Effects of Sodium Selenite Injection on Serum Metabolic Profiles in Women Diagnosed with Breast Cancer-Related Lymphedema—Secondary Analysis of a Randomized Placebo-Controlled Trial Using Global Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Samples for the Study
2.2. Study Design
2.3. Anthropometry, BIA, and WBSe
2.4. Serum Preparation for LC-MS/MS Analysis
2.5. LC-MS/MS Analysis
2.6. Data Processing and Identification of Metabolites
2.7. Pathway Analysis
2.8. Statistical Analysis
3. Results
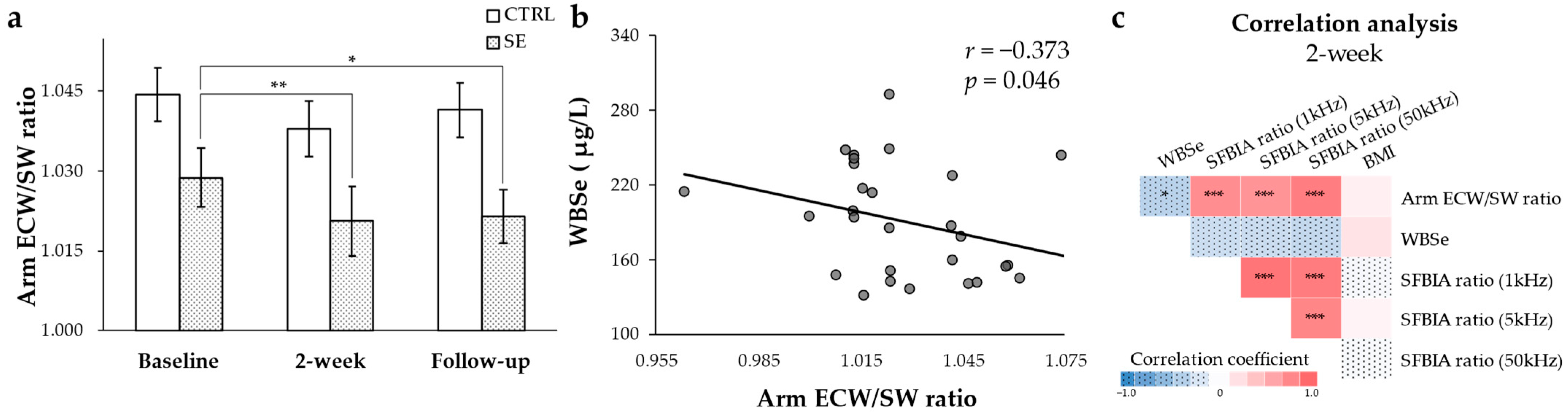
3.1. Reduced Arm ECW/SW Ratio after Se IV Injection and the Negative Correlation with WBSe in Patients with BCRL
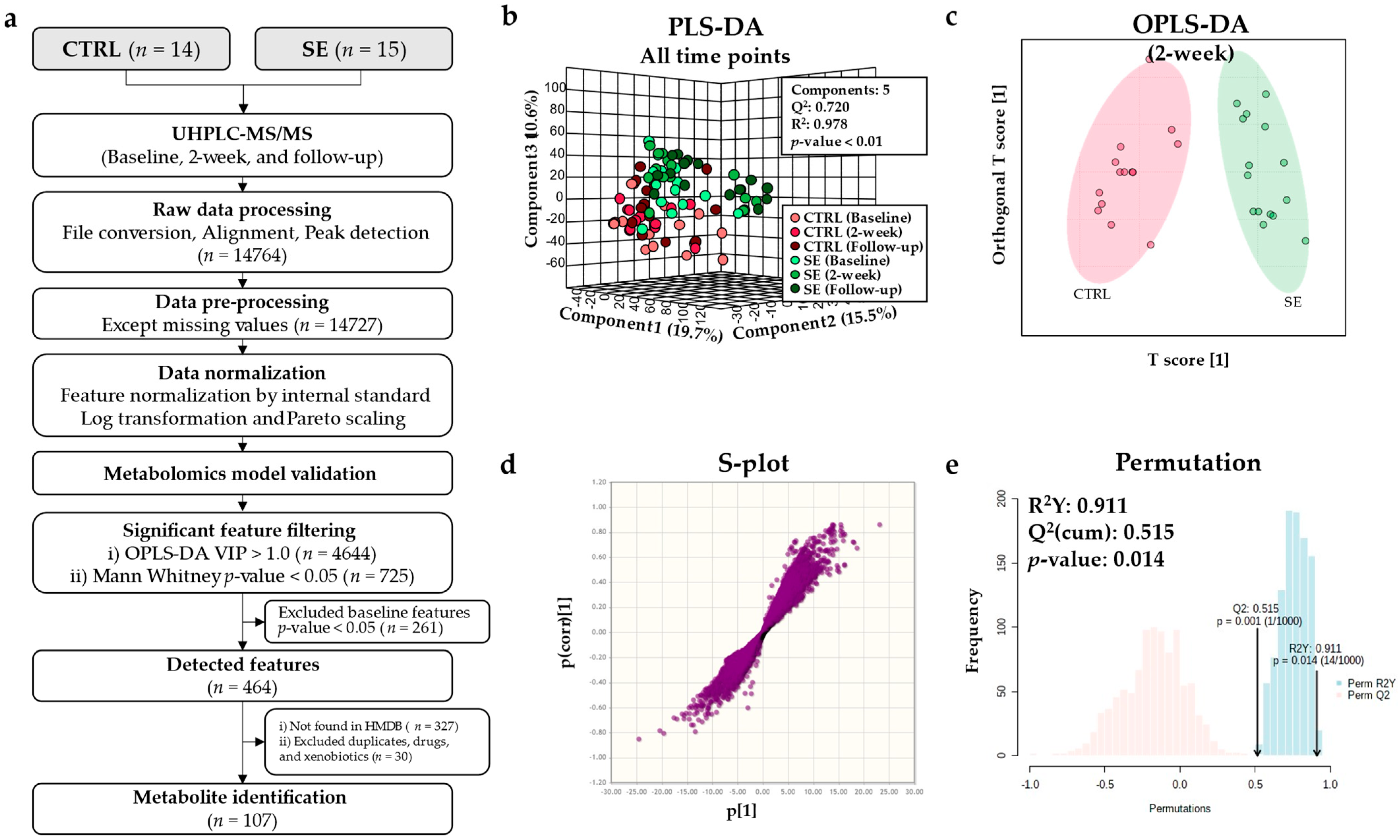
3.2. Metabolomic Model Validation
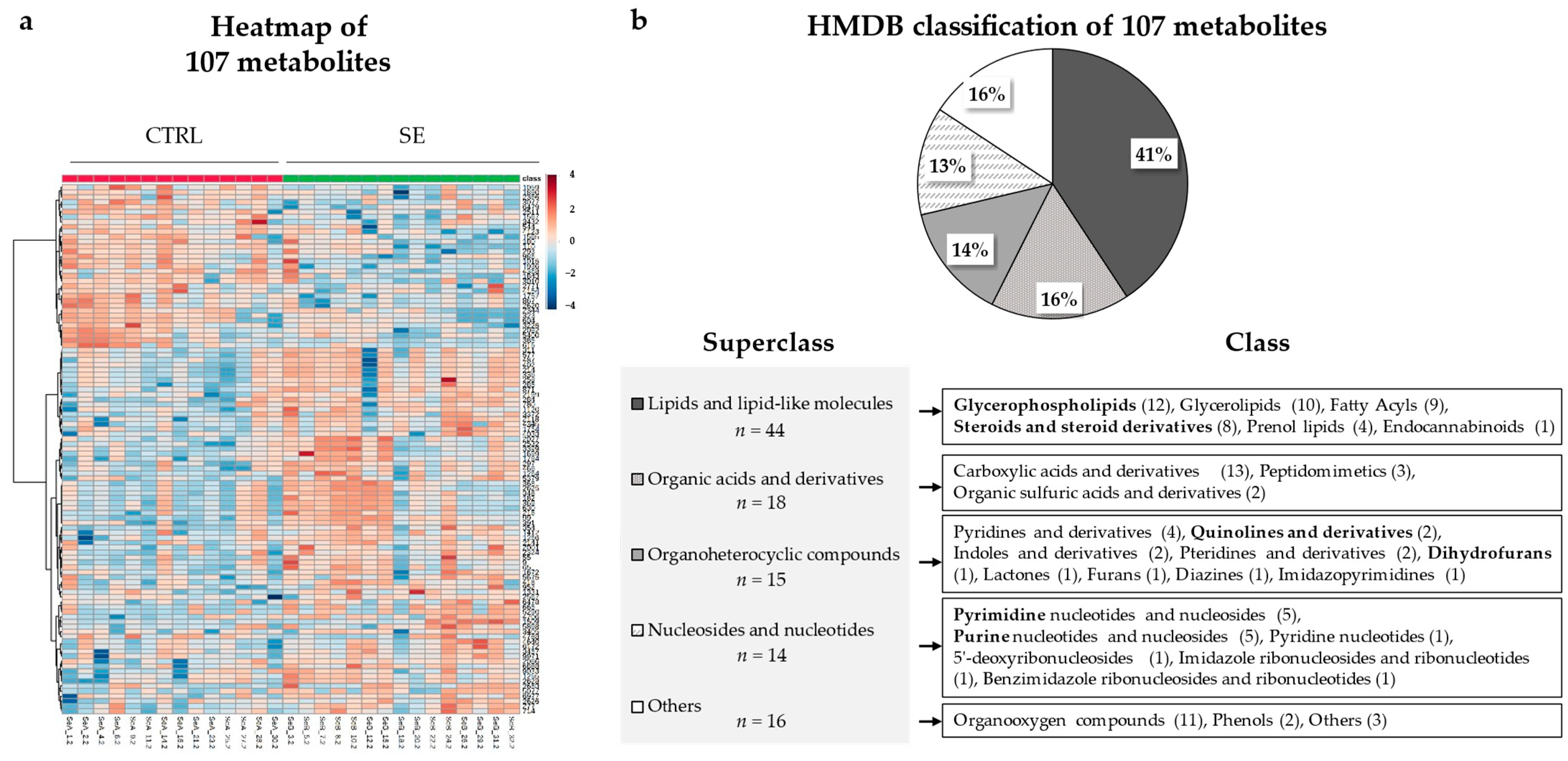
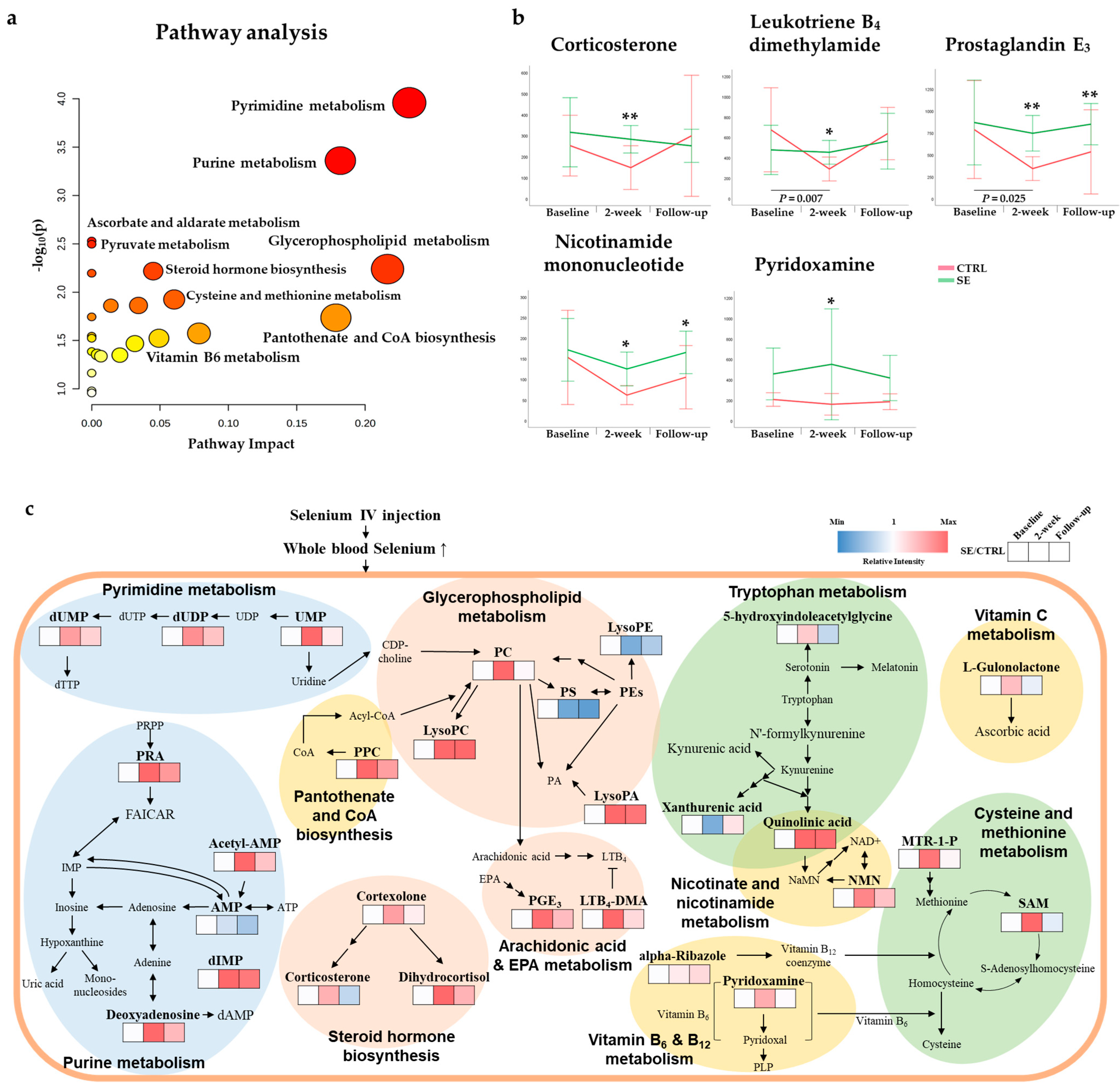
3.3. Differential Metabolites and Pathways Affected by Se IV Injection Compared to the Placebo Control
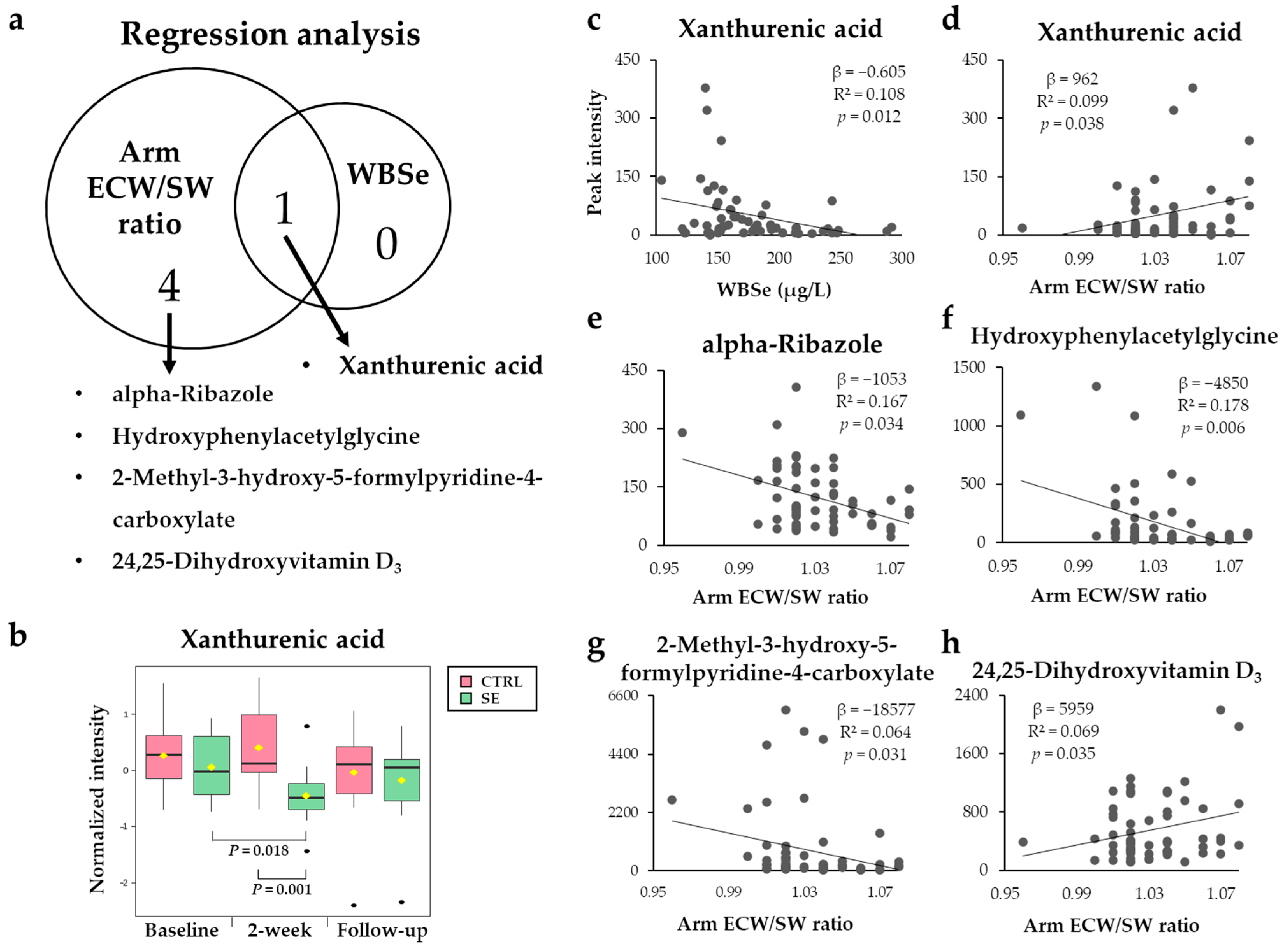
3.4. Metabolites Associated with WBSe or the Arm ECW/SW Ratio
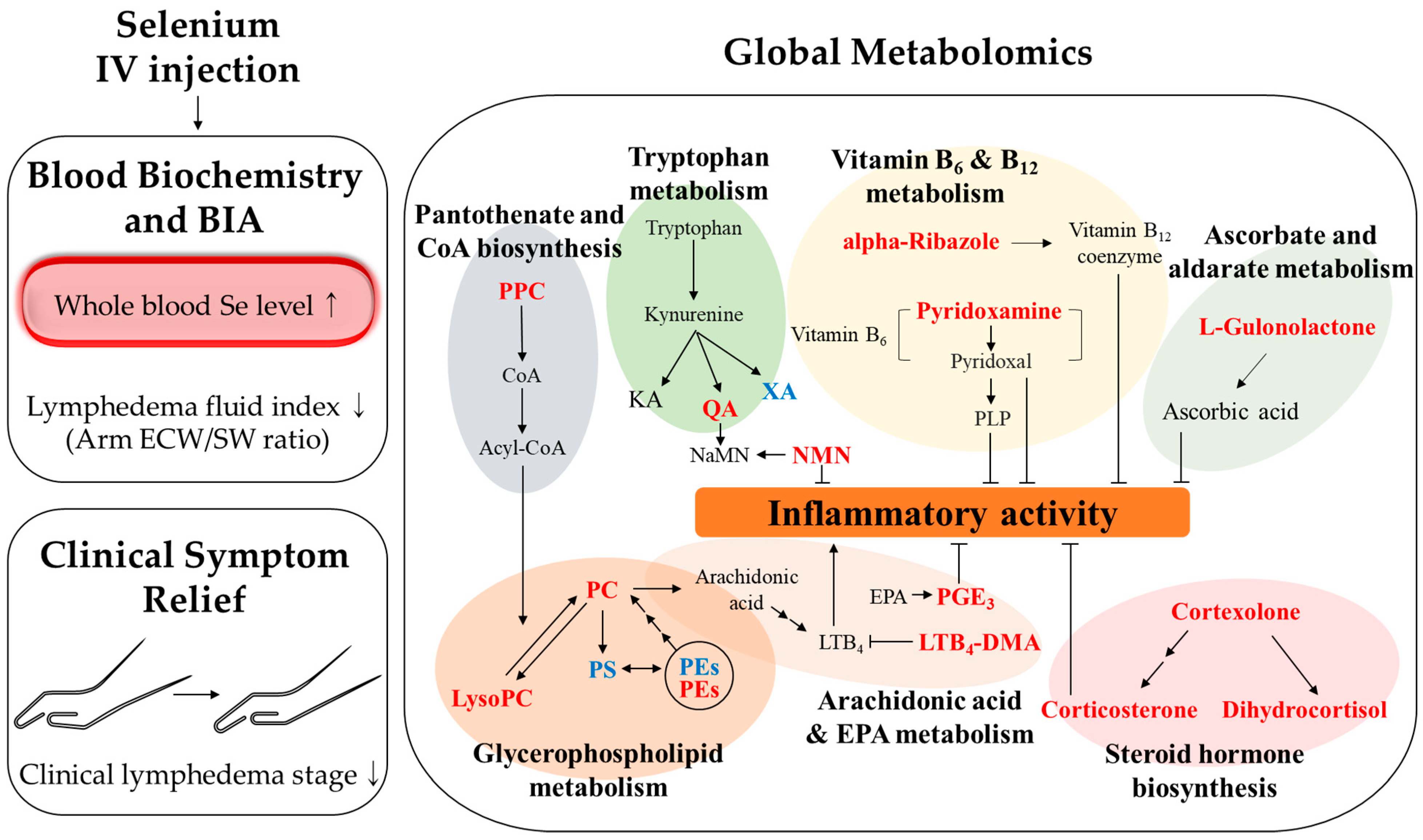
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Visser, J.; van Geel, M.; Cornelissen, A.J.M.; van der Hulst, R.R.W.J.; Qiu, S.S. Breast Cancer-Related Lymphedema and Genetic Predisposition: A Systematic Review of the Literature. Lymphat. Res. Biol. 2018, 17, 288–293. [Google Scholar] [CrossRef]
- Kasseroller, R.G.; Schrauzer, G.N. Treatment of secondary lymphedema of the arm with physical decongestive therapy and sodium selenite: A review. Am. J. Ther. 2000, 7, 273–279. [Google Scholar] [CrossRef]
- Ahmed, R.L.; Prizment, A.; Lazovich, D.; Schmitz, K.H.; Folsom, A.R. Lymphedema and quality of life in breast cancer survivors: The Iowa Women’s Health Study. J. Clin. Oncol. 2008, 26, 5689. [Google Scholar] [CrossRef]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Gärtner, R.; Jensen, M.-B.; Kronborg, L.; Ewertz, M.; Kehlet, H.; Kroman, N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment–a nationwide study of prevalence and associated factors. Breast 2010, 19, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Chachaj, A.; Małyszczak, K.; Pyszel, K.; Lukas, J.; Tarkowski, R.; Pudełko, M.; Andrzejak, R.; Szuba, A. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psycho-Oncology 2010, 19, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Amatya, B.; Pallant, J.F.; Rajapaksa, I. Factors associated with long-term functional outcomes and psychological sequelae in women after breast cancer. Breast 2012, 21, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Vassard, D.; Olsen, M.H.; Zinckernagel, L.; Vibe-Petersen, J.; Dalton, S.O.; Johansen, C. Psychological consequences of lymphoedema associated with breast cancer: A prospective cohort study. Eur. J. Cancer 2010, 46, 3211–3218. [Google Scholar] [CrossRef]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013, 27, 1114–1126. [Google Scholar] [CrossRef] [Green Version]
- Avraham, T.; Daluvoy, S.; Zampell, J.; Yan, A.; Haviv, Y.S.; Rockson, S.G.; Mehrara, B.J. Blockade of transforming growth factor-β1 accelerates lymphatic regeneration during wound repair. Am. J. Pathol. 2010, 177, 3202–3214. [Google Scholar] [CrossRef]
- Azhar, S.H.; Lim, H.Y.; Tan, B.-K.; Angeli, V. The unresolved pathophysiology of lymphedema. Front. Physiol. 2020, 11, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, K.; Kim, S.; Kang, H.; Ku, J.-M.; Park, K.W.; Lee, S. Sulfuretin has therapeutic activity against acquired lymphedema by reducing adipogenesis. Pharmacol. Res. 2017, 121, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Savetsky, I.L.; Torrisi, J.S.; Cuzzone, D.A.; Ghanta, S.; Albano, N.J.; Gardenier, J.C.; Joseph, W.J.; Mehrara, B.J. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H165–H172. [Google Scholar] [CrossRef] [Green Version]
- Michelini, S.; Ricci, M.; Serrani, R.; Stuppia, L.; Beccari, T.; Veselenyiova, D.; Kenanoglu, S.; Barati, S.; Kurti, D.; Baglivo, M. Possible Role of the RORC Gene in Primary and Secondary Lymphedema: Review of the Literature and Genetic Study of Two Rare Causative Variants. Lymphat. Res. Biol. 2021, 19, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.; Schulte, D.; Brice, G.; Simpson, M.A.; Roukens, M.G.; van Impel, A.; Connell, F.; Kalidas, K.; Jeffery, S.; Mortimer, P.S. Mutation in vascular endothelial growth factor-C, a ligand for vascular endothelial growth factor receptor-3, is associated with autosomal dominant milroy-like primary lymphedema. Circ. Res. 2013, 112, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Gousopoulos, E.; Proulx, S.T.; Bachmann, S.B.; Dieterich, L.C.; Scholl, J.; Karaman, S.; Bianchi, R.; Detmar, M. An important role of VEGF-C in promoting lymphedema development. J. Invest. Dermatol. 2017, 137, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Michelini, S.; Ricci, M.; Veselenyiova, D.; Kenanoglu, S.; Kurti, D.; Baglivo, M.; Fiorentino, A.; Basha, S.H.; Priya, S.; Serrani, R. TIE1 as a Candidate Gene for Lymphatic Malformations with or without Lymphedema. Int. J. Mol. Sci. 2020, 21, 6780. [Google Scholar] [CrossRef]
- Executive Committee. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Garza, R.; Skoracki, R.; Hock, K.; Povoski, S.P. A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies. BMC Cancer 2017, 17, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bruns, F.; Micke, O.; Bremer, M. Current status of selenium and other treatments for secondary lymphedema. J. Support. Oncol. 2003, 1, 121–130. [Google Scholar] [PubMed]
- Nakamura, K.; Radhakrishnan, K.; Wong, Y.M.; Rockson, S.G. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS ONE 2009, 4, e8380. [Google Scholar] [CrossRef] [Green Version]
- Gardenier, J.C.; Kataru, R.P.; Hespe, G.E.; Savetsky, I.L.; Torrisi, J.S.; Nores, G.D.G.; Jowhar, D.K.; Nitti, M.D.; Schofield, R.C.; Carlow, D.C. Topical tacrolimus for the treatment of secondary lymphedema. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Micke, O.; Bruns, F.; Mücke, R.; Schäfer, U.; Glatzel, M.; DeVries, A.F.; Schönekaes, K.; Kisters, K.; Büntzel, J. Selenium in the treatment of radiation-associated secondary lymphedema. Int. J. Radiat. 2003, 56, 40–49. [Google Scholar] [CrossRef]
- Zimmermann, T.; Leonhardt, H.; Kersting, S.; Albrecht, S.; Range, U.; Eckelt, U. Reduction of postoperative lymphedema after oral tumor surgery with sodium selenite. Biol. Trace Elem. Res. 2005, 106, 193–203. [Google Scholar] [CrossRef]
- Chandel, N.; Maltepe, E.; Goldwasser, E.; Mathieu, C.; Simon, M.; Schumacker, P. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 1998, 95, 11715–11720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfister, C.; Dawzcynski, H.; Schingale, F.-J. Sodium selenite and cancer related lymphedema: Biological and pharmacological effects. J. Trace Elem. Med. Biol. 2016, 37, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Kiremidjian-Schumacher, L.; Roy, M.; Glickman, R.; Schneider, K.; Rothstein, S.; Cooper, J.; Hochster, H.; Kim, M.; Newman, R. Selenium and immunocompetence in patients with head and neck cancer. Biol. Trace Elem. Res. 2000, 73, 97–111. [Google Scholar] [CrossRef]
- Bruns, F.; Büntzel, J.; Mücke, R.; Schönekaes, K.; Kisters, K.; Micke, O. Selenium in the treatment of head and neck lymphedema. Med. Princ Pract. 2004, 13, 185–190. [Google Scholar] [CrossRef]
- Han, H.W.; Yang, E.J.; Lee, S.-M. Sodium Selenite Alleviates Breast Cancer-Related Lymphedema Independent of Antioxidant Defense System. Nutrients 2019, 11, 1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickiewicz, B.; Villemaire, M.L.; Sandercock, L.E.; Jirik, F.R.; Vogel, H.J. Metabolic changes associated with selenium deficiency in mice. Biometals 2014, 27, 1137–1147. [Google Scholar] [CrossRef]
- Do, K.T.; Kastenmüller, G.; Mook-Kanamori, D.O.; Yousri, N.A.; Theis, F.J.; Suhre, K.; Krumsiek, J. Network-based approach for analyzing intra-and interfluid metabolite associations in human blood, urine, and saliva. J. Proteome Res. 2015, 14, 1183–1194. [Google Scholar] [CrossRef]
- Sedger, L.M.; Tull, D.L.; McConville, M.J.; De Souza, D.P.; Rupasinghe, T.W.; Williams, S.J.; Dayalan, S.; Lanzer, D.; Mackie, H.; Lam, T.C.J.P.O. Lipidomic profiling of adipose tissue reveals an inflammatory signature in cancer-related and primary Lymphedema. PLoS ONE 2016, 11, e0154650. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.H.; Clish, C.B.; Gladyshev, V.N. Selenium deficiency is associated with pro-longevity mechanisms. Cell Rep. 2019, 27, 2785–2797.e2783. [Google Scholar] [CrossRef] [Green Version]
- Alehagen, U.; Johansson, P.; Aaseth, J.; Alexander, J.; Surowiec, I.; Lundstedt-Enkel, K.; Lundstedt, T. Significant Changes in Metabolic Profiles after Intervention with Selenium and Coenzyme Q10 in an Elderly Population. Biomolecules 2019, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.-J.; Jo, G.-Y.; Park, J.-H.; Do, H.-K. Feasibility of segmental bioelectrical impedance analysis for mild-to moderate-degree breast cancer-related lymphedema: Correlation with circumferential volume measurement and phase angle. Medicine 2021, 100, e23722. [Google Scholar] [CrossRef]
- Sukackiene, D.; Laucyte-Cibulskiene, A.; Vickiene, A.; Rimsevicius, L.; Miglinas, M. Risk stratification for patients awaiting kidney transplantation: Role of bioimpedance derived edema index and nutrition status. Clin. Nutr. 2020, 39, 2759–2763. [Google Scholar] [CrossRef]
- Lim, S.M.; Han, Y.; Kim, S.I.; Park, H.S. Utilization of bioelectrical impedance analysis for detection of lymphedema in breast Cancer survivors: A prospective cross sectional study. BMC Cancer 2019, 19, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kim, L.; Jeon, J.Y.; Sung, I.Y.; Jeong, S.Y.; Do, J.H.; Kim, H.J. Prediction of treatment outcome with bioimpedance measurements in breast cancer related lymphedema patients. Ann. Rehabil. Med. 2011, 35, 687. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Jeon, J.Y.; Yun, G.J.; Yang, S.; Kwon, S.; Seo, Y.J. Reference values of bioelectrical impedance analysis for detecting breast cancer-related lymphedema. Medicine 2018, 97, e12945. [Google Scholar] [CrossRef]
- Shao, C.; Song, J.; Zhao, S.; Jiang, H.; Wang, B.; Chi, A. Therapeutic Effect and Metabolic Mechanism of A Selenium-Polysaccharide from Ziyang Green Tea on Chronic Fatigue Syndrome. Polymers 2018, 10, 1269. [Google Scholar] [CrossRef] [Green Version]
- Steinetz, B.; Dipasquale, G. Anti-granuloma and anti-edema properties of corticosterone in hyper-and hypothyroid rats. Proc. Soc. Exp. Biol. Med. 1967, 125, 673–676. [Google Scholar] [CrossRef]
- Showell, H.J.; Otterness, I.G.; Marfat, A.; Corey, E.J. Inhibition of leukotriene B4-induced neutrophil degranulation by leukotriene B4-dimethylamide. Biochem. Biophys. Res. Commun. 1982, 106, 741–747. [Google Scholar] [CrossRef]
- Jiang, X.; Nicolls, M.R.; Tian, W.; Rockson, S.G. Lymphatic dysfunction, leukotrienes, and lymphedema. Annu. Rev. Physiol. 2018, 80, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Rockson, S.G.; Jiang, X.; Kim, J.; Begaye, A.; Shuffle, E.M.; Tu, A.B.; Cribb, M.; Nepiyushchikh, Z.; Feroze, A.H. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Yamaguchi, H.; Mano, N. Transport of Eicosapentaenoic Acid-Derived PGE 3, PGF 3α, and TXB 3 by ABCC4. PLoS ONE 2014, 9, e109270. [Google Scholar] [CrossRef]
- Zhai, Q.; Xiao, Y.; Li, P.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Varied doses and chemical forms of selenium supplementation differentially affect mouse intestinal physiology. Food Funct. 2019, 10, 5398–5412. [Google Scholar] [CrossRef]
- YIN, S.-a.; SATO, I.; HOSOKAWA, Y.; NIIZEKI, S.; TOJO, H.; YAMAGUCHI, K. The necessity of dietary vitamin B6 to selenium biopotency for tissue selenium and glutathione peroxidase in rats. J. Nutr. Sci. Vitaminol. 1991, 37, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caton, P.W.; Kieswich, J.; Yaqoob, M.; Holness, M.; Sugden, M. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia 2011, 54, 3083–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Wei, J.C.; Wu, D.; Huang, Y. Vitamin B 6 supplementation improves pro-inflammatory responses in patients with rheumatoid arthritis. Eur. J. Clin. Nutr. 2010, 64, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Tsuchiya, K.; Kinoshita, T.; Kushiyama, H.; Suidasari, S.; Hatakeyama, M.; Imura, H.; Kato, N.; Suda, T. Vitamin B6 prevents IL-1β protein production by inhibiting NLRP3 inflammasome activation. J. Biol. Chem. 2016, 291, 24517–24527. [Google Scholar] [CrossRef] [Green Version]
- Yanaka, N.; Koyama, T.-A.; Komatsu, S.-I.; Nakamura, E.; Kanda, M.; Kato, N. Vitamin B6 suppresses NF-κB activation in LPS-stimulated mouse macrophages. Int. J. Mol. Med. 2005, 16, 1071–1075. [Google Scholar] [CrossRef]
- Casley-Smith, J.; Curri, S.; Földi, M. Topical treatment of acute hindlimb lymphedema of the rat using a troxerutin-phosphatidylcholine complex in liposomal-like microdispersion. Lymphology 1993, 26, 25–27. [Google Scholar] [PubMed]
- Ling, J.; Chaba, T.; Zhu, L.F.; Jacobs, R.L.; Vance, D.E. Hepatic ratio of phosphatidylcholine to phosphatidylethanolamine predicts survival after partial hepatectomy in mice. Hepatology 2012, 55, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Cansev, M.; Watkins, C.J.; van der Beek, E.M.; Wurtman, R.J. Oral uridine-5′-monophosphate (UMP) increases brain CDP-choline levels in gerbils. Brain Res. 2005, 1058, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Gurun, M.S.; Parker, R.; Eisenach, J.C.; Vincler, M. The effect of peripherally administered CDP-choline in an acute inflammatory pain model: The role of α7 nicotinic acetylcholine receptor. Anesth. Analg. 2009, 108, 1680–1687. [Google Scholar] [CrossRef]
- Yoshida, S.; Alksne, J.F.; Seelig, J.M.; Bailey, M.D.; Moore, S.S.; Kitamura, K. Effect of uridine 5’-diphosphate on cryogenic brain edema in rabbits. Stroke 1989, 20, 1694–1699. [Google Scholar] [CrossRef] [Green Version]
- Mafu, T.S.; September, A.V.; Shamley, D. The potential role of angiogenesis in the development of shoulder pain, shoulder dysfunction, and lymphedema after breast cancer treatment. Cancer Manag. 2018, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, N.; Shibata, R.; Walsh, K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ. Res. 2005, 96, 838–846. [Google Scholar] [CrossRef] [Green Version]
- Al-Daghri, N.M.; Rahman, S.; Sabico, S.; Yakout, S.; Wani, K.; Al-Attas, O.S.; Saravanan, P.; Tripathi, G.; McTernan, P.G.; Alokail, M.S. Association of vitamin B12 with pro-inflammatory cytokines and biochemical markers related to cardiometabolic risk in Saudi subjects. Nutrients 2016, 8, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggio-Hall, L.A.; Escalante-Semerena, J.C. In vitro synthesis of the nucleotide loop of cobalamin by Salmonella typhimurium enzymes. Proc. Natl. Acad. Sci. USA 1999, 96, 11798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowie, A.G. Vitamin C Inhibits NF-κB Activation by TNF Via the Activation of p38 Mitogen-Activated Protein Kinase1. J. Immunol. 2000, 165, 7180–7188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cárcamo, J.M.; Pedraza, A.; Bórquez-Ojeda, O.; Golde, D.W. Vitamin C suppresses TNFα-induced NFκB activation by inhibiting IκBα phosphorylation. Biochemistry 2002, 41, 12995–13002. [Google Scholar] [CrossRef] [PubMed]
- Brahma, B.; Forman, R.; Stewart, E.; Nicholson, C.; Rice, M. Ascorbate inhibits edema in brain slices. J. Neurochem. 2000, 74, 1263–1270. [Google Scholar] [CrossRef]
- Alishiri, A.; Mosavi, S.A. Ascorbic acid versus placebo in postoperative lid edema postphotorefractive keratectomy: A double-masked, randomized, prospective study. Oman J. Ophthalmol. 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Pfalzer, A.C.; Choi, S.-W.; Tammen, S.A.; Park, L.K.; Bottiglieri, T.; Parnell, L.D.; Lamon-Fava, S. S-adenosylmethionine mediates inhibition of inflammatory response and changes in DNA methylation in human macrophages. Physiol. Genom. 2014, 46, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Malina, H.Z.; Richter, C.; Mehl, M.; Hess, O.M. Pathological apoptosis by xanthurenic acid, a tryptophan metabolite: Activation of cell caspases but not cytoskeleton breakdown. BMC Physiol. 2001, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.-H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. (Landmark Ed.) 2015, 20, 1116–1143. [Google Scholar]
- Oxenkrug, G.F. Increased plasma levels of xanthurenic and kynurenic acids in type 2 diabetes. Mol. Neurobiol. 2015, 52, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Boyle, I.T.; Omdahl, J.L.; Gray, R.W.; DeLuca, H.F. The biological activity and metabolism of 24, 25-dihydroxyvitamin D3. J. Biol. Chem. 1973, 248, 4174–4180. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, J.; Sun, T.; Li, G.; Szeto, F.L.; Liu, W.; Deb, D.K.; Wang, Y.; Zhao, Q.; Thadhani, R. 1, 25-Dihydroxyvitamin D3 suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-κB activation. Arch. Biochem. 2011, 507, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Group Comparisons 2 | OPLS-DA 1 | |||
|---|---|---|---|---|
| Q2 (cum) | R2 (cum) | Q2 (cum)/R2 (cum) | Permutation Test (p-Value) | |
| Between groups (CTRL–SE) | ||||
| 0–0 | 0.543 | 0.988 | 0.550 | 0.21 |
| 2–2 | 0.515 | 0.911 | 0.565 | 0.01 |
| 6–6 | 0.509 | 0.987 | 0.516 | 0.14 |
| Within groups (CTRL–CTRL) | ||||
| 0–2 | 0.335 | 0.813 | 0.412 | 0.99 |
| 0–6 | 0.203 | 0.993 | 0.204 | 0.80 |
| 2–6 | 0.113 | 0.910 | 0.124 | 0.29 |
| Within groups (SE–SE) | ||||
| 0–2 | −0.309 | 0.996 | −0.310 | 0.23 |
| 0–6 | −0.104 | 0.911 | −0.114 | 0.01 |
| 2–6 | −0.325 | 0.691 | −0.470 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Lee, B.; Kim, Y.; Min, S.; Yang, E.; Lee, S. Effects of Sodium Selenite Injection on Serum Metabolic Profiles in Women Diagnosed with Breast Cancer-Related Lymphedema—Secondary Analysis of a Randomized Placebo-Controlled Trial Using Global Metabolomics. Nutrients 2021, 13, 3253. https://doi.org/10.3390/nu13093253
Lee H, Lee B, Kim Y, Min S, Yang E, Lee S. Effects of Sodium Selenite Injection on Serum Metabolic Profiles in Women Diagnosed with Breast Cancer-Related Lymphedema—Secondary Analysis of a Randomized Placebo-Controlled Trial Using Global Metabolomics. Nutrients. 2021; 13(9):3253. https://doi.org/10.3390/nu13093253
Chicago/Turabian StyleLee, Heeju, Bora Lee, Yeonhee Kim, Sohyun Min, Eunjoo Yang, and Seungmin Lee. 2021. "Effects of Sodium Selenite Injection on Serum Metabolic Profiles in Women Diagnosed with Breast Cancer-Related Lymphedema—Secondary Analysis of a Randomized Placebo-Controlled Trial Using Global Metabolomics" Nutrients 13, no. 9: 3253. https://doi.org/10.3390/nu13093253
APA StyleLee, H., Lee, B., Kim, Y., Min, S., Yang, E., & Lee, S. (2021). Effects of Sodium Selenite Injection on Serum Metabolic Profiles in Women Diagnosed with Breast Cancer-Related Lymphedema—Secondary Analysis of a Randomized Placebo-Controlled Trial Using Global Metabolomics. Nutrients, 13(9), 3253. https://doi.org/10.3390/nu13093253







