Assessing the Physiological Effects of Traditional Regional Diets Targeting the Prevention of Cardiovascular Disease: A Systematic Review of Randomized Controlled Trials Implementing Mediterranean, New Nordic, Japanese, Atlantic, Persian and Mexican Dietary Interventions
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question, PICO and Study Protocol
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Outcomes of Interest
2.5. Data Extraction
2.6. Risk of Bias in Individual Studies
3. Results
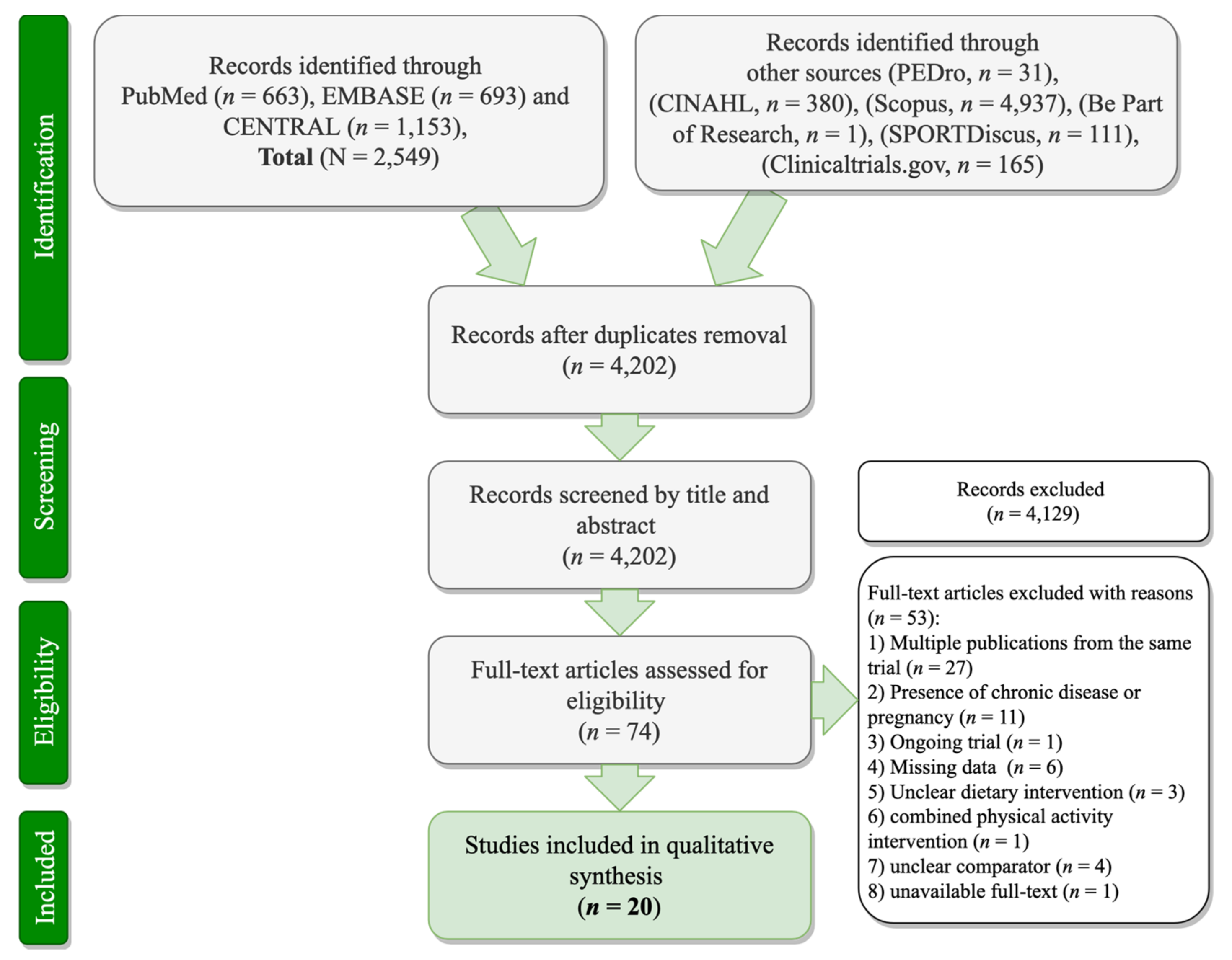
3.1. Search Results
3.2. Characteristics of the Included RCTs
3.2.1. Intervention and Comparator Arms
3.2.2. RCT Design, Masking and Duration
3.2.3. RCT Population
3.2.4. Outcomes of the Included Trials
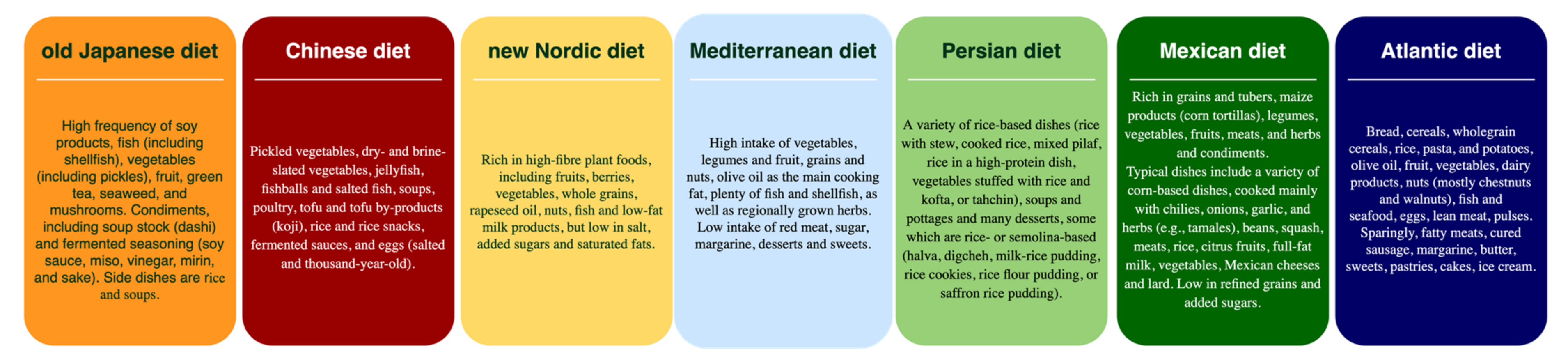
3.3. Characteristics of the Traditional Regional Diets
3.4. Effects of Regional Diets on CVD Prevention among Participants with Increased CVD Risk
3.4.1. Japanese Diet and CVD Prevention
3.4.2. Chinese Diet and CVD Prevention
3.4.3. New Nordic Diet and CVD Prevention
3.4.4. Mediterranean Diet and CVD Prevention
3.4.5. Mexican Diet and CVD Prevention
3.4.6. Traditional Persian Medicine Diet and CVD Prevention
3.4.7. Traditional Southern European Atlantic Diet and CVD Prevention
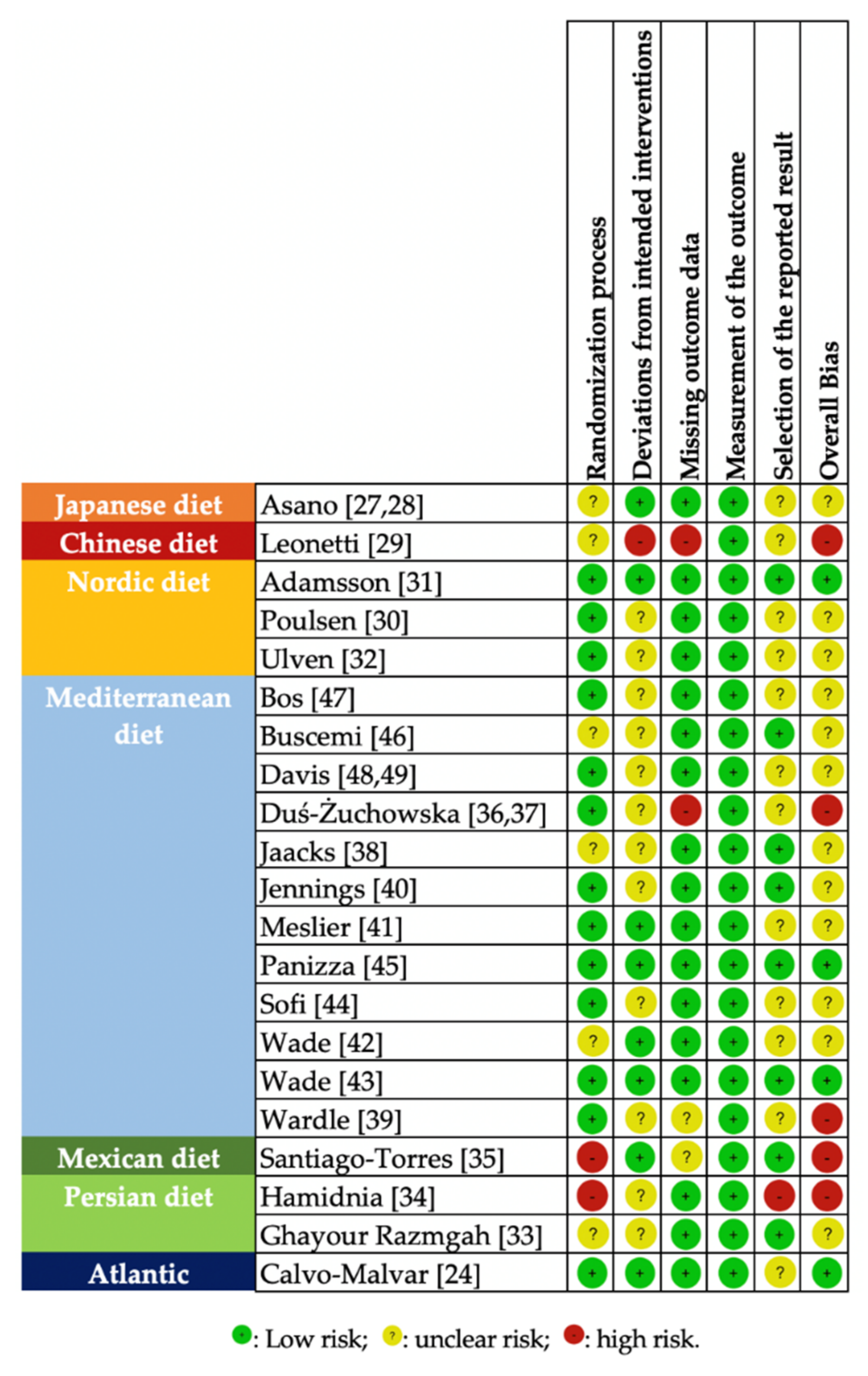
3.5. Risk of Bias
3.6. Synthesis of the Outcomes
4. Discussion
4.1. Do Health Benefits Lie in the Constituents/Nutrients of Regional Diets?
4.2. Concerns Regarding the Methodology of the Included RCTs
4.3. Health Benefits of Regional Diets beyond CVD Risk
4.4. Environmental Benefits of Regional Diets
4.5. Limitations of the Present Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases: Country Profiles 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Vandenberghe, D.; Albrecht, J. The financial burden of non-communicable diseases in the European Union: A systematic review. Eur. J. Public Health 2020, 30, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Palafox, B.; Walli-Attaei, M.; Powell-Jackson, T.; Rangarajan, S.; Alhabib, K.F.; Avezum, A.J.; Calik, K.B.T.; Chifamba, J.; Choudhury, T.; et al. The household economic burden of non-communicable diseases in 18 countries. BMJ Glob. Health 2020, 5, e002040. [Google Scholar] [CrossRef]
- Richards, N.C.; Gouda, H.N.; Durham, J.; Rampatige, R.; Rodney, A.; Whittaker, M. Disability, noncommunicable disease and health information. Bull. World Health Organ. 2016, 94, 230–232. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Stein, D.J.; Benjet, C.; Gureje, O.; Lund, C.; Scott, K.M.; Poznyak, V.; van Ommeren, M. Integrating mental health with other non-communicable diseases. BMJ 2019, 364, l295. [Google Scholar] [CrossRef] [PubMed]
- Kishore, S.P.; Reddy, K.S. Non-communicable Diseases: Equity, Action and Targets. In Manson’s Tropical Infectious Diseases; Farrar, J., Ed.; W.B. Saunders: Oxford, UK, 2014; pp. 848–853.e1. [Google Scholar]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Branca, F.; Lartey, A.; Oenema, S.; Aguayo, V.; Stordalen, G.A.; Richardson, R.; Arvelo, M.; Afshin, A. Transforming the food system to fight non-communicable diseases. BMJ 2019, 364, 1296. [Google Scholar] [CrossRef]
- World Health Organization. Globalization, Diets and Noncommunicable Diseases World Health Organization; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Popkin, B.M. The shift in stages of the nutrition transition in the developing world differs from past experiences! Public Health Nutr. 2002, 5, 205–214. [Google Scholar] [CrossRef]
- Tsakiraki, M.; Grammatikopoulou, M.G.; Stylianou, C.; Tsigga, M. Nutrition transition and health status of Cretan women: Evidence from two generations. Public Health Nutr. 2011, 14, 793–800. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Maraki, M.I.; Giannopoulou, D.; Poulimeneas, D.; Sidossis, L.S.; Tsigga, M. Similar Mediterranean diet adherence but greater central adiposity is observed among Greek diaspora adolescents living in Istanbul, compared to Athens. Ethn. Health 2018, 23, 221–232. [Google Scholar] [CrossRef]
- Cano, A.; Marshall, S.; Zolfaroli, I.; Bitzer, J.; Ceausu, I.; Chedraui, P.; Durmusoglu, F.; Erkkola, R.; Goulis, D.G.; Hirschberg, A.L.; et al. The Mediterranean diet and menopausal health: An EMAS position statement. Maturitas 2020, 139, 90–97. [Google Scholar] [CrossRef]
- Liu, Y.; Milner, M.; Klonizakis, M. Physiological effects of a short-term lifestyle intervention based on the Mediterranean diet: Comparison between older and younger healthy, sedentary adults. Nutrition 2018, 55–56, 185–191. [Google Scholar] [CrossRef]
- Alkhatib, A.; Klonizakis, M. Effects of exercise training and Mediterranean diet on vascular risk reduction in post-menopausal women. Clin. Hemorheol. Microcirc. 2014, 57, 33–47. [Google Scholar] [CrossRef]
- Klonizakis, M.; Alkhatib, A.; Middleton, G.; Smith, M.F. Mediterranean diet- and exercise-induced improvement in age-dependent vascular activity. Clin. Sci. 2013, 124, 579–587. [Google Scholar] [CrossRef]
- Klonizakis, M.; Grammatikopoulou, M.G.; Theodoridis, X.; Milner, M.; Liu, Y.; Chourdakis, M. Effects of Long-Versus Short-Term Exposure to the Mediterranean Diet on Skin Microvascular Function and Quality of Life of Healthy Adults in Greece and the UK. Nutrients 2019, 11, 2487. [Google Scholar] [CrossRef]
- Rogerson, D.; McNeill, S.; Könönen, H.; Klonizakis, M. Encouraging effects of a short-term, adapted Nordic diet intervention on skin microvascular function and skin oxygen tension in younger and older adults. Nutrition 2018, 49, 96–101. [Google Scholar] [CrossRef]
- Jalilpiran, Y.; Jayedi, A.; Djafarian, K.; Shab-Bidar, S. The Nordic diet and the risk of non-communicable chronic disease and mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2020, 1–13. [Google Scholar] [CrossRef]
- Niu, K.; Momma, H.; Kobayashi, Y.; Guan, L.; Chujo, M.; Otomo, A.; Ouchi, E.; Nagatomi, R. The traditional Japanese dietary pattern and longitudinal changes in cardiovascular disease risk factors in apparently healthy Japanese adults. Eur. J. Nutr. 2016, 55, 267–279. [Google Scholar] [CrossRef]
- Calvo-Malvar, M.; Benítez-Estévez, A.J.; Sánchez-Castro, J.; Leis, R.; Gude, F. Effects of a Community-Based Behavioral Intervention with a Traditional Atlantic Diet on Cardiometabolic Risk Markers: A Cluster Randomized Controlled Trial (“The GALIAT Study”). Nutrients 2021, 13, 1211. [Google Scholar] [CrossRef]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the Traditional Mexican Diet and Its Role in Health: A Systematic Review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.; Elbers, R.; Blencowe, N.; Boutron, I.; Cates, C.; Cheng, H.-Y.; Corbett, M.; Eldridge, S.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Br. Med. J. 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Kushida, M.; Yamamoto, K.; Tomata, Y.; Tsuji, I.; Tsuduki, T. Abdominal Fat in Individuals with Overweight Reduced by Consumption of a 1975 Japanese Diet: A Randomized Controlled Trial. Obesity 2019, 27, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Kushida, M.; Iwagaki, Y.; Asano, M.; Yamamoto, K.; Tomata, Y.; Tsuji, I.; Tsuduki, T. The 1975 Type Japanese Diet Improves Lipid Metabolic Parameters in Younger Adults: A Randomized Controlled Trial. J. Oleo Sci. 2018, 67, 599–607. [Google Scholar] [CrossRef]
- Leonetti, F.; Liguori, A.; Petti, F.; Rughini, S.; Silli, L.; Liguori, S.; Bangrazi, S. Effects of basic traditional Chinese diet on body mass index, lean body mass, and eating and hunger behaviours in overweight or obese individuals. J. Tradit. Chin. Med. 2016, 36, 456–463. [Google Scholar] [CrossRef]
- Poulsen, S.K.; Due, A.; Jordy, A.B.; Kiens, B.; Stark, K.D.; Stender, S.; Holst, C.; Astrup, A.; Larsen, T.M. Health effect of the New Nordic Diet in adults with increased waist circumference: A 6-mo randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 35–45. [Google Scholar] [CrossRef]
- Adamsson, V.; Reumark, A.; Fredriksson, I.-B.; Hammarström, E.; Vessby, B.; Johansson, G.; Risérus, U. Effects of a healthy Nordic diet on cardiovascular risk factors in hypercholesterolaemic subjects: A randomized controlled trial (NORDIET). J. Intern. Med. 2011, 269, 150–159. [Google Scholar] [CrossRef]
- Ulven, S.M.; Leder, L.; Elind, E.; Ottestad, I.; Christensen, J.J.; Telle-Hansen, V.H.; Skjetne, A.J.; Raael, E.; Sheikh, N.A.; Holck, M.; et al. Exchanging a few commercial, regularly consumed food items with improved fat quality reduces total cholesterol and LDL-cholesterol: A double-blind, randomised controlled trial. Br. J. Nutr. 2016, 116, 1383–1393. [Google Scholar] [CrossRef]
- Ghayour Razmgah, G.R.; Hosseini, S.M.-R.; Nematy, M.; Esmaily, H.; Yousefi, M.; Kamalinejad, M.; Mosavat, S.H. Efficacy of Traditional Persian Medicine-Based Diet on Non-Alcoholic Fatty Liver Disease: A Randomized, Controlled, Clinical Trial. Galen Med. J. 2017, 6, 208–216. [Google Scholar] [CrossRef]
- Hamidnia, L.; Nematy, M.; Salari, R.; Taghipour, A.; Motavasselian, M. Comparing the efficacy of therapeutic packages in Persian Medicine with Classical Medicine in overweight patients: A randomized clinical trial. Electron. Physician 2018, 10, 6892–6903. [Google Scholar] [CrossRef][Green Version]
- Santiago-Torres, M.; Kratz, M.; Lampe, J.W.; Tapsoba, J.D.D.; Breymeyer, K.L.; Levy, L.; Villaseñor, A.; Wang, C.-Y.; Song, X.; Neuhouser, M.L. Metabolic responses to a traditional Mexican diet compared with a commonly consumed US diet in women of Mexican descent: A randomized crossover feeding trial1,2. Am. J. Clin. Nutr. 2016, 103, 366–374. [Google Scholar] [CrossRef]
- Duś-Żuchowska, M.; Bajerska, J.; Krzyżanowska, P.; Chmurzyńska, A.; Miśkiewicz-Chotnicka, A.; Muzsik, A.; Walkowiak, J. The Central European diet as an alternative to the Mediterranean diet in atherosclerosis prevention in postmenopausal obese women with a high risk of metabolic syndrome—A randomized nutrition-al trial. Acta Sci. Pol. Technol. Aliment. 2018, 17, 399–407. [Google Scholar] [CrossRef]
- Bajerska, J.; Chmurzynska, A.; Muzsik, A.; Krzyżanowska, P.; Mądry, E.; Malinowska, A.M.; Walkowiak, J. Weight loss and metabolic health effects from energy-restricted Mediterranean and Central-European diets in postmenopausal women: A randomized controlled trial. Sci. Rep. 2018, 8, 11170. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Sher, S.; Staercke, C.; Porkert, M.; Alexander, W.R.; Jones, D.P.; Vaccarino, V.; Ziegler, T.R.; Quyyumi, A.A. Pilot randomized controlled trial of a Mediterranean diet or diet supplemented with fish oil, walnuts, and grape juice in overweight or obese US adults. BMC Nutr. 2018, 4, 26. [Google Scholar] [CrossRef]
- Wardle, J.; Rogers, P.; Judd, P.; Taylor, M.A.; Rapoport, L.; Green, M.; Nicholson Perry, K. Randomized trial of the effects of cholesterol-lowering dietary treatment on psychological function. Am. J. Med. 2000, 108, 547–553. [Google Scholar] [CrossRef]
- Jennings, A.; Berendsen, A.M.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Brzozowska, A.; Sicinska, E.; Pietruszka, B.; Meunier, N.; Caumon, E.; Malpuech-Brugère, C.; et al. Mediterranean-Style Diet Improves Systolic Blood Pressure and Arterial Stiffness in Older Adults. Hypertension 2019, 73, 578–586. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Murphy, K.J. Effects of Mediterranean diet supplemented with lean pork on blood pressure and markers of cardiovascular risk: Findings from the MedPork trial. Br. J. Nutr. 2019, 122, 873–883. [Google Scholar] [CrossRef]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Murphy, K.J. A Mediterranean diet supplemented with dairy foods improves markers of cardiovascular risk: Results from the MedDairy randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 1166–1182. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile: CARDIVEG Study (Cardiovascular Prevention with Vegetarian Diet). Circulation 2018, 137, 1103–1113. [Google Scholar] [CrossRef]
- Panizza, C.E.; Lim, U.; Yonemori, K.M.; Cassel, K.D.; Wilkens, L.R.; Harvie, M.N.; Maskarinec, G.; Delp, E.J.; Lampe, J.W.; Shepherd, J.A.; et al. Effects of Intermittent Energy Restriction Combined with a Mediterranean Diet on Reducing Visceral Adiposity: A Randomized Active Comparator Pilot Study. Nutrients 2019, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Verga, S.; Tranchina, M.R.; Cottone, S.; Cerasola, G. Effects of hypocaloric very-low-carbohydrate diet vs. Mediterranean diet on endothelial function in obese women. Eur. J. Clin. Investig. 2009, 39, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.B.; de Vries, J.H.; Feskens, E.J.; van Dijk, S.J.; Hoelen, D.W.; Siebelink, E.; Heijligenberg, R.; de Groot, L.C. Effect of a high monounsaturated fatty acids diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.R.; Hodgson, J.M.; Woodman, R.; Bryan, J.; Wilson, C.; Murphy, K.J. A Mediterranean diet lowers blood pressure and improves endothelial function: Results from the MedLey randomized intervention trial. Am. J. Clin. Nutr. 2017, 105, 1305–1313. [Google Scholar] [CrossRef]
- Davis, C.R.; Bryan, J.; Hodgson, J.M.; Woodman, R.; Murphy, K.J. A Mediterranean Diet Reduces F 2-Isoprostanes and Triglycerides among Older Australian Men and Women after 6 Months. J. Nutr. 2017, 147, 1348–1355. [Google Scholar] [CrossRef]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Keage, H.A.D.; Murphy, K.J. Including pork in the Mediterranean diet for an Australian population: Protocol for a randomised controlled trial assessing cardiovascular risk and cognitive function. Nutr. J. 2017, 16, 84. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shuang, E.; Hatakeyama, Y.; Sakamoto, Y.; Honma, T.; Jibu, Y.; Kawakami, Y.; Tsuduki, T. The Japanese diet from 1975 delays senescence and prolongs life span in SAMP8 mice. Nutrition 2016, 32, 122–128. [Google Scholar] [CrossRef]
- Li, J.; Hsieh, Y.-H.P. Traditional Chinese food technology and cuisine. Asia Pac. J. Clin. Nutr. 2004, 13, 147–155. [Google Scholar]
- Mithril, C.; Dragsted, L.O.; Meyer, C.; Blauert, E.; Holt, M.K.; Astrup, A. Guidelines for the New Nordic Diet. Public Health Nutr. 2012, 15, 1941–1947. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Nigdelis, M.P.; Theodoridis, X.; Gkiouras, K.; Tranidou, A.; Papamitsou, T.; Bogdanos, D.P.; Goulis, D.G. How fragile are Mediterranean diet interventions? A research-on-research study of randomized controlled trials. BMJ Nutr. Prev. Health 2021, 4, 115–131. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Lampropoulou, M.; Goulis, D.G. Mediterranean diet and female fertility: Cross-talk of an evidence-based approach. In The Mediterranean Diet: An Evidence-Based Approach; Preedy, V.R., Watson, R.R., Eds.; Elsevier: London, UK, 2020; pp. 473–483. [Google Scholar]
- Middleton, G.; Keegan, R.; Smith, M.F.; Alkhatib, A.; Klonizakis, M. Brief Report: Implementing a Mediterranean Diet Intervention into a RCT: Lessons Learned from a Non-Mediterranean Based Country. J Nutr. Health Aging 2015, 19, 1019–1022. [Google Scholar] [CrossRef]
- Karizaki, V.M. Ethnic and traditional Iranian rice-based foods. J. Ethn. Foods 2016, 3, 124–134. [Google Scholar] [CrossRef]
- Santiago-Torres, M.; Tinker, L.F.; Allison, M.A.; Breymeyer, K.L.; Garcia, L.; Kroenke, C.H.; Lampe, J.W.; Shikany, J.M.; van Horn, L.; Neuhouser, M.L. Development and use of a traditional Mexican diet score in relation to systemic inflammation and insulin resistance among women of Mexican descent. J. Nutr. 2015, 145, 2732–2740. [Google Scholar] [CrossRef]
- Carballo-Casla, A.; Ortolá, R.; García-Esquinas, E.; Oliveira, A.; Sotos-Prieto, M.; Lopes, C.; Lopez-Garcia, E.; Rodríguez-Artalejo, F. The Southern European Atlantic Diet and all-cause mortality in older adults. BMC Med. 2021, 19, 36. [Google Scholar] [CrossRef]
- Velho, M.V.; Pinheiro, R.; Rodrigues, A.S. The Atlantic diet—Origin and features. Int. J. Food Stud. 2016, 5, 106–119. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef]
- Djoussé, L.; Arnett, D.K.; Coon, H.; Province, M.A.; Moore, L.L.; Ellison, R.C. Fruit and vegetable consumption and LDL cholesterol: The National Heart, Lung, and Blood Institute Family Heart Study. Am. J. Clin. Nutr. 2004, 79, 213–217. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Khan, T.A.; Tayyiba, M.; Agarwal, A.; Mejia, S.B.; de Souza, R.J.; Wolever, T.M.S.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of Total Sugars, Sucrose, Fructose, and Added Sugars with the Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Mayo Clin. Proc. 2019, 94, 2399–2414. [Google Scholar] [CrossRef]
- Carbone, S.; Billingsley, H.E.; Lavie, C.J. The Effects of Dietary Sugars on Cardiovascular Disease and Cardiovascular Disease–Related Mortality: Finding the Sweet Spot. Mayo Clin. Proc. 2019, 94, 2375–2377. [Google Scholar] [CrossRef]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2013, 346, e7492. [Google Scholar] [CrossRef]
- Ruiz-Núñez, B.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Poulimeneas, D.; Grammatikopoulou, M.G.; Devetzi, P.; Petrocheilou, A.; Kaditis, A.G.; Papamitsou, T.; Doudounakis, S.E.; Vassilakou, T. Adherence to dietary recommendations, nutrient intake adequacy and diet quality among pediatric cystic fibrosis patients: Results from the greeCF study. Nutrients 2020, 12, 3126. [Google Scholar] [CrossRef]
- Kang, Z.-Q.; Yang, Y.; Xiao, B. Dietary saturated fat intake and risk of stroke: Systematic review and dose-response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 179–189. [Google Scholar] [CrossRef]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019, 18, 91. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 6, CD011737. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Willett, W.C.; Volek, J.S.; Neuhouser, M.L. Dietary fat: From foe to friend? Science 2018, 362, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.E.; Russell, J.; Gorman, E.; Hanich, Q.; Delisle, A.; Campbell, B.; Bell, J. Fish, food security and health in Pacific Island countries and territories: A systematic literature review. BMC Public Health 2016, 16, 285. [Google Scholar] [CrossRef]
- Marushka, L.; Kenny, T.-A.; Batal, M.; Cheung, W.W.L.; Fediuk, K.; Golden, C.D.; Salomon, A.K.; Sadik, T.; Weatherdon, L.V.; Chan, H.M. Potential impacts of climate-related decline of seafood harvest on nutritional status of coastal First Nations in British Columbia, Canada. PLoS ONE 2019, 14, e0211473. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, K.; Cai, J.; Ma, A. Fish Consumption and Coronary Heart Disease: A Meta-Analysis. Nutrients 2020, 12, 2278. [Google Scholar] [CrossRef]
- Qin, Z.-Z.; Xu, J.-Y.; Chen, G.-C.; Ma, Y.-X.; Qin, L.-Q. Effects of fatty and lean fish intake on stroke risk: A meta-analysis of prospective cohort studies. Lipids Health Dis. 2018, 17, 1–7. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Ioannidis, J.P. Is everything we eat associated with cancer? A systematic cookbook review. Am. J. Clin. Nutr. 2013, 97, 127–134. [Google Scholar] [CrossRef]
- Kebbe, M.; Sparks, J.R.; Flanagan, E.W.; Redman, L.M. Beyond weight loss: Current perspectives on the impact of calorie restriction on healthspan and lifespan. Expert Rev. Endocrinol. Metab. 2021, 16, 95–108. [Google Scholar] [CrossRef]
- Bales, C.W.; Kraus, W.E. Caloric restriction: Implications for human cardiometabolic health. J. Cardiopulm. Rehabil. Prev. 2013, 33, 201–208. [Google Scholar] [CrossRef]
- Caristia, S.; De Vito, M.; Sarro, A.; Leone, A.; Pecere, A.; Zibetti, A.; Filigheddu, N.; Zeppegno, P.; Prodam, F.; Faggiano, F.; et al. Is caloric restriction associated with better healthy aging outcomes? A systematic review and meta- analysis of randomized controlled trials. Nutrients 2020, 12, 2290. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Beka, V.; Prado, C.M. The effect of caloric restriction on blood pressure and cardiovascular function: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2021, 40, 728–739. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; La Vecchia, C.; Bamia, C. Mediterranean diet and its components in relation to all-cause mortality: Meta-analysis. Br. J. Nutr. 2018, 120, 1081–1097. [Google Scholar] [CrossRef]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The effect of the mediterranean diet on metabolic health: A systematic review and meta-analysis of controlled trials in adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef] [PubMed]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Vamvakis, A.; Gkaliagkousi, E.; Lazaridis, A.; Grammatikopoulou, M.G.; Triantafyllou, A.; Nikolaidou, B.; Koletsos, N.; Anyfanti, P.; Tzimos, C.; Zebekakis, P.; et al. Impact of intensive lifestyle treatment (Diet plus exercise) on endothelial and vascular function, arterial stiffness and blood pressure in stage 1 hypertension: Results of the HINTreat randomized controlled trial. Nutrients 2020, 12, 1326. [Google Scholar] [CrossRef] [PubMed]
- Tsigalou, C.; Paraschaki, A.; Karvelas, A.; Kantartzi, K.; Gagali, K.; Tsairidis, D.; Bezirtzoglou, E. Gut microbiome and Mediterranean diet in the context of obesity. Current knowledge, perspectives and potential therapeutic targets. Metab. Open 2021, 9, 100081. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to Mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Chaimani, A.; Hoffmann, G.; Schwedhelm, C.; Boeing, H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur. J. Epidemiol. 2018, 33, 157. [Google Scholar] [CrossRef]
- Zimorovat, A.; Mohammadi, M.; Ramezani-Jolfaie, N.; Salehi-Abargouei, A. The healthy Nordic diet for blood glucose control: A systematic review and meta-analysis of randomized controlled clinical trials. Acta Diabetol. 2019, 57, 1–12. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, G.; Lai, L.; Xu, L.; Shen, Q.; Wang, Y.; Fan, M.; Shen, L. Traditional chinese medicine diet paratherapy for alleviating toxicity in chemotherapy and radiotherapy in cancer patients: A meta-analysis. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Wong, A.R.; Yang, A.W.H.; Li, K.; Gill, H.; Li, M.; Lenon, G.B. Chinese Herbal Medicine for Weight Management: A Systematic Review and Meta-Analyses of Randomised Controlled Trials. J. Obes. 2021, 2021, 3250723. [Google Scholar] [CrossRef]
- Abe, C.; Imai, T.; Sezaki, A.; Miyamoto, K.; Kawase, F.; Shirai, Y.; Sanada, M.; Inden, A.; Kato, T.; Shimokata, H. A longitudinal association between the traditional Japanese diet score and incidence and mortality of breast cancer—An ecological study. Eur. J. Clin. Nutr. 2021, 75, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Okada, E.; Nakamura, K.; Ukawa, S.; Wakai, K.; Date, C.; Iso, H.; Tamakoshi, A. The Japanese food score and risk of all-cause, CVD and cancer mortality: The Japan Collaborative Cohort Study. Br. J. Nutr. 2018, 120, 464–471. [Google Scholar] [CrossRef]
- Gussow, J.D.; Clancy, K.L. Dietary guidelines for sustainability. J. Nutr. Educ. 1986, 18, 1–5. [Google Scholar] [CrossRef]
- Dernini, S.; Berry, E.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G.; et al. Med Diet 4.0: The Mediterranean diet with four sustainable benefits. Public Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef]
- Clarke, E.D.; Rollo, M.E.; Collins, C.E.; Wood, L.; Callister, R.; Philo, M.; Kroon, P.A.; Haslam, R.L. The Relationship between Dietary Polyphenol Intakes and Urinary Polyphenol Concentrations in Adults Prescribed a High Vegetable and Fruit Diet. Nutrients 2020, 12, 3431. [Google Scholar] [CrossRef]
- Yamori, Y. Food factors for atherosclerosis prevention: Asian perspective derived from analyses of worldwide dietary biomarkers. Exp. Clin. Cardiol. 2006, 11, 94–98. [Google Scholar]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017, 105, 600–608. [Google Scholar] [CrossRef]
- Cuparencu, C.; Praticó, G.; Hemeryck, L.Y.; Sri Harsha, P.S.C.; Noerman, S.; Rombouts, C.; Xi, M.; Vanhaecke, L.; Hanhineva, K.; Brennan, L.; et al. Biomarkers of meat and seafood intake: An extensive literature review. Genes Nutr. 2019, 14, 35. [Google Scholar] [CrossRef]
- Aresta, A.M.; De Vietro, N.; Clodoveo, M.L.; Amirante, R.; Corbo, F.; Schena, F.P.; Zambonin, C. Determination of hydroxytyrosol and tyrosol in human urine after intake of extra virgin olive oil produced with an ultrasounds-based technology. J. Pharm. Biomed. Anal. 2021, 203, 114204. [Google Scholar] [CrossRef]
- Caruso, D.; Visioli, F.; Patelli, R.; Galli, C.; Galli, G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism 2001, 50, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
| PICO Components | Determinants |
|---|---|
| Population: | Adults at high risk for CVD (age ≥ 55 years old and at least one of the following risk factors 1. post-menopausal, 2. with hyperglycemia or prediabetes, 3. hypertension, 4. hyperlipidemia, 5. overweight/obesity, 6. current cigarette smokers, or 7. excessive alcohol drinkers, 8. physically inactive) |
| Intervention: | Any regional diet, including the Mediterranean, Persian, Southern European Atlantic, Japanese, Chinese, Nordic, or other. |
| Comparison: | Habitual diet, sham diet, any other dietary pattern, or no intervention |
| Outcome: | Any CVD outcome, including BW, BMI, WC, body fat (% BW), waist-to-hip ratio, blood pressure, insulin resistance, fasting blood glucose/insulin, HbA1c, blood lipids, FMD or LDF |
| 1 | “Mediterranean diet”[Abstract] AND (“fatness”[Abstract] OR “body fat”[Abstract] OR “adiposity”[Abstract] OR “obesity”[Abstract] OR “obese”[Abstract] OR “BMI”[Abstract] OR “blood pressure”[Abstract] OR “glucose”[Abstract] OR “insulin”[Abstract] OR “cholesterol”[Abstract] OR “flow mediated dilation”[Abstract] OR “laser doppler”[Abstract] OR “weight”[Abstract] OR “circulation”[Abstract]) |
| 2 | mexican diet[Abstract] AND (fatness[Abstract] OR body fat[Abstract] OR adiposity[Abstract] OR obesity[Abstract] OR obese[Abstract] OR BMI[Abstract] OR blood pressure[Abstract] OR glucose[Abstract] OR insulin[Abstract] OR cholesterol[Abstract] OR flow mediated dilation[Abstract] OR laser doppler[Abstract] OR weight[Abstract] OR circulation[Abstract]) |
| 3 | persian diet[Abstract] AND (fatness[Abstract] OR body fat[Abstract] OR adiposity[Abstract] OR obesity[Abstract] OR obese[Abstract] OR BMI[Abstract] OR blood pressure[Abstract] OR glucose[Abstract] OR insulin[Abstract] OR cholesterol[Abstract] OR flow mediated dilation[Abstract] OR laser doppler[Abstract] OR weight[Abstract] OR circulation[Abstract]) |
| 4 | chinese diet[Abstract] AND (fatness[Abstract] OR body fat[Abstract] OR adiposity[Abstract] OR obesity[Abstract] OR obese[Abstract] OR BMI[Abstract] OR blood pressure[Abstract] OR glucose[Abstract] OR insulin[Abstract] OR cholesterol[Abstract] OR flow mediated dilation[Abstract] OR laser doppler[Abstract] OR weight[Abstract] OR circulation[Abstract]) |
| 5 | (“New Nordic diet”[Abstract] OR “Nordic diet”[Abstract]) AND (fatness[Abstract] OR “body fat”[Abstract] OR “adiposity”[Abstract] OR “obesity”[Abstract] OR “obese”[Abstract] OR “BMI”[Abstract] OR “blood pressure”[Abstract] OR “glucose”[Abstract] OR “insulin”[Abstract] OR “cholesterol”[Abstract] OR “flow mediated dilation”[Abstract] OR “laser doppler”[Abstract] OR “weight”[Abstract] OR “circulation”[Abstract]) |
| 6 | “Japanese diet”[Abstract] AND (“fatness”[Abstract] OR “body fat”[Abstract] OR “adiposity”[Abstract] OR “obesity”[Abstract] OR “obese”[Abstract] OR “BMI”[Abstract] OR “blood pressure”[Abstract] OR “glucose”[Abstract] OR “insulin”[Abstract] OR “cholesterol”[Abstract] OR “flow mediated dilation”[Abstract] OR “laser doppler”[Abstract] OR “weight”[Abstract] OR “circulation”[Abstract]) |
| 7 | “Atlantic diet”[Abstract] AND (“fatness”[Abstract] OR “body fat”[Abstract] OR “adiposity”[Abstract] OR “obesity”[Abstract] OR “obese”[Abstract] OR “BMI”[Abstract] OR “blood pressure”[Abstract] OR “glucose”[Abstract] OR “insulin”[Abstract] OR “cholesterol”[Abstract] OR “flow mediated dilation”[Abstract] OR “laser doppler”[Abstract] OR “weight”[Abstract] OR “circulation”[Abstract]) |
| Regional Diet | First author | Trial name | Design | Masking | Participant Characteristics | Intervention | Comparator(s) | Intervention Duration | Outcomes | Significant Findings Favoring Intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| Japanese | Asano [27,28] | - | Parallel | Single-blind | n = 60 patients with overweight/obesity (20–70 years) | Older (circa 1975) Japanese diet (n = 30) | Modern Japanese diet (n = 30) | 28 days | BW, BF mass, BMI, WC, SBP, DBP, TC, HDL, CRP, LDL, TG, ALT, ALP, γ-GT, AST, HbA1c, FPG, HOMA-IR, IRI, PLT, Hb, MCV, MCH, RBC, WBC, LD, UA, BUN, Cre, CK, TP, Fe, K, Na, Cl, Mg | BW, BF mass, BMI, WC, CRP, HDL, LDL, HbA1c |
| Chinese | Leonetti [29] | - | Parallel | NR | n = 284 patients with overweight/obesity (25–70 years) | Hypocaloric Chinese diet (1200 kcal) (n = 142) | Hypocaloric typical Western diet (1200 kcal) (n = 142) | 6 weeks | BMI, LBM, hunger, PHI, MHI | BMI, LBM, hunger, PHI, MHI |
| Nordic | Adamsson [31] | NORDIET | Parallel | Open label | n = 86 patients with mild hypercholesterolemia (25–65 years) | Nordic diet (n = 44) (BMI: 26.3 kg/m2) | Usual Western diet (n = 42) (BMI: 26.5 kg/m2) | 6 weeks | BW, BMI, SBP, DBP, Ins, Glu, HDL/LDL, TC, TG, LDL, HDL, apoB/apoA1, ApoB, ApoA1 | BW, BMI, SBP, Ins, TC, LDL, HDL, LDL/HDL, apoB/apoA1, ApoB, ApoA1 |
| Poulsen [30] | - | Parallel | Open label | n = 181 men and women with abdominal obesity (18–65 years) | New Nordic diet based on 15 food groups (n = 113) | Average Danish diet (n = 68) | 26 weeks | BW, BF mass, BF (% BW), BMI, WC, HC, SBP, DBP, TG, LDL, HDL, VLDL, FPG, Ins, HOMA-IR, Matsuda Index, CRP, fructosamine, Sagittal diameter, physical fitness | BW, BF mass, BF (% BW), BMI, WC, HC, SBP, HOMA-IR, Matsuda Index, TG, CRP, Saggital diameter | |
| Ulven [32] | - | Parallel | Double blind | n = 99 participants with elevated hs-CRP and TC (25–29 years) | Nordic diet with higher fiber and FA content (n = 47) | Typical modern Nordic diet (n = 52) | 8 weeks | BW, TC, TG, LDL, HDL, FPG, Ins, HbA1c, SBP, DBP, hs-CRP, IL-6, ALT, IFNγ, sTNFR1, LpA, ApoB, ApoA1 | BW, TC, TG, LDL, HDL | |
| MedD | Bos [47] | - | Parallel | Open label | n = 39 non-diabetic, BMI ≥ 25 kg/m2 or WC ≥ 94 cm for men, ≥ 80 cm for women (40–65 years) | MedD (n = 19) (BMI: 26.1 kg/m2) | Western-style diet (n = 20) (BMI: 28.3 kg/m2) | 10 weeks | BW, WC, TC, HDL, LDL, TG, TC:HDL, FPG, Ins | None |
| Buscemi [46] | - | Parallel | Open label | n = 20 women with overweight/obesity (30–50 years) (BMI: 27–39.9 kg/m2) | Hypo-caloric MedD (n = 10) (BMI = 34 kg/m2) | Atkins (very low CHO) diet (n = 10) (BMI: 34.5 kg/m2) | 2 months | FMD (%), TNF-α, Il-6, 8-iso-PGF2a, BW, BF (% BW), SBP, UA | BW, FMD | |
| Davis [48,49] | MedLey | Parallel | Open label | n = 149 older adults (≥65 years) | MedD (n = 80) (BMI: 26.7 kg/m2) | Usual diet (n = 69) (BMI: 27.1 kg/m2) | 6 months | SBP, DBP, FMD (%), hs-CRP, LDL, HDL, TG, F2-IsoPs, Ins, FPG | SBP (3, 6 months), DBP (6 months), FMD, TG (3, 6 months), F2-IsoPs (3, 6 months) | |
| Duś-Żuchowska [36,37] | Parallel | Single blind | n = 131 post-menopausal women with obesity, at a risk of MetS | Hypo-caloric (−700 kcal/day) MedD (n = 68) (BMI: 33.8 kg/m2) | Energy-restricted Central European diet (n = 63) (BMI: 33.6 kg/m2) | 16 weeks | hs-CRP, ADMA, BW, BF (mass), FFM, VAT, WC | BW, BF, FFM, VAT | ||
| Jaacks [38] | - | Parallel | Open label | n = 30 patients with overweight/obesity (35 < BMI ≥ 28 kg/m2) | MedD (n = 11) | (1) Usual diet (n = 9) (2) Usual diet + fish oil, walnuts, and grape juice (n = 10) | 8 weeks | BW, WC, FMD (%), Ins, TC, TG, LDL, HDL | BW, TC (at 4 weeks), LDL (at 4 weeks) | |
| Jennings [40] | - | Parallel | Open label | n = 1142 older adults (≥65 years) | MedD (n = 574) (BMI: 26.7 kg/m2) | Habitual diet (n = 568) (BMI: 26.6 kg/m2) | 12 months | SBP, DBP | SBP | |
| Sofi [44] | CARDIVEG | Cross-over | Open label | n = 118 patients with BMI ≥ 25 kg/m2 and ≥1 of the following: TC > 190 mg/dL, LDL > 115 mg/dL, TG > 150 mg/dL, 110 ≤ FPG < 126 mg/dL (BMI: 30.6 kg/m2) | Hypo-caloric MedD (n = 118) | Hypo-caloric lacto-ovo vegetarian diet (n = 118) | 3 months | BW, BMI, BF mass, TC, HDL, LDL, TG, FPG, Ins | LDL, TG | |
| Panizza [45] | Multiethnic Cohort Adiposity Phenotype | Parallel | Double blind | n = 60 patients with overweight/obesity (30–50 years) (BMI: 25–40 kg/m2) | IER MedD (n = 30) (BMI: 30.5 kg/m2) | Iso-energetic DASH diet (n = 30) (BMI: 30.8 kg/m2) | 12 weeks | BW, BMI, WC, BF mass, BF (% BW), TC, HDL, LDL, TG, SBP, DBP, VAT, ALT | BW, VAT, ALT, BMI, WC, BF mass, BF (% BW) | |
| Wade [42] | MedPork | Cross-over (8 wk interval) | Open label | n = 33 patients at risk for CVD (SBP > 120 mmHg and ≥2 of the following: BMI ≥ 25 kg/m2; dyslipidemia (TC ≥5.5 mmol/L, TG > 2.0 mmol/L, LDL ≥ 3.5 mmol/L, HDL ≤ 0.9 mmol/L for men or ≤1.0 mmol/L for women); IFG (6.1–7.8 mmol/L); family history of CVD/T2DM) (BMI: 30.6 kg/m2) | MedD supplemented with 2–3 serv/wk of fresh, lean pork (n = 33) | Low-fat diet (n = 33) | 8 weeks | BW, BMI, CRP, WC, BF mass, BF (% BW), TC, HDL, LDL, TG, SBP, DBP, FPG, Ins | BW, BMI, WC | |
| Wade [43] | MedDairy | Cross-over (8 wk wash-out) | Open label | n = 41 patients with hypertension and ≥2 of the following: overweight, IFG, dyslipi-demia, family history of CVD/T2DM (BMI: 30.8 kg/m2) | MedD with 3–4 serv of dairy/day (n = 41) | Low-fat diet (n = 41) | 8 weeks | BW, BMI, WC, BF mass, BF %, TC, HDL, LDL, TG, SBP, DBP, FPG, Ins | SBP, TG, HDL, BW, BF mass, BF (% BW) | |
| Wardle [39] | - | Parallel | Single blind | n = 155 patients with mildly/moderately elevated TC (adults) | MedD (n = 53) | (1) Usual diet (n = 50) (2) Low-fat diet (n = 52) | 12 weeks | BW, TC, LDL, HDL, TG, stress, cognitive function, well-being, depression, etc. | TC, LDL, BW | |
| Mexican | Santiago-Torres [35] | - | Cross-over (28 d wash-out) | Single blind | n = 26 women with overweight/obesity (BMI: 30 kg/m2) | Traditional MexD (pre-1940s), based on data from historical records (n = 26) | US diet (n = 26) | 24 d (each arm) | FPG, Ins, CRP, IGF-1, IGFBP-3, adiponectin, IL-6, HOMA-IR | Ins |
| Persian | Ghayour Razmgah [33] | Parallel | Open label | n = 43 patients with NAFLD (grades 1–2), diagnosed by US imaging (20–60 years) | TPM diet (n = 21) (BMI: 26 kg/m2) | Low-fat hypo-caloric diet (n = 22) (BMI: 24.4 kg/m2) | 12 weeks | BMI, ALT, AST, changes in NAFLD grade | BMI, NAFLD grade, AST (6 weeks), ALT (6 weeks) | |
| Hamidnia [34] | Parallel | Single blind | n = 69 women with overweight (BMI: 27–29.9 kg/m2, WC > 88 cm) | Hypocaloric TPM diet (1200–1600 kcal/day) (n = 23) (BMI: 29.1 kg/m2) | (1) Hypo-caloric diet (1200–1600 kcal/day) + orlistat (120 mg/day) (n = 23) (BMI: 29.4 kg/m2) (2) Hypo-caloric diet (1200–1600 kcal/day) + MDB ONS (2 × 5 g/day), (n = 23) (BMI: 28.5 kg/m2) | 12 weeks | BW, BMI, WC, BF (mass and % BW), TC, HDL, LDL, TG, Ins | None | ||
| Atlantic | Calvo-Malvar [24] | GALIAT | Parallel | Open label | n = 250 families (720 adults and children) | Educational sessions, cooking classes, written supporting material, and foods that form part of the Atlantic diet | Habitual lifestyle | 6 months | BW, BMI, BF, HWR, ΤC, HDL, LDL-C, CRP, TNF-α, FPG, HOMA-IR, SBP, DBP | BW, BF, BMI, HWR, TC, LDL |
| Regional Dietary Pattern | Reference | Anthropometric Indices | Inflammation Markers | Blood Lipid Concentrations | Blood Pressure | FMD (%) | ADMA (nmol/mL) | Glucose Metabolism | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW (kg) | BMI (kg/m2) | BF (% BW or kg) | VAT (cm2) | WC (cm) | FFM/LBM (% BW or kg) | HWR | CRP (mg/L) | hs-CRP (mg/L) | TNF-α (pg/mL) | sTNFR1 (pg/mL) | IL-6 (pg/mL) | IFNγ (pg/mL) | TC (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | TG (mg/dL) | apoA1 (g/L) | apoB (g/L) | apoB/apoA1 | SBP (mm Hg) | DBP (mm Hg) | Ins (mg/dL) | HbA1c (%) | FPG (mg/dL) | IR * | ||||
| Japanese | Asano [27,28] | ↓ | ↓ | ↓ | ↓ | - | ↓ | ↓ | ↑ | ↓ | - | - | - | ↓ | - | - | |||||||||||||
| Chinese | Leonetti [29] | ↓ | ↓ | ||||||||||||||||||||||||||
| Nordic | Adamsson [31] | - | ↓ | ↓ | ↓ | - | ↓ | ↓ | ↓ | ↓ | - | ↓ | - | ↓ | |||||||||||||||
| Poulsen [30] | ↓ | ↓ | ↓ | ↓ | ↓ | - | - | - | ↓ | ↓ | ↓ | - | - | - | |||||||||||||||
| Ulven [32] | ↓ | - | - | - | - | ↓ | ↓ | ↓ | ↓ | - | ↓ | - | - | - | - | - | |||||||||||||
| MedD | Bos [47] | - | - | - | - | - | - | - | - | ||||||||||||||||||||
| Buscemi [46] | ↓ | - | - | - | - | ↑ | |||||||||||||||||||||||
| Davis [48,49] | - | - | - | ↓ | ↓ | ↓ | ↑ | - | - | ||||||||||||||||||||
| Duś-Żuchowska [36,37] | ↓ | ↓ | ↓ | - | ↓ | - | ↓ | ||||||||||||||||||||||
| Jaacks [38] | ↓ | - | ↓ | - | ↓ | - | - | - | |||||||||||||||||||||
| Jennings [40] | ↓ | - | |||||||||||||||||||||||||||
| Sofi [44] | - | - | - | - | - | ↓ | ↓ | - | - | ||||||||||||||||||||
| Panizza [45] | ↓ | ↓ | ↓ | ↓ | ↓ | - | - | - | - | - | - | ||||||||||||||||||
| Wade [42] | ↓ | ↓ | - | ↓ | - | - | - | - | - | - | - | - | - | ||||||||||||||||
| Wade [43] | ↓ | - | ↓ | - | - | ↓ | - | ↓ | ↓ | - | - | - | |||||||||||||||||
| Wardle [39] | ↓ | ↓ | - | ↓ | - | ||||||||||||||||||||||||
| Mexican | Santiago-Torres [35] | - | - | ↓ | - | - | |||||||||||||||||||||||
| Persian | Ghayour Razmgah [33] | ↓ | |||||||||||||||||||||||||||
| Hamidnia [34] | - | - | - | - | - | - | - | - | - | ||||||||||||||||||||
| Atlantic | Calvo-Malvar [24] | ↓ | ↓ | ↓ | ↓ | - | - | ↓ | - | ↓ | - | - | - | - | - | ||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klonizakis, M.; Bugg, A.; Hunt, B.; Theodoridis, X.; Bogdanos, D.P.; Grammatikopoulou, M.G. Assessing the Physiological Effects of Traditional Regional Diets Targeting the Prevention of Cardiovascular Disease: A Systematic Review of Randomized Controlled Trials Implementing Mediterranean, New Nordic, Japanese, Atlantic, Persian and Mexican Dietary Interventions. Nutrients 2021, 13, 3034. https://doi.org/10.3390/nu13093034
Klonizakis M, Bugg A, Hunt B, Theodoridis X, Bogdanos DP, Grammatikopoulou MG. Assessing the Physiological Effects of Traditional Regional Diets Targeting the Prevention of Cardiovascular Disease: A Systematic Review of Randomized Controlled Trials Implementing Mediterranean, New Nordic, Japanese, Atlantic, Persian and Mexican Dietary Interventions. Nutrients. 2021; 13(9):3034. https://doi.org/10.3390/nu13093034
Chicago/Turabian StyleKlonizakis, Markos, Alex Bugg, Beatrice Hunt, Xenophon Theodoridis, Dimitrios P. Bogdanos, and Maria G. Grammatikopoulou. 2021. "Assessing the Physiological Effects of Traditional Regional Diets Targeting the Prevention of Cardiovascular Disease: A Systematic Review of Randomized Controlled Trials Implementing Mediterranean, New Nordic, Japanese, Atlantic, Persian and Mexican Dietary Interventions" Nutrients 13, no. 9: 3034. https://doi.org/10.3390/nu13093034
APA StyleKlonizakis, M., Bugg, A., Hunt, B., Theodoridis, X., Bogdanos, D. P., & Grammatikopoulou, M. G. (2021). Assessing the Physiological Effects of Traditional Regional Diets Targeting the Prevention of Cardiovascular Disease: A Systematic Review of Randomized Controlled Trials Implementing Mediterranean, New Nordic, Japanese, Atlantic, Persian and Mexican Dietary Interventions. Nutrients, 13(9), 3034. https://doi.org/10.3390/nu13093034







