Irritable Bowel Syndrome, Depression, and Neurodegeneration: A Bidirectional Communication from Gut to Brain
Abstract
1. Introduction
2. Irritable Bowel Syndrome and Gut Dysbiosis
3. Irritable Bowel Syndrome and Depression
4. Cognition and Neurology in Irritable Bowel Syndrome
5. Neurodegeneration in Irritable Bowel Syndrome: Roles of Enteric Nervous System
6. Neurodegeneration in IBS: Roles of Central Nervous System
7. Therapeutic Interventions in IBS: The Role of Antidepressants
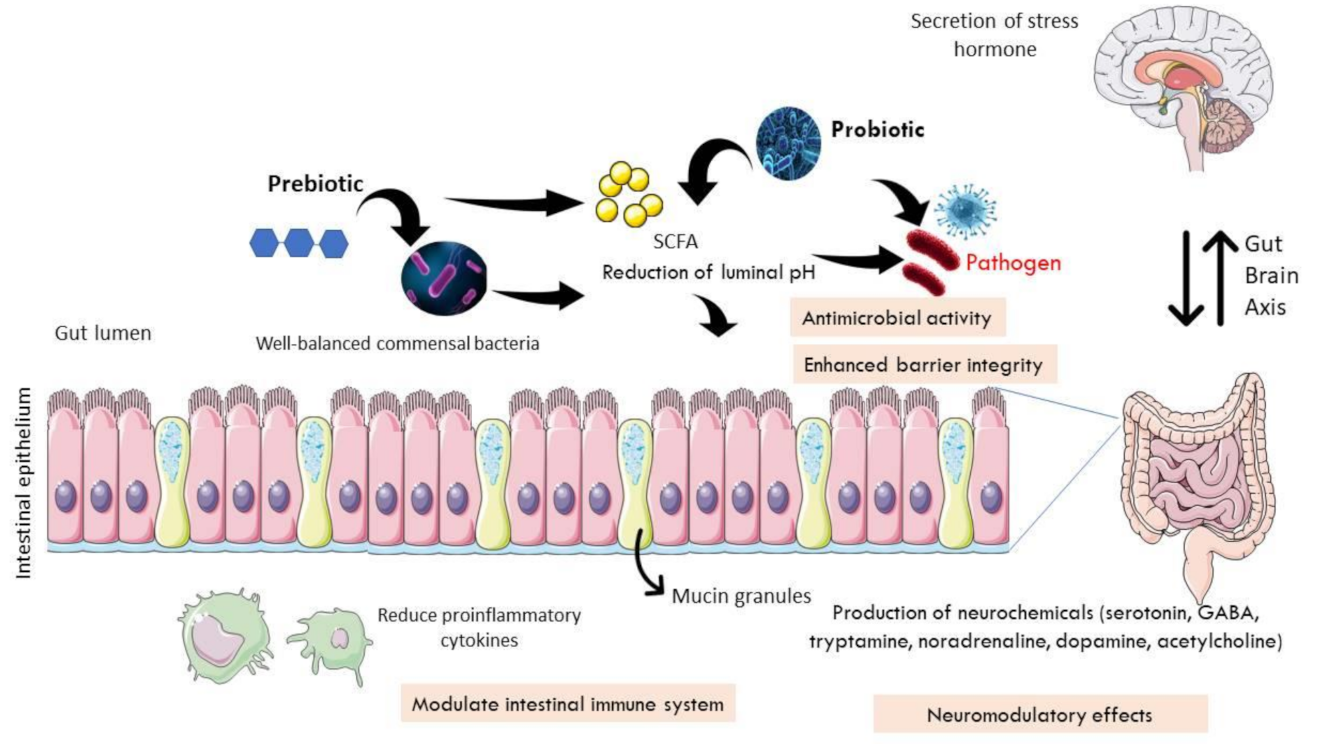
8. Therapeutic Intervention in IBS: Roles of Prebiotics, Probiotics, and Psychobiotics
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef]
- Silvernale, C.; Kuo, B.; Staller, K. Racial disparity in healthcare utilization among patients with Irritable Bowel Syndrome: Results from a multicenter cohort. Neurogastroenterol. Motil. 2020, 33, e14039. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Tan, Y.-M.; Goh, K.L.; Muhidayah, R.; Ooi, C.L.; Salem, O. Prevalence of irritable bowel syndrome in young adult Malaysians: A survey among medical students. J. Gastroenterol. Hepatol. 2003, 18, 1412–1416. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Waid, A.; Tan, H.J.; Chua, A.S.B.; Whitehead, W.E. Rome III survey of irritable bowel syndrome among ethnic Malays. World J. Gastroenterol. 2012, 18, 6475–6480. [Google Scholar] [CrossRef]
- Rajendra, S.; Alahuddin, S. Prevalence of irritable bowel syndrome in a multi-ethnic Asian population. Aliment. Pharmacol. Ther. 2004, 19, 704–706. [Google Scholar] [CrossRef]
- Oświęcimska, J.; Szymlak, A.; Roczniak, W.; Girczys-Połedniok, K.; Kwiecień, J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv. Med. Sci. 2017, 62, 17–30. [Google Scholar] [CrossRef]
- Hadjivasilis, A.; Tsioutis, C.; Michalinos, A.; Ntourakis, D.; Christodoulou, D.K.; Agouridis, A.P. New insights into irritable bowel syndrome: From pathophysiology to treatment. Ann. Gastroenterol. 2019, 32, 554–564. [Google Scholar] [CrossRef]
- Defrees, D.N.; Bailey, J. Irritable bowel syndrome. Prim. Care: Clin. Off. Pr. 2017, 44, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Grad, S.; Dumitrascu, D.L. Irritable bowel syndrome subtypes: New names for old medical conditions. Dig. Dis. 2019, 38, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.; Ford, A.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Abdollahi, M.; Rahimi, R. The Role of visceral hypersensitivity in irritable bowel syndrome: Pharmacological targets and novel treatments. J. Neurogastroenterol. Motil. 2016, 22, 558–574. [Google Scholar] [CrossRef]
- GonzálezCastro, A.M.; Martínez, C.; SalvoRomero, E.; Fortea, M.; PardoCamacho, C.; Perez, M.V.; AlonsoCotoner, C.; Santos, J.; Vicario, M. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2017, 32, 53–63. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Meerveld, B.G.-V.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Canavan, C.; West, J.; Card, T. Review article: The economic impact of the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2014, 40, 1023–1034. [Google Scholar] [CrossRef]
- Zhang, F.; Xiang, W.; Li, C.-Y.; Li, S.-C. Economic burden of irritable bowel syndrome in China. World J. Gastroenterol. 2016, 22, 10450–10460. [Google Scholar] [CrossRef]
- Drossman, D.A.; Chang, L.; Schneck, S.; Blackman, C.; Norton, W.F.; Norton, N.J. A Focus Group Assessment of Patient Perspectives on Irritable Bowel Syndrome and Illness Severity. Dig. Dis. Sci. 2009, 54, 1532–1541. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 35–56. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C.; Rinninella, E.; Raoul, P.; Cintoni, M.; et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, G.; Qin, X.; Huang, S.; Wang, B.; Cao, H. The potential role of gut mycobiome in irritable bowel syndrome. Front. Microbiol. 2019, 10, 1894. [Google Scholar] [CrossRef] [PubMed]
- Herndon, C.C.; Wang, Y.; Lu, C. Targeting the gut microbiota for the treatment of irritable bowel syndrome. Kaohsiung J. Med. Sci. 2019, 36, 160–170. [Google Scholar] [CrossRef]
- Andrews, C.N.; Sidani, S.; Marshall, J.K. Clinical management of the microbiome in irritable bowel syndrome. J. Can. Assoc. Gastroenterol. 2020, 4, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Carco, C.; Young, W.; Gearry, R.B.; Talley, N.J.; McNabb, W.C.; Roy, N.C. Increasing evidence that irritable bowel syndrome and functional gastrointestinal disorders have a microbial pathogenesis. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Fallah, F.; Pormohammad, A.; Taghipour, A.; Safari, H.; Chirani, A.S.; Sabour, S.; AlizadehSani, M.; Azimi, T. The possible role of bacteria, viruses, and parasites in initiation and exacerbation of irritable bowel syndrome. J. Cell. Physiol. 2018, 234, 8550–8569. [Google Scholar] [CrossRef] [PubMed]
- Dayananda, P.; Wilcox, M.H. Irritable bowel syndrome following Clostridium difficile infection. Curr. Opin. Gastroenterol. 2019, 35, 1–5. [Google Scholar] [CrossRef]
- Kim, Y.-A.; Cho, Y.J.; Kwak, S.G. The association between Helicobacter pylori infection and irritable bowel syndrome: A meta-analysis. Int. J. Environ. Res. Public Health 2020, 17, 2524. [Google Scholar] [CrossRef]
- Dogan, B.; Belcher-Timme, H.F.; I Dogan, E.; Jiang, Z.-D.; Dupont, H.L.; Synder, N.; Yang, S.; Chandler, B.; Scherl, E.J.; Simpson, K.W. Evaluation of Escherichia coli pathotypes associated with Irritable Bowel Syndrome. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Dinan, T.G.; Kennedy, P.J.; Beers, S.; Scott, K.; et al. Transferring the blues: Depres-sion-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, A.; Huang, T.; Lai, J.; Li, J.; Sublette, M.E.; Lu, H.; Lu, Q.; Du, Y.; Hu, Z.; et al. Gut microbiota changes in patients with bipolar depression. Adv. Sci. 2019, 6, 1900752. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal mi-crobiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- WHO. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Gardner, A.; Boles, R.G. Beyond the serotonin hypothesis: Mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 730–743. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Bahrudin, M.F.; Ghani, N.A.; Rani, R.A.; Ali, R.A.R. Prevalence of subthreshold depression among constipation-predominant irritable bowel syndrome patients. Front. Psychol. 2020, 11, 1936. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef]
- Roohafza, H.; Bidaki, E.Z.; Hasanzadeh-Keshteli, A.; Daghaghzade, H.; Afshar, H.; Adibi, P. Anxiety, depression and distress among irritable bowel syndrome and their subtypes: An epidemiological population based study. Adv. Biomed. Res. 2016, 5, 183. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Doo, E.; Choi, J.M.; Jang, S.H.; Ryu, H.S.; Lee, J.Y.; Oh, J.H.; Park, J.H.; Kim, Y.S.; Brain-Gut Axis Research Group of Korean Society of, N.; et al. The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: Systematic review and meta-analysis. J. Neurogastroenterol. Motil. 2017, 23, 349–362. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic review with meta-analysis: The prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Zhang, Q.-E.; Wang, F.; Zheng, W.; Ng, C.H.; Ungvari, G.S.; Wang, G.; Xiang, Y.-T. Comparison of comorbid depression between irritable bowel syndrome and inflammatory bowel disease: A meta-analysis of comparative studies. J. Affect. Disord. 2018, 237, 37–46. [Google Scholar] [CrossRef]
- Midenfjord, I.; Polster, A.; Sjövall, H.; Törnblom, H.; Simrén, M. Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol. Motil. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Sibelli, A.; Chalder, T.; Everitt, H.; Workman, P.; Windgassen, S.; Moss-Morris, R. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol. Med. 2016, 46, 3065–3080. [Google Scholar] [CrossRef]
- Takajo, T.; Tomita, K.; Tsuchihashi, H.; Enomoto, S.; Tanichi, M.; Toda, H.; Okada, Y.; Furuhashi, H.; Sugihara, N.; Wada, A.; et al. Depression promotes the onset of irritable bowel syndrome through unique dysbiosis in rats. Gut Liver 2019, 13, 325–332. [Google Scholar] [CrossRef]
- Attree, E.A.; Dancey, C.P.; Keeling, D.; Wilson, C. Cognitive function in people with chronic illness: Inflammatory bowel disease and irritable bowel syndrome. Appl. Neuropsychol. 2003, 10, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Hestad, K. Cognitive functions and depression in patients with irritable bowel syndrome. Gastroenterol. Res. Pr. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lam, N.C.-Y.; Yeung, H.-Y.; Li, W.-K.; Lo, H.-Y.; Yuen, C.-F.; Chang, R.C.-C.; Ho, Y.-S. Cognitive impairment in irritable bowel syndrome (IBS): A systematic review. Brain Res. 2019, 1719, 274–284. [Google Scholar] [CrossRef]
- Blankstein, U.; Chen, J.; Diamant, N.E.; Davis, K.D. Altered brain structure in irritable bowel syndrome: Potential contributions of pre-existing and disease-driven factors. Gastroenterology 2010, 138, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Yang, W.; Meng, P.; Huang, W.; Zheng, Q.; Xia, Y.; Liu, F. Changes of the postcentral cortex in irritable bowel syndrome patients. Brain Imaging Behav. 2019, 14, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Seminowicz, D.A.; Labus, J.S.; Bueller, J.A.; Tillisch, K.; Naliboff, B.D.; Bushnell, M.C.; Mayer, E.A. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 2010, 139, 48–57.e2. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, S.; Tian, J.; Jiang, G.; Wen, H.; Wang, T.; Fang, J.; Zhan, W.; Xu, Y. Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: A resting-state fMRI study. Clin. Neurophysiol. 2015, 126, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Liu, C.; Ke, J.; Xu, Q.; Ye, Y.; Jia, L.; Wang, F.; Zhang, L.; Lu, G. Abnormal amygdala resting-state functional connectivity in irritable bowel syndrome. Am. J. Neuroradiol. 2016, 37, 1139–1145. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Naliboff, B.; Labus, J.S.; Gupta, A.; Kilpatrick, L.A.; Ashe-McNalley, C.; Stains, J.; Heendeniya, N.; Smith, S.R.; Tillisch, K.; et al. Altered brain responses in subjects with irritable bowel syndrome during cued and uncued pain expectation. Neurogastroenterol. Motil. 2015, 28, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Tillisch, K.; Mayer, E.A.; Labus, J.S. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011, 140, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Mayer, E.; Harris, R.J.; Ashe-McNally, C.; Naliboff, B.D.; Labus, J.S.; Tillisch, K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain 2013, 154, 1528–1541. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, C.S.; Becerra, L.; Heinz, N.; Ludwick, A.; Rasooly, T.; Yendiki, A.; Wu, R.; Schechter, N.L.; Nurko, S.; Borsook, D. Microstructural white matter abnormalities in the dorsal cingulum of adolescents with IBS. eNeuro 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Kempton, M.J.; Salvador, Z.; Munafo, M.; Geddes, J.R.; Simmons, A.; Frangou, S.; Williams, S. Structural neuroimaging studies in major depressive disorder. Arch. Gen. Psychiatry 2011, 68, 675–690. [Google Scholar] [CrossRef]
- Lai, C.-H. Gray matter volume in major depressive disorder: A meta-analysis of voxel-based morphometry studies. Psychiatry Res. Neuroimaging 2013, 211, 37–46. [Google Scholar] [CrossRef]
- Lener, M.S.; Iosifescu, D.V. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: A review of the literature. Ann. N. Y. Acad. Sci. 2015, 1344, 50–65. [Google Scholar] [CrossRef]
- Bremner, J.D.; Narayan, M.; Anderson, E.R.; Staib, L.; Miller, H.L.; Charney, D.S. Hippocampal volume reduction in major depression. Am. J. Psychiatry 2000, 157, 115–118. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, M.C.; Yucel, K.; Nazarov, A.; MacQueen, G.M. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J. Psychiatry Neurosci. 2009, 34, 41–54. [Google Scholar]
- Schmaal, L.; for the ENIGMA-Major Depressive Disorder Working Group; Hibar, D.; Sämann, P.; Hall, G.; Baune, B.; Jahanshad, N.; Cheung, J.; Van Erp, T.; Bos, D.; et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Mol. Psychiatry 2016, 22, 900–909. [Google Scholar] [CrossRef] [PubMed]
- De Giorgio, R.; Volta, U.; Stanghellini, V.; Cogliandro, R.F.; Barbara, G.; Corinaldesi, R.; Towns, R.; Guo, C.; Hong, S.; Wiley, J.W. Neurogenic chronic intestinal pseudo-obstruction: Antineuronal antibody-mediated activation of autophagy via fas. Gastroenterology 2008, 135, 601–609. [Google Scholar] [CrossRef]
- Ostertag, D.; Buhner, S.; Michel, K.; Pehl, C.; Kurjak, M.; Gotzberger, M.; Schulte-Frohlinde, E.; Frieling, T.; Enck, P.; Phillip, J.; et al. Reduced responses of submucous neurons from irritable bowel syndrome patients to a cocktail containing histamine, serotonin, TNFalpha, and Tryptase (IBS-Cocktail). Front. Neurosci. 2015, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fei, G.; Fang, X.; Yang, X.; Sun, X.; Qian, J.; Wood, J.D.; Ke, M. Changes in enteric neurons of small intestine in a rat model of irritable bowel syndrome with diarrhea. J. Neurogastroenterol. Motil. 2016, 22, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.S.; Morteau, O.; Bozymski, E.M.; Woosley, J.T.; Sartor, R.B.; Davies, M.J.; Johnson, D.A.; Turner, M.J.; Whitehead, W.E. Elevated vasoactive intestinal peptide concentrations in patients with irritable bowel syndrome. Dig. Dis. Sci. 2004, 49, 1236–1243. [Google Scholar] [CrossRef]
- Ye, L.; Li, G.; Goebel, A.; Raju, A.V.; Kong, F.; Lv, Y.; Li, K.; Zhu, Y.; Raja, S.; He, P.; et al. Caspase-11–mediated enteric neuronal pyroptosis underlies Western diet–induced colonic dysmotility. J. Clin. Investig. 2020, 130, 3621–3636. [Google Scholar] [CrossRef]
- Fan, W.; Fei, G.; Li, X.; Wang, X.; Hu, C.; Xin, H.; Sun, X.; Li, Y.; Wood, J.D.; Fang, X. Sera with anti-enteric neuronal antibodies from patients with irritable bowel syndrome promote apoptosis in myenteric neurons of guinea pigs and human SH-Sy5Y cells. Neurogastroenterol. Motil. 2018, 30, e13457. [Google Scholar] [CrossRef] [PubMed]
- Pittock, S.J.; Lennon, V.A.; Dege, C.L.; Talley, N.J.; Locke, G.R. Neural autoantibody evaluation in functional gastrointestinal disorders: A population-based case–control study. Dig. Dis. Sci. 2010, 56, 1452–1459. [Google Scholar] [CrossRef]
- Lai, S.-W.; Liao, K.-F.; Lin, C.-L.; Sung, F.-C. Irritable bowel syndrome correlates with increased risk of Parkinson’s disease in Taiwan. Eur. J. Epidemiol. 2014, 29, 57–62. [Google Scholar] [CrossRef]
- Liu, B.; Sjolander, A.; Pedersen, N.L.; Ludvigsson, J.F.; Chen, H.; Fang, F.; Wirdefeldt, K. Irritable bowel syndrome and Parkinson’s disease risk: Register-based studies. NPJ Parkinsons Dis. 2021, 7, 1–7. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, C.-L.; Kao, C.-H. Irritable bowel syndrome is associated with an increased risk of dementia: A nationwide population-based study. PLoS ONE 2016, 11, e0144589. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Qi, R.; Liu, C.; Ke, J.; Xu, Q.; Wang, F.; Zhang, L.J.; Lu, G.M. Disrupted functional connectivity density in irritable bowel syndrome patients. Brain Imaging Behav. 2016, 11, 1812–1822. [Google Scholar] [CrossRef]
- Fang, J.; Li, S.; Li, M.; Chan, Q.; Ma, X.; Su, H.; Wang, T.; Zhan, W.; Yan, J.; Xu, M.; et al. Altered white matter microstructure identified with tract-based spatial statistics in irritable bowel syndrome: A diffusion tensor imaging study. Brain Imaging Behav. 2016, 11, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Edlow, B.L.; Copen, W.A.; Izzy, S.; van der Kouwe, A.; Glenn, M.B.; Greenberg, S.M.; Greer, D.M.; Wu, O. Longitudinal Diffusion Tensor Imaging Detects Recovery of Fractional Anisotropy Within Traumatic Axonal Injury Lesions. Neurocrit. Care 2016, 24, 342–352. [Google Scholar] [CrossRef]
- Moayedi, M.; Weissman-Fogel, I.; Salomons, T.V.; Crawley, A.P.; Goldberg, M.B.; Freeman, B.V.; Tenenbaum, H.C.; Davis, K.D. White matter brain and trigeminal nerve abnormalities in temporomandibular disorder. Pain 2012, 153, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Jarman, J.; Fernandez, M.; Davies, P.T.; Glover, V.; Steiner, T.J.; Thompson, C.; Rose, F.C.; Sandler, M. High incidence of endogenous depression in migraine: Confirmation by tyramine test. J. Neurol. Neurosurg. Psychiatry 1990, 53, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Zhang, L.; Chen, Q.; Zong, N.; Zhang, P.; Ji, X.; Ma, S.; Zhang, Y.; Huang, W.; Du, Z.; et al. White matter microstructural similarity and diversity of functional constipation and constipation-predominant irritable bowel syndrome. J. Neurogastroenterol. Motil. 2018, 24, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qian, S.; Liu, K.; Li, B.; Li, M.; Xin, K.; Sun, G. Reduced white matter integrity and its correlation with clinical symptom in first-episode, treatment-naive generalized anxiety disorder. Behav. Brain Res. 2016, 314, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Choi, S.; Kang, J.; Kim, A.; Han, K.M.; Chang, H.S.; Tae, W.S.; Son, K.R.; Joe, S.H.; Lee, M.S.; et al. Association between reduced white matter integrity in the corpus callosum and serotonin transporter gene DNA methylation in medication-naive patients with major depressive disorder. Transl. Psychiatry 2016, 6, e866. [Google Scholar] [CrossRef]
- Benedetti, F.; Yeh, P.H.; Bellani, M.; Radaelli, D.; Nicoletti, M.A.; Poletti, S.; Falini, A.; Dallaspezia, S.; Colombo, C.; Scotti, G.; et al. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol. Psychiatry 2011, 69, 309–317. [Google Scholar] [CrossRef]
- Elahi, S.; Bachman, A.H.; Lee, S.H.; Sidtis, J.J.; Ardekani, B.A.; Alzheimer’s Disease Neuroimaging, I. Corpus callosum atrophy rate in mild cognitive impairment and prodromal Alzheimer’s disease. J. Alzheimers Dis. 2015, 45, 921–931. [Google Scholar] [CrossRef]
- Di Paola, M.; Phillips, O.; Orfei, M.D.; Piras, F.; Cacciari, C.; Caltagirone, C.; Spalletta, G. Corpus callosum structure is topographically correlated with the early course of cognition and depression in Alzheimer’s disease. J. Alzheimers Dis. 2015, 45, 1097–1108. [Google Scholar] [CrossRef]
- Fabri, M.; Pierpaoli, C.; Barbaresi, P.; Polonara, G. Functional topography of the corpus callosum investigated by DTI and fMRI. World J. Radiol. 2014, 6, 895–906. [Google Scholar] [CrossRef]
- Fenlon, L.; Richards, L.J. Contralateral targeting of the corpus callosum in normal and pathological brain function. Trends Neurosci. 2015, 38, 264–272. [Google Scholar] [CrossRef]
- Gershon, M.D. Plasticity in serotonin control mechanisms in the gut. Curr. Opin. Pharmacol. 2003, 3, 600–607. [Google Scholar] [CrossRef]
- Creed, F. How do SSRIs help patients with irritable bowel syndrome? Gut 2006, 55, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, D.; Drukker, M.; Mujagic, Z.; Vork, L.; Rutten, B.P.F.; van Os, J.; Masclee, A.A.M.; Kruimel, J.W.; Leue, C. Symptom-network dynamics in irritable bowel syndrome with comorbid panic disorder using electronic momentary assessment: A randomized controlled trial of escitalopram vs. placebo. J. Psychosom Res. 2021, 141, 110351. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E. The immune system, depression and the action of antidepressants. Prof. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 767–780. [Google Scholar] [CrossRef]
- Galecki, P.; Mossakowska-Wojcik, J.; Talarowska, M. The anti-inflammatory mechanism of antidepressants—SSRIs, SNRIs. Prof. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 291–294. [Google Scholar] [CrossRef]
- Sitges, M.; Gomez, C.D.; Aldana, B.I. Sertraline reduce IL-1β and TNF-α mRNA expression and overcomes their rise induced by seizures in the rat hippocampus. PLoS ONE 2014, 9, e111665. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, L.; Hajhashemi, V.; Javanmard, S.H. Fluvoxaminne inhibits some inflammatory gene expression in LPS/stimulated human endothelial cells, U937 macrophages, and carrageenan-induced paw edema in rat. Iran. J. Basic Med. Sci. 2016, 19, 977–984. [Google Scholar]
- Dubovicky, M.; Csaszar, E.; Melichercikova, K.; Rackova, L. Modulation of microglial function by the antidepressant drug venlafaxine. Interdiscip. Toxicol. 2014, 7, 201–207. [Google Scholar]
- Vara, E.J.; Brokstad, K.A.; Hausken, T.; Lied, G.A. Altered levels of cytokines in patients with irritable bowel syndrome are not correlated with fatigue. Int. J. Gen. Med. 2018, 11, 285–291. [Google Scholar] [CrossRef]
- Heefner, J.D.; Wilder, R.M.; Wilson, I.D. Irritable colon and depression. Psychosomatics 1978, 19, 540–547. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Harris, L.A.; Quigley, E.M.M.; Moayyedi, P. Effect of antidepressants and psychological therapies in irritable bowel syndrome: An updated systematic review and meta-analysis. Am. J. Gastroenterol. 2019, 114, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Brennan, B.P.; Fogarty, K.V.; Roberts, J.L.; Reynolds, K.A.; Pope, H.G., Jr.; Hudson, J.I. Duloxetine in the treatment of irritable bowel syndrome: An open-label pilot study. Hum. Psychopharmacol. Clin. Exp. 2009, 24, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Franzen, M.D.; Nickell, P.V.; Ransom, D.; Lebovitz, P.J. An open-label trial of duloxetine in patients with irritable bowel syndrome and comorbid generalized anxiety disorder. Int. J. Psychiatry Clin. Pr. 2014, 18, 11–15. [Google Scholar] [CrossRef]
- Seddighnia, A.; Tadayon Najafabadi, B.; Ghamari, K.; Noorbala, A.A.; Ebrahimi Daryani, N.; Kashani, L.; Akhondzadeh, S. Vortioxetine effects on quality of life of irritable bowel syndrome patients: A randomized, double-blind, placebo-controlled trial. J. Clin. Pharm. Ther. 2020, 45, 97–104. [Google Scholar] [CrossRef]
- Mayer, E.A.; Bradesi, S. Alosetron and irritable bowel syndrome. Expert Opin. Pharm. 2003, 4, 2089–2098. [Google Scholar] [CrossRef]
- Matsueda, K.; Harasawa, S.; Hongo, M.; Hiwatashi, N.; Sasaki, D. A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand. J. Gastroenterol. 2008, 43, 1202–1211. [Google Scholar] [CrossRef]
- Khalilian, A.; Ahmadimoghaddam, D.; Saki, S.; Mohammadi, Y.; Mehrpooya, M. A randomized, double-blind, placebo-controlled study to assess efficacy of mirtazapine for the treatment of diarrhea predominant irritable bowel syndrome. Biopsychosoc. Med. 2021, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Propst, E.L.; Flickinger, E.A.; Bauer, L.L.; Merchen, N.R.; Fahey, G.C., Jr. A dose-response experiment evaluating the effects of oligofructose and inulin on nutrient digestibility, stool quality, and fecal protein catabolites in healthy adult dogs. J. Anim. Sci. 2003, 81, 3057–3066. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Ford, A.C.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Moayyedi, P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561; quiz 1546, 1562. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of action of prebiotics and their effects on gastro-intestinal disorders in adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Collins, S.; Reid, G. Distant site effects of ingested prebiotics. Nutrients 2016, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K. Probiotics and prebiotics in the management of irritable bowel syndrome: A review of recent clinical trials and systematic reviews. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Bahrudin, M.F.; Abdul Rani, R.; Tamil, A.M.; Mokhtar, N.M.; Raja Ali, R.A. Effectiveness of sterilized symbiotic drink containing lactobacillus helveticus comparable to probiotic alone in patients with constipation-predominant irritable bowel syndrome. Dig. Dis. Sci. 2020, 65, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.M.; Gibson, G.R.; Rowland, I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.; O’Mahony, L.; O’Callaghan, L.; Sheil, B.; Vaughan, E.E.; Fitzsimons, N.; Fitzgibbon, J.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut 2003, 52, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Ohman, L.; Simren, M. Pathogenesis of IBS: Role of inflammation, immunity and neuroimmune interactions. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 163–173. [Google Scholar] [CrossRef]
- Brenner, D.M.; Moeller, M.J.; Chey, W.D.; Schoenfeld, P.S. The utility of probiotics in the treatment of irritable bowel syndrome: A systematic review. Am. J. Gastroenterol. 2009, 104, 1033–1049; quiz 1050. [Google Scholar] [CrossRef]
- Yuan, F.; Ni, H.; Asche, C.V.; Kim, M.; Walayat, S.; Ren, J. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: A meta-analysis. Curr. Med. Res. Opin. 2017, 33, 1191–1197. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Han, K.; Wang, J.; Seo, J.G.; Kim, H. Efficacy of double-coated probiotics for irritable bowel syndrome: A randomized double-blind controlled trial. J. Gastroenterol. 2017, 52, 432–443. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
| Factors | Interpretation | References |
|---|---|---|
| Dietary Sensitivity |
| Oświęcimska et al., 2017 [8] |
| Inflammation |
| Defrees and Bailey, 2017 [10]; Oświęcimska et al., 2017 [8] |
| Genetics |
| Black and Ford, 2020 [1]; Oświęcimska et al., 2017 [8]; Holtmann et al., 2016 [12] |
| Infection |
| Black and Ford, 2020 [1]; Defrees and Bailey, 2017 [10]; Oświęcimska et al., 2017 [8] |
| Visceral Hypersensitivity (VH) |
| Defrees and Bailey, 2017 [10]; Farzaei et al., 2016 [13] |
| Increased Intestinal Permeability |
| González-Castro et al., 2017 [14]; Oświęcimska et al., 2017 [8]; Camilleri et al., 2012 [15] |
| Gut Dysbiosis |
| Hadjivasilis et al., 2019 [9]; Oświęcimska et al., 2017 [8] |
| Psychosocial Distress |
| Black and Ford, 2020 [1]; Hadjivasilis et al., 2019 [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, M.N.M.; Kumar, J.; Muhammad Nawawi, K.N.; Raja Ali, R.A.; Mokhtar, N.M. Irritable Bowel Syndrome, Depression, and Neurodegeneration: A Bidirectional Communication from Gut to Brain. Nutrients 2021, 13, 3061. https://doi.org/10.3390/nu13093061
Aziz MNM, Kumar J, Muhammad Nawawi KN, Raja Ali RA, Mokhtar NM. Irritable Bowel Syndrome, Depression, and Neurodegeneration: A Bidirectional Communication from Gut to Brain. Nutrients. 2021; 13(9):3061. https://doi.org/10.3390/nu13093061
Chicago/Turabian StyleAziz, Muhammad Nazirul Mubin, Jaya Kumar, Khairul Najmi Muhammad Nawawi, Raja Affendi Raja Ali, and Norfilza M. Mokhtar. 2021. "Irritable Bowel Syndrome, Depression, and Neurodegeneration: A Bidirectional Communication from Gut to Brain" Nutrients 13, no. 9: 3061. https://doi.org/10.3390/nu13093061
APA StyleAziz, M. N. M., Kumar, J., Muhammad Nawawi, K. N., Raja Ali, R. A., & Mokhtar, N. M. (2021). Irritable Bowel Syndrome, Depression, and Neurodegeneration: A Bidirectional Communication from Gut to Brain. Nutrients, 13(9), 3061. https://doi.org/10.3390/nu13093061







