A Long-Term Energy-Rich Diet Increases Prefrontal BDNF in Sprague-Dawley Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Behavioral Procedures
2.2. Tissue Sampling
2.3. BDNF and TrkB Receptor
2.4. Western Blotting
2.5. Oxidative Stress Markers and Monoamine Metabolites
2.6. Statistical Analysis
3. Results
3.1. Body Weight
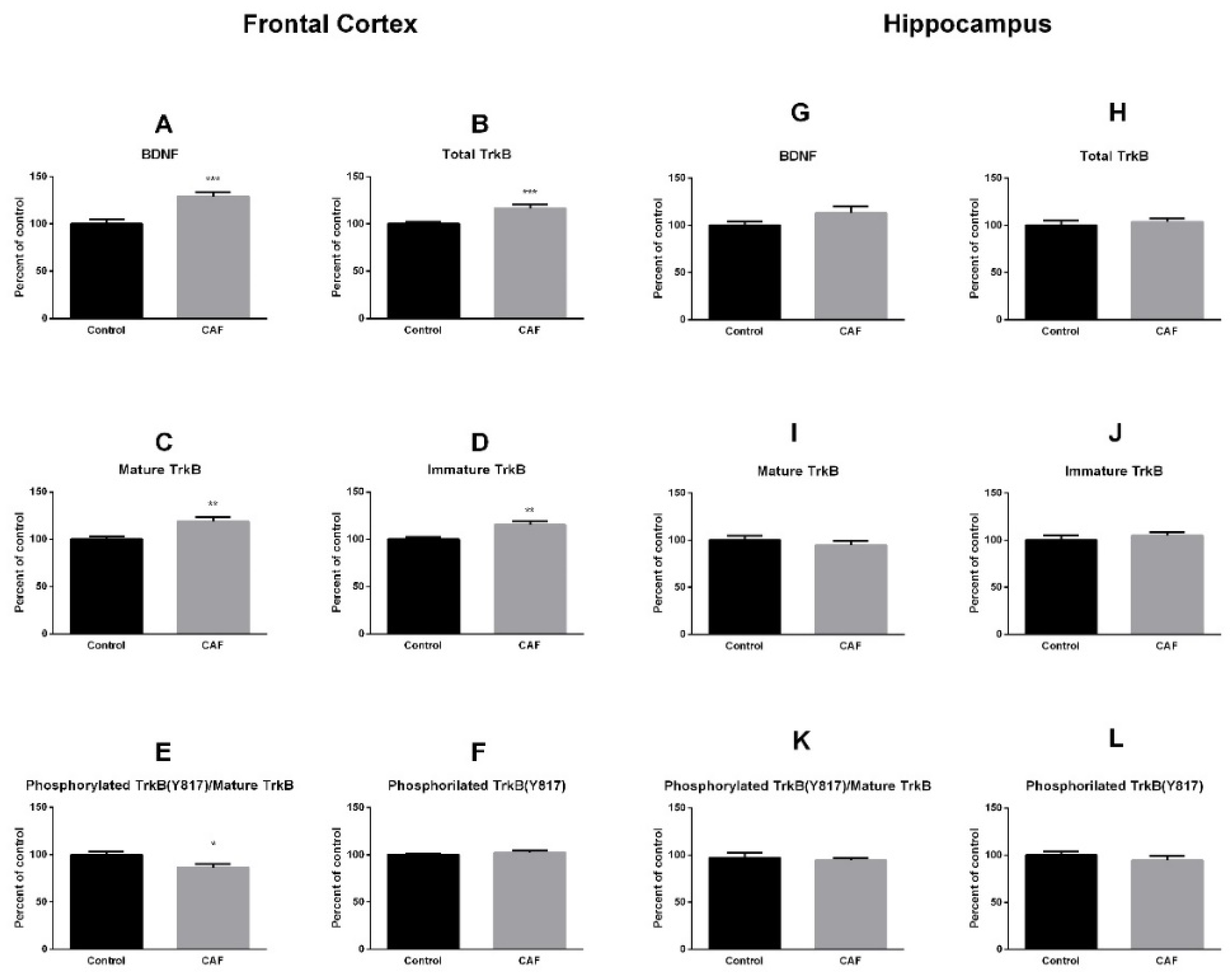
3.2. BDNF and TrkB Receptor
3.3. Monoamine and Metabolites
3.4. Oxidative Stress Markers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bake, T.; Morgan, D.G.A.; Mercer, J.G. Feeding and metabolic consequences of scheduled consumption of large, binge-type meals of high fat diet in the Sprague–Dawley rat. Physiol. Behav. 2014, 128, 70–79. [Google Scholar] [CrossRef]
- Ghibaudi, L.; Cook, J.; Farley, C.; van Heek, M.; Hwa, J.J. Fat Intake Affects Adiposity, Comorbidity Factors, and Energy Metabolism of Sprague-Dawley Rats. Obes. Res. 2002, 10, 956–963. [Google Scholar] [CrossRef]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav. Immun. 2014, 37, 134–141. [Google Scholar] [CrossRef]
- Winocur, G.; Greenwood, C.E.; Piroli, G.G.; Grillo, C.A.; Reznikov, L.R.; Reagan, L.P.; McEwen, B.S. Memory impairment in obese Zucker rats: An investigation of cognitive function in an animal model of insulin resistance and obesity. Behav. Neurosci. 2005, 119, 1389–1395. [Google Scholar] [CrossRef]
- Buchenauer, T.; Behrendt, P.; Bode, F.J.; Horn, R.; Brabant, G.; Stephan, M.; Nave, H. Diet-induced obesity alters behavior as well as serum levels of corticosterone in F344 rats. Physiol. Behav. 2009, 98, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Warneke, W.; Klaus, S.; Fink, H.; Langley-Evans, S.C.; Voigt, J.-P. The impact of cafeteria diet feeding on physiology and anxiety-related behaviour in male and female Sprague–Dawley rats of different ages. Pharm. Biochem. Behav. 2014, 116, 45–54. [Google Scholar] [CrossRef]
- Jurdak, N.; Lichtenstein, A.H.; Kanarek, R.B. Diet-induced obesity and spatial cognition in young male rats. Nutr. Neurosci. 2008, 11, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef]
- Molteni, R.; Wu, A.; Vaynman, S.; Ying, Z.; Barnard, R.J.; Gómez-Pinilla, F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience 2004, 123, 429–440. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Norman, E.D.; Lee, K.; Cutler, R.G.; Telljohann, R.S.; Egan, J.M.; Mattson, M.P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008, 18, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Tyler, W.J.; Alonso, M.; Bramham, C.R.; Pozzo-Miller, L.D. From acquisition to consolidation: On the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem. 2002, 9, 224–237. [Google Scholar] [CrossRef]
- Camer, D.; Yu, Y.; Szabo, A.; Fernandez, F.; Dinh, C.H.L.; Huang, X.-F. Bardoxolone methyl prevents high-fat diet-induced alterations in prefrontal cortex signalling molecules involved in recognition memory. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2015, 59, 68–75. [Google Scholar] [CrossRef]
- Chao, M.V.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health and disease. Clin. Sci. 2006, 110, 167–173. [Google Scholar] [CrossRef]
- Jullien, J.; Guili, V.; Reichardt, L.F.; Rudkin, B.B. Molecular Kinetics of Nerve Growth Factor Receptor Trafficking and Activation. J. Biol. Chem. 2002, 277, 38700–38708. [Google Scholar] [CrossRef]
- Rajagopal, R.; Chen, Z.-Y.; Lee, F.S.; Chao, M.V. Transactivation of Trk Neurotrophin Receptors by G-Protein-Coupled Receptor Ligands Occurs on Intracellular Membranes. J. Neurosci. 2004, 24, 6650–6658. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, A.; Constantine-Paton, M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010, 70, 304–322. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Brambilla, R.; Thomas, K. A simple role for BDNF in learning and memory? Front. Mol. NeuroSci. 2010, 3, 1. [Google Scholar] [CrossRef]
- Deinhardt, K.; Jeanneteau, F. More than Just an OFF-Switch: The Essential Role of Protein Dephosphorylation in the Modulation of BDNF Signaling Events, Protein Phosphorylation in Human Health. In IntechOpen Book Series; Huang, C., Ed.; InTechOpen: London, UK, 2012. [Google Scholar] [CrossRef][Green Version]
- Davis, J.F.; Tracy, A.L.; Schurdak, J.D.; Tschöp, M.H.; Lipton, J.W.; Clegg, D.J.; Benoit, S.C. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav. Neurosci. 2008, 122, 1257–1263. [Google Scholar] [CrossRef]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef]
- Markianos, M.; Evangelopoulos, M.-E.; Koutsis, G.; Sfagos, C. Elevated CSF serotonin and dopamine metabolite levels in overweight subjects. Obesity 2013, 21, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef]
- Martire, S.I.; Maniam, J.; South, T.; Holmes, N.; Westbrook, R.F.; Morris, M.J. Extended exposure to a palatable cafeteria diet alters gene expression in brain regions implicated in reward, and withdrawal from this diet alters gene expression in brain regions associated with stress. Behav. Brain Res. 2014, 265, 132–141. [Google Scholar] [CrossRef]
- Leffa, D.D.; Valvassori, S.S.; Varela, R.B.; Lopes-Borges, J.; Daumann, F.; Longaretti, L.M.; Dajori, A.L.F.; Quevedo, J.; Andrade, V.M. Effects of palatable cafeteria diet on cognitive and noncognitive behaviors and brain neurotrophins’ levels in mice. Metab. Brain Dis. 2015, 30, 1073–1082. [Google Scholar] [CrossRef]
- Maniam, J.; Morris, M.J. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: Role of hippocampus. Psychoneuroendocrinology 2010, 35, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Zeeni, N.; Daher, C.; Fromentin, G.; Tome, D.; Darcel, N.; Chaumontet, C. A cafeteria diet modifies the response to chronic variable stress in rats. Stress 2013, 16, 211–219. [Google Scholar] [CrossRef]
- Mendoza, J.; Angeles-Castellanos, M.; Escobar, C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience 2005, 133, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Van der Harst, J.E.; Kapteijn, C.M.; Baars, A.J.M.; Spruijt, B.M.; Ramakers, G.M.J. Announced reward counteracts the effects of chronic social stress on anticipatory behavior and hippocampal synaptic plasticity in rats. Exp. Brain Res. 2010, 201, 641–651. [Google Scholar] [CrossRef][Green Version]
- Van der Harst, J.E.; Baars, A.-M.; Spruijt, B.M. Announced rewards counteract the impairment of anticipatory behaviour in socially stressed rats. Behav. Brain Res. 2005, 161, 183–189. [Google Scholar] [CrossRef]
- Virtuoso, A.; Forkman, B.; Sarruf, D.A.; Tveden-Nyborg, P.; Sorensen, D.B. A cafeteria diet alters the decision making strategy and metabolic markers in Sprague-Dawley male rats. Appl. Anim. Behav. Sci. 2018, 199, 35–44. [Google Scholar] [CrossRef]
- Charnov, E.L. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 1976, 9, 129–136. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. Determination of ascorbic acid and dehydroascorbic acid in biological samples by high-performance liquid chromatography using subtraction methods: Reliable reduction with tris 2-carboxyethyl phosphine hydrochloride. Anal. Biochem. 2000, 282, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. Increased oxidative damage in vitamin C deficiency is accompanied by induction of ascorbic acid recycling capacity in young but not mature guinea pigs. Free Radic. Res. 2002, 36, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. Ascorbate and dehydroascorbic acid as reliable biomarkers of oxidative stress: Analytical reproducibility and long-term stability of plasma samples subjected to acidic deproteinization. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2513–2516. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: Comparison with ultraviolet-visible spectrophotometry. Clin. Chem. 2001, 47, 1725–1727. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Hasselholt, S.; Tveden-Nyborg, P.; Lykkesfeldt, J. Guinea pig ascorbate status predicts tetrahydrobiopterin plasma concentration and oxidation ratio in vivo. Nutr. Res. 2013, 33, 859–867. [Google Scholar] [CrossRef]
- Schou-Pedersen, A.M.V.; Hansen, S.N.; Tveden-Nyborg, P.; Lykkesfeldt, J. Simultaneous quantification of monoamine neurotransmitters and their biogenic metabolites intracellularly and extracellularly in primary neuronal cell cultures and in sub-regions of guinea pig brain. J. Chromatogr. B 2016, 1028, 222–230. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef]
- Arcego, D.M.; Krolow, R.; Lampert, C.; Toniazzo, A.P.; Berlitz, C.; Lazzaretti, C.; Schmitz, F.; Rodrigues, A.F.; Wyse, A.T.S.; Dalmaz, C. Early life adversities or high fat diet intake reduce cognitive function and alter BDNF signaling in adult rats: Interplay of these factors changes these effects. Int. J. Dev. Neurosci. 2016, 50, 16–25. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Meisel, R.L.; Mullins, A.J.; Davidson, T.L. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav. Brain Res. 2007, 182, 57–66. [Google Scholar] [CrossRef]
- White, C.L.; Pistell, P.J.; Purpera, M.N.; Gupta, S.; Fernandez-Kim, S.-O.; Hise, T.L.; Keller, J.N.; Ingram, D.K.; Morrison, C.D.; Bruce-Keller, A.J. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: Contributions of maternal diet. Neurobiol. Dis. 2009, 35, 3–13. [Google Scholar] [CrossRef]
- Macedo, I.C.; Rozisky, J.R.; Oliveira, C.; Oliveira, C.M.; Laste, G.; Nonose, Y.; Santos, V.S.; Marques, P.R.; Ribeiro, M.F.; Caumo, W.; et al. Chronic stress associated with hypercaloric diet changes the hippocampal BDNF levels in male Wistar rats. Neuropeptides 2015, 51, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Seilhamer, J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis 1997, 18, 533–537. [Google Scholar] [CrossRef]
- Pradet-Balade, B.; Boulmé, F.; Beug, H.; Müllner, E.W.; Garcia-Sanz, J.A. Translation control: Bridging the gap between genomics and proteomics? Trends Biochem. Sci. 2001, 26, 225–229. [Google Scholar] [CrossRef]
- Tian, Q.; Stepaniants, S.B.; Mao, M.; Weng, L.; Feetham, M.C.; Doyle, M.J.; Yi, E.C.; Dai, H.; Thorsson, V.; Eng, J.; et al. Integrated Genomic and Proteomic Analyses of Gene Expression in Mammalian Cells. Mol. Cell Proteom. 2004, 3, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Nithianantharajah, J.; Hannan, A.J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 2006, 7, 697–709. [Google Scholar] [CrossRef]
- Bechara, R.G.; Kelly, Á.M. Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behav. Brain Res. 2013, 245, 96–100. [Google Scholar] [CrossRef]
- Ickes, B.R.; Pham, T.M.; Sanders, L.A.; Albeck, D.S.; Mohammed, A.H.; Granholm, A.-C. Long-Term Environmental Enrichment Leads to Regional Increases in Neurotrophin Levels in Rat Brain. Exp. Neurol. 2000, 164, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mosaferi, B.; Babri, S.; Mohaddes, G.; Khamnei, S.; Mesgari, M. Post-weaning environmental enrichment improves BDNF response of adult male rats. Int. J. Dev. Neurosci. 2015, 46, 108–114. [Google Scholar] [CrossRef]
- Zeeni, N.; Bassil, M.; Fromentin, G.; Chaumontet, C.; Darcel, N.; Tome, D.; Daher, C.F. Environmental enrichment and cafeteria diet attenuate the response to chronic variable stress in rats. Physiol. Behav. 2015, 139, 41–49. [Google Scholar] [CrossRef]
- Molteni, R.; Rossetti, A.C.; Savino, E.; Racagni, G.; Calabrese, F. Chronic Mild Stress Modulates Activity-Dependent Transcription of BDNF in Rat Hippocampal Slices. Neural Plast. 2016, 2016, 2592319. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Imbe, H.; Morikawa, Y.; Kubo, C.; Senba, E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res. 2005, 53, 129–139. [Google Scholar] [CrossRef]
- Larsen, M.H.; Mikkelsen, J.D.; Hay-Schmidt, A.; Sandi, C. Regulation of brain-derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. J. Psychiatr. Res. 2010, 44, 808–816. [Google Scholar] [CrossRef]
- Marmigère, F.; Givalois, L.; Rage, F.; Arancibia, S.; Tapia-Arancibia, L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus 2003, 13, 646–655. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 5, 16–18. [Google Scholar] [CrossRef]
- García-Krauss, A.; Ferrada, L.; Astuya, A.; Salazar, K.; Cisternas, P.; Martínez, F.; Ramírez, E.; Nualart, F. Dehydroascorbic Acid Promotes Cell Death in Neurons Under Oxidative Stress: A Protective Role for Astrocytes. Mol. Neurobiol. 2016, 53, 5847–5863. [Google Scholar] [CrossRef]
- Yu, Z.F.; Bruce-Keller, A.J.; Goodman, Y.; Mattson, M.P. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res. 1998, 53, 613–625. [Google Scholar] [CrossRef]
- Llull, L.; Amaro, S.; Chamorro, Á. Administration of Uric Acid in the Emergency Treatment of Acute Ischemic Stroke. Curr. Neurol. Neurosci. Rep. 2015, 16, 4. [Google Scholar] [CrossRef]
- Romanos, E.; Planas, A.M.; Amaro, S.; Chamorro, Á. Uric Acid Reduces Brain Damage and Improves the Benefits of rt-PA in a Rat Model of Thromboembolic Stroke. J. Cereb. Blood Flow Metab. 2007, 27, 14–20. [Google Scholar] [CrossRef]
- Mateos, R.; Lecumberri, E.; Ramos, S.; Goya, L.; Bravo, L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress: Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J. Chromatogr. B 2005, 827, 76–82. [Google Scholar] [CrossRef]
- Nevin, K.G.; Rajamohan, T. Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clin. Biochem. 2004, 37, 830–835. [Google Scholar] [CrossRef]
- Yeap, S.K.; Beh, B.K.; Ali, N.M.; Yusof, H.M.; Ho, W.Y.; Koh, S.P.; Alitheen, N.B.; Long, K. Antistress and antioxidant effects of virgin coconut oil in vivo. Exp. Med. 2015, 9, 39–42. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Birck, M.M.; Ipsen, D.H.; Thiessen, T.; Feldmann, L.d.B.; Lindblad, M.M.; Jensen, H.E.; Lykkesfeldt, J. Diet-induced dyslipidemia leads to nonalcoholic fatty liver disease and oxidative stress in guinea pigs. Transl. Res. 2016, 168, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Oswald, K.D.; Murdaugh, D.L.; King, V.L.; Boggiano, M.M. Motivation for palatable food despite consequences in an animal model of binge eating. Int. J. Eat. Disord. 2011, 44, 203–211. [Google Scholar] [CrossRef]
- Shomaker, L.B.; Tanofsky-Kraff, M.; Zocca, J.M.; Courville, A.; Kozlosky, M.; Columbo, K.M.; Wolkoff, L.E.; Brady, S.M.; Crocker, M.K.; Ali, A.H.; et al. Eating in the absence of hunger in adolescents: Intake after a large-array meal compared with that after a standardized meal. Am. J. Clin. Nutr. 2010, 92, 697–703. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; White, M.A.; Masheb, R.M.; Morgan, P.T.; Crosby, R.D.; Grilo, C.M. An examination of the food addiction construct in obese patients with binge eating disorder. Int. J. Eat. Disord. 2012, 45, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Ziauddeen, H.; Fletcher, P.C. Is food addiction a valid and useful concept? Obes. Rev. 2013, 14, 19–28. [Google Scholar] [CrossRef]
- Lopez-Esparza, S.; Berumen, L.C.; Padilla, K.; Miledi, R.; García-Alcocer, G. Expression of hippocampal serotonin receptors 5-HT2C and 5-HT5A in a rat model of diet-induced obesity supplemented with tryptophan. Int. J. Dev. Neurosci. 2015, 42, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, B.; Würbel, H. Reproducibility Crisis: Are We Ignoring Reaction Norms? Trends Pharmacol. Sci. 2016, 37, 509–510. [Google Scholar] [CrossRef]
- Meredith, M.E.; May, J.M. Regulation of embryonic neurotransmitter and tyrosine hydroxylase protein levels by ascorbic acid. Brain Res. 2013, 1539, 7–14. [Google Scholar] [CrossRef]
- Miwa, S.; Watanabe, Y.; Hayaishi, O. 6R-l-Erythro-5,6,7,8-tetrahydrobiopterin as a regulator of dopamine and serotonin biosynthesis in the rat brain. Arch. Biochem. Biophys. 1985, 239, 234–241. [Google Scholar] [CrossRef]
| Per 100 g | Altromin 1324 | CAF |
|---|---|---|
| Energy (kcal) | 318.8 | 534 |
| Fat (g) | 4.1 | 36.3 |
| Saturated fat (g) | 0.46 | 21.3 |
| Carbohydrates (g) | 40.8 | 47.4 |
| Sugar (g) | 4.9 | 27.4 |
| Protein (g) | 19.2 | 3.8 |
| Water content (g) | 10 | 16 |
| Dopamine | DOPAC | HVA | ||||
|---|---|---|---|---|---|---|
| Fraction | PFC | HIP | PFC | HIP | PFC | HIP |
| CAF | 7.61 ± 5.29 | n/a | 5.22 ± 2.92 | n/a | 1.82 ± 1.97 | n/a |
| Control | 9.22 ± 4.76 | n/a | 6.28 ± 2.70 | n/a | 0.78 ± 0.49 | n/a |
| Serotonin | 5-HIAA | Norepinephrine | ||||
| Fraction | PFC | HIP | PFC | HIP | PFC | HIP |
| CAF | 2.20 ± 0.84 | 0.99 ± 0.19 | 3.16 ± 0.65 | 2.88 ± 0.66 | 1.87 ± 0.31 | 2.21 ± 0.57 |
| Control | 2.65 ± 0.69 | 1.04 ± 0.30 | 3.20 ± 0.61 | 2.64 ± 0.46 | 1.91 ± 0.43 | 2.04 ± 0.49 |
| Vit C | DHA | %DHA of Vit C | Uric Acid | MDA | |
|---|---|---|---|---|---|
| CAF | 2417.00 ± 119.50 | 96.11 ± 56.97 * | 3.99 ± 2.38 ** | 263.80 ± 21.88 | 134.30 ± 38.98 * |
| Control | 2486.00 ± 441.30 | 54.98 ± 42.84 | 2.16 ± 1.59 | 253.90 ± 36.76 | 169.80 ± 46.97 |
| BH4 | BH2 | Total Biopterin Conc. | BH2/BH4 | |
|---|---|---|---|---|
| CAF | 0.444 ± 0.063 ** | 0.036 ± 0.008 * | 0.480 ± 0.067 ** | 0.081 ± 0.017 |
| Control | 0.512 ± 0.089 | 0.045 ± 0.013 | 0.557 ± 0.098 | 0.086 ± 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virtuoso, A.; Tveden-Nyborg, P.; Schou-Pedersen, A.M.V.; Lykkesfeldt, J.; Müller, H.K.; Elfving, B.; Sørensen, D.B. A Long-Term Energy-Rich Diet Increases Prefrontal BDNF in Sprague-Dawley Rats. Nutrients 2022, 14, 126. https://doi.org/10.3390/nu14010126
Virtuoso A, Tveden-Nyborg P, Schou-Pedersen AMV, Lykkesfeldt J, Müller HK, Elfving B, Sørensen DB. A Long-Term Energy-Rich Diet Increases Prefrontal BDNF in Sprague-Dawley Rats. Nutrients. 2022; 14(1):126. https://doi.org/10.3390/nu14010126
Chicago/Turabian StyleVirtuoso, Alessandro, Pernille Tveden-Nyborg, Anne Marie Voigt Schou-Pedersen, Jens Lykkesfeldt, Heidi Kaastrup Müller, Betina Elfving, and Dorte Bratbo Sørensen. 2022. "A Long-Term Energy-Rich Diet Increases Prefrontal BDNF in Sprague-Dawley Rats" Nutrients 14, no. 1: 126. https://doi.org/10.3390/nu14010126
APA StyleVirtuoso, A., Tveden-Nyborg, P., Schou-Pedersen, A. M. V., Lykkesfeldt, J., Müller, H. K., Elfving, B., & Sørensen, D. B. (2022). A Long-Term Energy-Rich Diet Increases Prefrontal BDNF in Sprague-Dawley Rats. Nutrients, 14(1), 126. https://doi.org/10.3390/nu14010126







