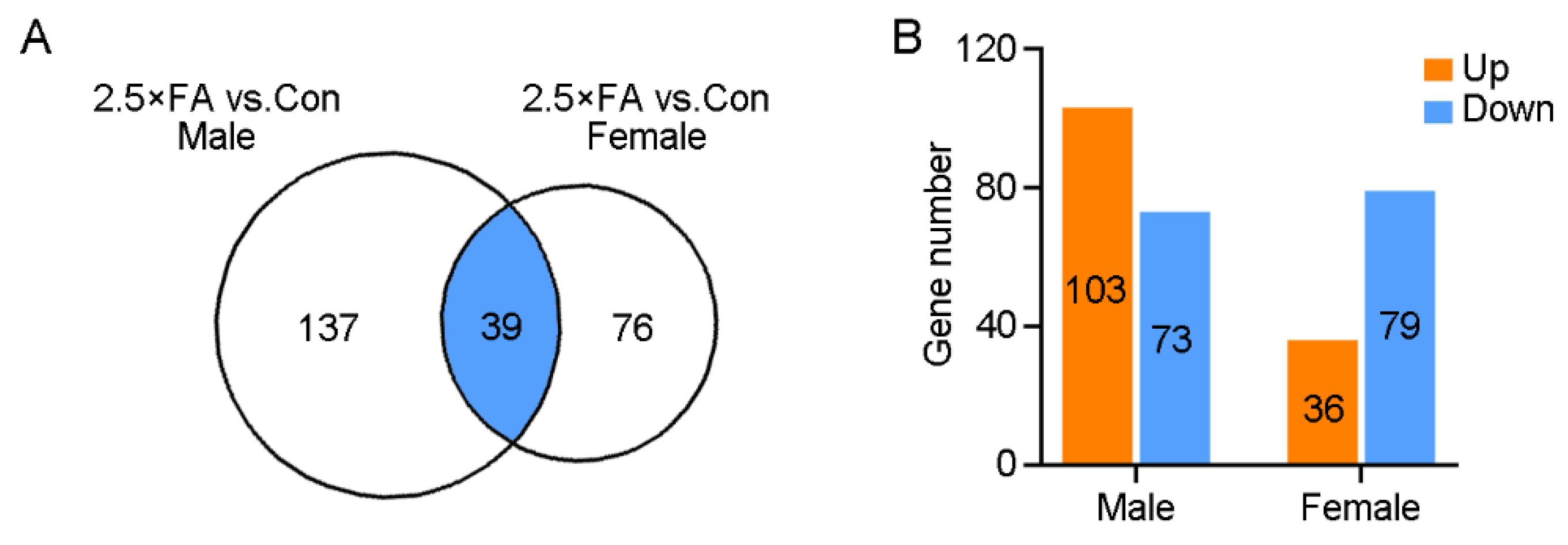
3.2. Excess FA Alters Brain Transcriptome of the Female Offspring at Weaning
3.3. Analysis of Pathway and Gene Ontology Enrichment of the DEGs in 2.5 × FA Brains
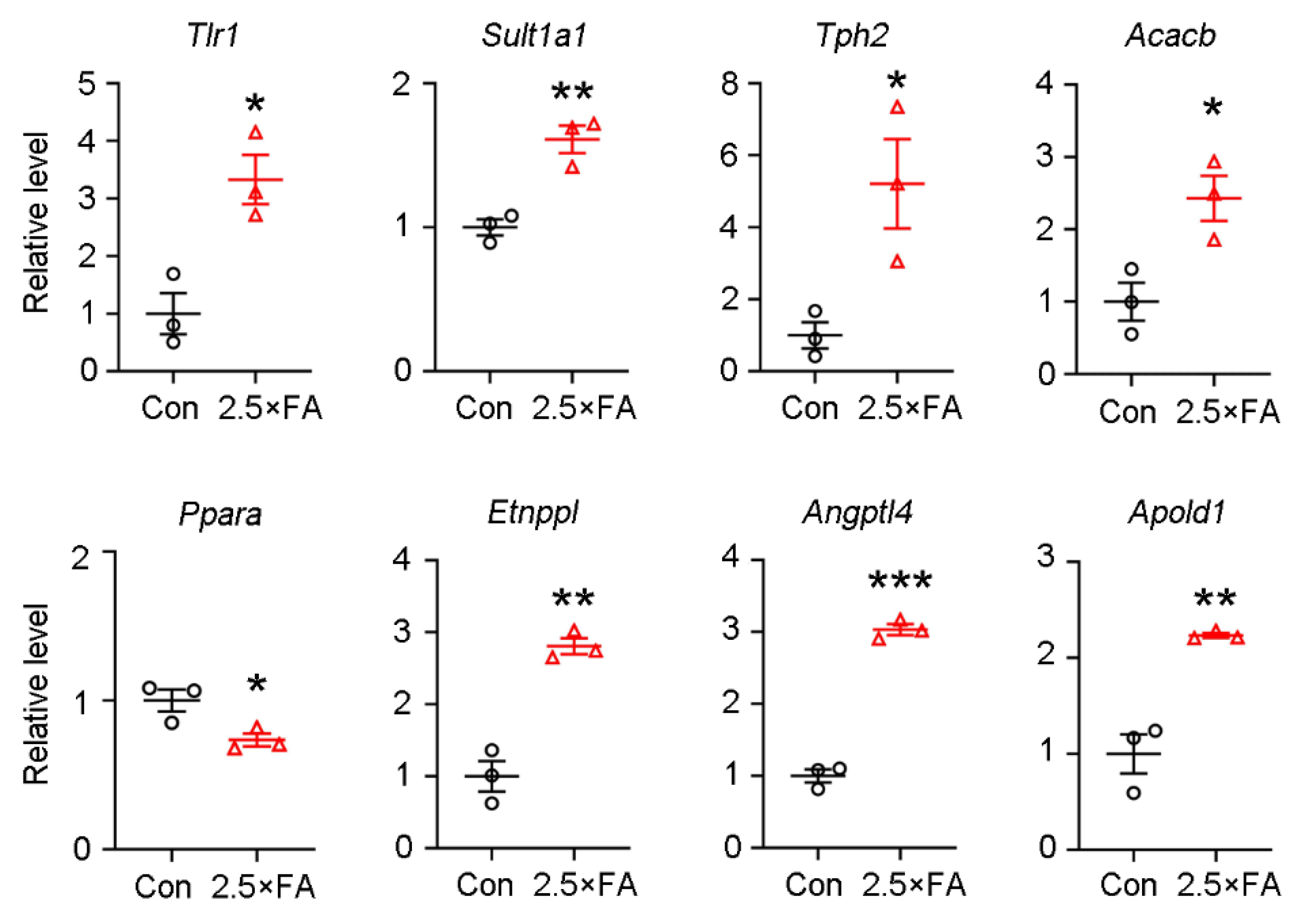
3.4. The Expression of Genes Enriched in the GO Category of Cellular Component Were Changed in 2.5 × FA Brains at Weaning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naninck, E.F.G.; Stijger, P.C.; Brouwer-Brolsma, E.M. The Importance of Maternal Folate Status for Brain Development and Function of Offspring. Adv. Nutr. 2019, 10, 502–519. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Treiman, K.A.; Kish-Doto, J.; Middleton, J.C.; Coker-Schwimmer, E.J.; Nicholson, W.K. Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017, 317, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic Acid Food Fortification—Its History, Effect, Concerns, and Future Directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, R.; Robichaud, A.; Arbuckle, T.E.; Fraser, W.D.; MacFarlane, A.J. Total folate and unmetabolized folic acid in the breast milk of a cross-section of Canadian women. Am. J. Clin. Nutr. 2017, 105, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.L.; Mistry, K.B.; Wang, G.; Zuckerman, B.; Wang, X. Folate Nutrition Status in Mothers of the Boston Birth Cohort, Sample of a US Urban Low-Income Population. Am. J. Public Health 2018, 108, 799–807. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Hughes, J.P.; Lacher, D.A.; Bailey, R.L.; Berry, R.J.; Zhang, M.; Yetley, E.A.; Rader, J.I.; Sempos, C.T.; Johnson, C.L. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988-2010. J. Nutr. 2012, 142, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.K.; Smith, M.J.; Jadavji, N.M. Maternal oversupplementation with folic acid and its impact on neurodevelopment of offspring. Nutr. Rev. 2018, 76, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Valera-Gran, D.; Navarrete-Munoz, E.M.; Garcia de la Hera, M.; Fernandez-Somoano, A.; Tardon, A.; Ibarluzea, J.; Balluerka, N.; Murcia, M.; Gonzalez-Safont, L.; Romaguera, D.; et al. Effect of maternal high dosages of folic acid supplements on neurocognitive development in children at 4–5 y of age: The prospective birth cohort Infancia y Medio Ambiente (INMA) study. Am. J. Clin. Nutr. 2017, 106, 878–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desoto, M.C.; Hitlan, R.T. Synthetic folic acid supplementation during pregnancy may increase the risk of developing autism. J. Pediatr. Biochem. 2012, 2, 251–261. [Google Scholar]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef]
- Egorova, O.; Myte, R.; Schneede, J.; Hagglof, B.; Bolte, S.; Domellof, E.; Ivars A’roch, B.; Elgh, F.; Ueland, P.M.; Silfverdal, S.A. Maternal blood folate status during early pregnancy and occurrence of autism spectrum disorder in offspring: A study of 62 serum biomarkers. Mol. Autism 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickell, L.; Brown, K.; Li, D.; Wang, X.L.; Deng, L.; Wu, Q.; Selhub, J.; Luo, L.; Jerome-Majewska, L.; Rozen, R. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res. Part. A Clin. Mol. Teratol. 2011, 91, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Chadman, K.K.; Kuizon, S.; Buenaventura, D.; Stapley, N.W.; Ruocco, F.; Begum, U.; Guariglia, S.R.; Brown, W.T.; Junaid, M.A. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PLoS ONE 2014, 9, e101674. [Google Scholar] [CrossRef]
- Girotto, F.; Scott, L.; Avchalumov, Y.; Harris, J.; Iannattone, S.; Drummondmain, C.; Tobias, R.; Belloespinosa, L.; Rho, J.M.; Davidsen, J. High dose folic acid supplementation of rats alters synaptic transmission and seizure susceptibility in offspring. Sci. Rep. 2013, 3, 1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henzel, K.S.; Ryan, D.P.; Schroder, S.; Weiergraber, M.; Ehninger, D. High-dose maternal folic acid supplementation before conception impairs reversal learning in offspring mice. Sci. Rep. 2017, 7, 3098. [Google Scholar] [CrossRef]
- Huot, P.S.; Ly, A.; Szeto, I.M.; Reza-Lopez, S.A.; Cho, D.; Kim, Y.I.; Anderson, G.H. Maternal and postweaning folic acid supplementation interact to influence body weight, insulin resistance, and food intake regulatory gene expression in rat offspring in a sex-specific manner. Appl. Physiol. Nutr. Metab. 2016, 41, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barua, S.; Kuizon, S.; Brown, W.T.; Junaid, M.A. DNA Methylation Profiling at Single-Base Resolution Reveals Gestational Folic Acid Supplementation Influences the Epigenome of Mouse Offspring Cerebellum. Front. Neurosci. 2016, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.; Li, L.; Jiang, Y.; Tan, J.; Ji, J.; Zhang, Y.; Jin, N.; Liu, F. Excess Folic Acid Supplementation Before and During Pregnancy and Lactation Activates Fos Gene Expression and Alters Behaviors in Male Mouse Offspring. Front. Neurosci. 2019, 13, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compan Gabucio, L.M.; Garcia de la Hera, M.; Torres Collado, L.; Fernandez-Somoano, A.; Tardon, A.; Guxens, M.; Vrijheid, M.; Rebagliato, M.; Murcia, M.; Ibarluzea, J.; et al. The Use of Lower or Higher Than Recommended Doses of Folic Acid Supplements during Pregnancy Is Associated with Child Attentional Dysfunction at 4–5 Years of Age in the INMA Project. Nutrients 2021, 13, 327. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127 (Suppl. S5), 838S–841S. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Takano, M.; Sugano, N.; Ohtsu, M.; Tsunoda, K.; Koshi, R.; Yoshinuma, N. The effect of B vitamin supplementation on wound healing in type 2 diabetic mice. J. Clin. Biochem. Nutr. 2016, 58, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qipshidze, N.; Metreveli, N.; Lominadze, D.; Tyagi, S.C. Folic acid improves acetylcholine-induced vasoconstriction of coronary vessels isolated from hyperhomocysteinemic mice: An implication to coronary vasospasm. J. Cell. Physiol. 2011, 226, 2712–2720. [Google Scholar] [CrossRef] [Green Version]
- Huerkamp, M.J.; Dowdy, M.R. Diet replenishment for ad-libitum-fed mice housed in social groups is compatible with shelf life. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 47–50. [Google Scholar]
- Bachmanov, A.A.; Reed, D.R.; Beauchamp, G.K.; Tordoff, M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002, 32, 435–443. [Google Scholar] [CrossRef]
- Kim, Y.I. Folic acid supplementation and cancer risk: Point. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2220–2225. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Hu, W.; Xie, S.; Gong, C.X.; Iqbal, K.; Liu, F. Rapid alteration of protein phosphorylation during postmortem: Implication in the study of protein phosphorylation. Sci. Rep. 2015, 5, 15709. [Google Scholar] [CrossRef] [Green Version]
- Moy, S.S.; Nadler, J.J.; Perez, A.; Barbaro, R.P.; Johns, J.M.; Magnuson, T.R.; Piven, J.; Crawley, J.N. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004, 3, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Harlan De Crescenzo, A.; Panoutsopoulos, A.A.; Tat, L.; Schaaf, Z.; Racherla, S.; Henderson, L.; Leung, K.Y.; Greene, N.D.E.; Green, R.; Zarbalis, K.S. Deficient or Excess Folic Acid Supply During Pregnancy Alter Cortical Neurodevelopment in Mouse Offspring. Cereb. Cortex 2021, 31, 635–649. [Google Scholar] [CrossRef]
- Cosin-Tomas, M.; Luan, Y.; Leclerc, D.; Malysheva, O.V.; Lauzon, N.; Bahous, R.H.; Christensen, K.E.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism. Nutrients 2020, 12, 1716. [Google Scholar] [CrossRef]
- Wiens, D.; DeSoto, M.C. Is High Folic Acid Intake a Risk Factor for Autism?—A Review. Brain Sci. 2017, 7, 149. [Google Scholar] [CrossRef] [Green Version]
- Wiens, D.; DeWitt, A.; Kosar, M.; Underriner, C.; Finsand, M.; Freese, M. Influence of Folic Acid on Neural Connectivity during Dorsal Root Ganglion Neurogenesis. Cells Tissues Organs 2016, 201, 342–353. [Google Scholar] [CrossRef]
- Schaevitz, L.; Berger-Sweeney, J.; Ricceri, L. One-carbon metabolism in neurodevelopmental disorders: Using broad-based nutraceutics to treat cognitive deficits in complex spectrum disorders. Neurosci. Biobehav. Rev. 2014, 46, 270–284. [Google Scholar] [PubMed]
- Plumptre, L.; Masih, S.P.; Ly, A.; Aufreiter, S.; Sohn, K.J.; Croxford, R.; Lausman, A.Y.; Berger, H.; O’Connor, D.L.; Kim, Y.I. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 2015, 102, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, M.; Xiao, Y.; Zhang, X.; Cui, S.; Huang, G. Folic acid inhibits tau phosphorylation through regulation of PP2A methylation in SH-SY5Y cells. J. Nutr. Health Aging 2015, 19, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Q.; Zhou, X.; Zhao, J.; Song, A.; Li, W.; Liu, H.; Xu, W.; Huang, G. Effects of folic acid supplementation on cognitive function and Abeta-related biomarkers in mild cognitive impairment: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Kuizon, S.; Chadman, K.K.; Brown, W.T.; Junaid, M.A. Microarray analysis reveals higher gestational folic Acid alters expression of genes in the cerebellum of mice offspring-a pilot study. Brain Sci. 2015, 5, 14–31. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.W.; Ayling, J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar]
- Ismail, S.; Senna, A.A.; Behiry, E.G.; Ashaat, E.A.; Zaki, M.S.; Ashaat, N.A.; Salah, D.M. Study of C677T variant of methylene tetrahydrofolate reductase gene in autistic spectrum disorder Egyptian children. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2019, 180, 305–309. [Google Scholar] [CrossRef]
- Sun, Y.; Qian, L.; Xu, L.; Hunt, S.; Sah, P. Somatostatin neurons in the central amygdala mediate anxiety by disinhibition of the central sublenticular extended amygdala. Mol. Psychiatry 2020, 1–12. [Google Scholar] [CrossRef]
- Zhang, R.; Asai, M.; Mahoney, C.E.; Joachim, M.; Shen, Y.; Gunner, G.; Majzoub, J.A. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol. Psychiatry 2017, 22, 733–744. [Google Scholar] [CrossRef]
- Reznikov, R.; Bambico, F.R.; Diwan, M.; Raymond, R.J.; Nashed, M.G.; Nobrega, J.N.; Hamani, C. Prefrontal Cortex Deep Brain Stimulation Improves Fear and Anxiety-Like Behavior and Reduces Basolateral Amygdala Activity in a Preclinical Model of Posttraumatic Stress Disorder. Neuropsychopharmacology 2018, 43, 1099–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.T.; Huang, Y.; Masood, A.; Stolinski, L.R.; Li, Y.; Zhang, L.; Dlaboga, D.; Jin, S.L.; Conti, M.; O’Donnell, J.M. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B). Neuropsychopharmacology 2008, 33, 1611–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatten, M.E.; Roussel, M.F. Development and cancer of the cerebellum. Trends Neurosci. 2011, 34, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Fjell, A.M.; Mcevoy, L.; Holland, D.; Dale, A.M.; Walhovd, K.B. What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog. Neurobiol. 2014, 117, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Riches, Z.; Bloomer, J.C.; Coughtrie, M.W. Comparison of 2-aminophenol and 4-nitrophenol as in vitro probe substrates for the major human hepatic sulfotransferase, SULT1A1, demonstrates improved selectivity with 2-aminophenol. Biochem. Pharm. 2007, 74, 352–358. [Google Scholar] [CrossRef]
- Cook, I.; Wang, T.; Girvin, M.; Leyh, T.S. The structure of the catechin-binding site of human sulfotransferase 1A1. Proc. Natl. Acad. Sci. USA 2016, 113, 14312–14317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alnouti, Y.; Klaassen, C.D. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol. Sci. 2006, 93, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Salman, E.D.; Kadlubar, S.A.; Falany, C.N. Expression and localization of cytosolic sulfotransferase (SULT) 1A1 and SULT1A3 in normal human brain. Drug Metab. Dispos. 2009, 37, 706–709. [Google Scholar] [CrossRef] [Green Version]
- Tsapakis, E.M.; Fernandes, C.; Moran-Gates, T.; Basu, A.; Sugden, K.; Aitchison, K.J.; Tarazi, F.I. Effects of antidepressant drug exposure on gene expression in the developing cerebral cortex. Synapse 2014, 68, 209–220. [Google Scholar] [CrossRef]
- Sidharthan, N.P.; Minchin, R.F.; Butcher, N.J. Cytosolic sulfotransferase 1A3 is induced by dopamine and protects neuronal cells from dopamine toxicity: Role of D1 receptor-N-methyl-D-aspartate receptor coupling. J. Biol. Chem. 2013, 288, 34364–34374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, I.; Wang, T.; Leyh, T.S. Tetrahydrobiopterin regulates monoamine neurotransmitter sulfonation. Proc. Natl. Acad. Sci. USA 2017, 114, E5317–E5324. [Google Scholar] [CrossRef] [Green Version]
- Bader, M. Inhibition of serotonin synthesis: A novel therapeutic paradigm. Pharm. Ther. 2020, 205, 107423. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.X.; Liu, S.Y.; Zheng, X.; Chen, X.; Lu, L.X.; Chen, B.; Xiong, X.Y.; Shu, H.F.; Yang, Q.W.; Yang, H. TLR1 expression in mouse brain was increased in a KA-induced seizure model. Inflamm. Res. 2015, 64, 487–495. [Google Scholar] [CrossRef]
- Madar, R.; Rotter, A.; Waldman Ben-Asher, H.; Mughal, M.R.; Arumugam, T.V.; Wood, W.H., 3rd; Becker, K.G.; Mattson, M.P.; Okun, E. Postnatal TLR2 activation impairs learning and memory in adulthood. Brain Behav. Immun. 2015, 48, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Daniele, S.G.; Beraud, D.; Davenport, C.; Cheng, K.; Yin, H.; Maguire-Zeiss, K.A. Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci. Signal. 2015, 8, ra45. [Google Scholar] [CrossRef] [Green Version]
- Wada, Y.; Maekawa, M.; Ohnishi, T.; Balan, S.; Matsuoka, S.; Iwamoto, K.; Iwayama, Y.; Ohba, H.; Watanabe, A.; Hisano, Y.; et al. Peroxisome proliferator-activated receptor alpha as a novel therapeutic target for schizophrenia. EBioMedicine 2020, 62, 103130. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Aleshin, S.E.; Astakhova, A.A.; Sergeeva, M.G.; Reiser, G. Regulation of peroxisome proliferator-activated receptors (PPAR) alpha and -gamma of rat brain astrocytes in the course of activation by toll-like receptor agonists. J. Neurochem. 2015, 134, 113–124. [Google Scholar] [CrossRef]
- Meher, A.; Joshi, A.; Joshi, S. Differential regulation of hepatic transcription factors in the Wistar rat offspring born to dams fed folic acid, vitamin B12 deficient diets and supplemented with omega-3 fatty acids. PLoS ONE 2014, 9, e90209. [Google Scholar] [CrossRef] [PubMed]
- White, C.J.; Ellis, J.M.; Wolfgang, M.J. The role of ethanolamine phosphate phospholyase in regulation of astrocyte lipid homeostasis. J. Biol. Chem. 2021, 297, 100830. [Google Scholar] [CrossRef] [PubMed]
- Leventoux, N.; Augustus, M.; Azar, S.; Riquier, S.; Villemin, J.P.; Guelfi, S.; Falha, L.; Bauchet, L.; Goze, C.; Ritchie, W.; et al. Transformation Foci in IDH1-mutated Gliomas Show STAT3 Phosphorylation and Downregulate the Metabolic Enzyme ETNPPL, a Negative Regulator of Glioma Growth. Sci. Rep. 2020, 10, 5504. [Google Scholar] [CrossRef]
- Diaz-Canestro, C.; Bonetti, N.R.; Wust, P.; Nageswaran, V.; Liberale, L.; Beer, J.H.; Montecucco, F.; Luscher, T.F.; Bohacek, J.; Camici, G.G. Apold1 deficiency associates with increased arterial thrombosis in vivo. Eur. J. Clin. Investig. 2020, 50, e13191. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Madrid, B.S.; Hernandez, P.; Blasco, A.; Haley, C.S.; Fontanesi, L.; Santacreu, M.A.; Pena, R.N.; Navarro, P.; Ibanez-Escriche, N. Genomic regions influencing intramuscular fat in divergently selected rabbit lines. Anim. Genet. 2020, 51, 58–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushing, E.M.; Chi, X.; Sylvers, K.L.; Shetty, S.K.; Potthoff, M.J.; Davies, B.S.J. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol. Metab. 2017, 6, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Kamermans, A.; van Het Hof, B.; Castricum, K.; Aanhane, E.; van Horssen, J.; Thijssen, V.L.; Scheltens, P.; Teunissen, C.E.; Fontijn, R.D.; et al. Angiopoietin like-4 as a novel vascular mediator in capillary cerebral amyloid angiopathy. Brain 2018, 141, 3377–3388. [Google Scholar] [CrossRef]



| Gene | Forward Sequence | Reverse Sequence | Amplicon Length (bp) |
|---|---|---|---|
| Tlr1 | GAGGCATGAAGAGAGCGGAA | TAGGGGTGTCCACAATTGCC | 292 |
| Sult1a1 | GCTAGATAAGTGTGGCCGGG | TTCGGGCAACGTAGATCACC | 194 |
| Tph2 | CAATCGAGTTCGGCCTTTGC | CTGCGTGTAGGGGTTGAAGT | 275 |
| Acacb | CCGCTCAAGATCGAGGAGTC | ATGCAGGCTACCTTGCTTGT | 255 |
| Ppara | TGTGAACTGACGTTTGTGGC | CCACAGAGCGCTAAGCTGT | 70 |
| Etnppl | CGGTCATGTGCGAGCTCTAT | GTCGTGGAGGAAGCGAGAAT | 263 |
| Angptl4 | GGGGACCTTAACTGTGCCAA | CTGCAGAGGATAGTAGCGGC | 165 |
| Apold1 | CCGTCCTGAAGGCCAAGATT | AGAAAAACAACGCTGCGTCC | 168 |
| Female | Male | |
|---|---|---|
| 1 | Alcoholism | Amphetamine addiction |
| 2 | Amphetamine addiction | Dopaminergic synapse |
| 3 | Cocaine addiction | Cocaine addiction |
| 4 | Histidine metabolism | Alcoholism |
| 5 | Tyrosine metabolism | Neuroactive ligand–receptor interaction |
| Biological Process | Female | Male |
|---|---|---|
| 1 | Regulation of RNA metabolic process | Cell-cell signaling |
| 2 | Regulation of transcription, DNA-templated | Muscle tissue development |
| 3 | Regulation of nucleic acid-templated transcription | Feeding behavior |
| 4 | Phenol-containing compound metabolic process | Positive regulation of amine transport |
| 5 | Phenol-containing compound biosynthetic process | Positive regulation of anion transport |
| Cellular component | Female | Male |
| 1 | Cellular_component | Plasma membrane part |
| 2 | Cytosol | Cell projection |
| 3 | Neuron projection | Neuron projection |
| 4 | Cytoplasmic membrane-bounded vesicle | Neuron part |
| 5 | Synapse part | Integral component of plasma membrane |
| Molecular function | Female | Male |
| 1 | Sequence-specific DNA binding transcription factor activity | Receptor binding |
| 2 | Nucleic acid binding transcription factor activity | Enzyme inhibitor activity |
| 3 | Organic acid binding | Hormone activity |
| 4 | Carboxylic acid binding | Drug binding |
| 5 | Amino acid binding | Neuropeptide hormone activity |
| 2.5 × FA vs. Control | ||
|---|---|---|
| Female | Male [18] | |
| Body weight | NS a | Increased |
| Open field | Decreased exploration Increased anxiety | NS a |
| Three-chamber sociability | NS a | Decreased sociability |
| Three-chamber social novelty | NS a | NS a |
| Elevated plus-maze | NS a | Increased anxiety |
| Accelerating rotarod | Impaired motor learning | Impaired motor learning |
| Morris water maze | Impaired spatial memory | Delay in spatial learning |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Sun, W.; Wu, Q.; Lin, H.; Lu, Z.; Shen, X.; Chen, Y.; Zhou, Y.; Huang, L.; Wu, F.; et al. Excess Folic Acid Supplementation before and during Pregnancy and Lactation Alters Behaviors and Brain Gene Expression in Female Mouse Offspring. Nutrients 2022, 14, 66. https://doi.org/10.3390/nu14010066
Yang X, Sun W, Wu Q, Lin H, Lu Z, Shen X, Chen Y, Zhou Y, Huang L, Wu F, et al. Excess Folic Acid Supplementation before and during Pregnancy and Lactation Alters Behaviors and Brain Gene Expression in Female Mouse Offspring. Nutrients. 2022; 14(1):66. https://doi.org/10.3390/nu14010066
Chicago/Turabian StyleYang, Xingyue, Wenyan Sun, Qian Wu, Hongyan Lin, Zhixing Lu, Xin Shen, Yongqi Chen, Yan Zhou, Li Huang, Feng Wu, and et al. 2022. "Excess Folic Acid Supplementation before and during Pregnancy and Lactation Alters Behaviors and Brain Gene Expression in Female Mouse Offspring" Nutrients 14, no. 1: 66. https://doi.org/10.3390/nu14010066
APA StyleYang, X., Sun, W., Wu, Q., Lin, H., Lu, Z., Shen, X., Chen, Y., Zhou, Y., Huang, L., Wu, F., Liu, F., & Chu, D. (2022). Excess Folic Acid Supplementation before and during Pregnancy and Lactation Alters Behaviors and Brain Gene Expression in Female Mouse Offspring. Nutrients, 14(1), 66. https://doi.org/10.3390/nu14010066






