The Carbohydrate Threshold in Pregnancy and Gestational Diabetes: How Low Can We Go?
Abstract
:1. Introduction
2. Materials and Methods
3. Results & Discussion
3.1. Question 1: Does a LC Diet in Pregnancy Compromise the Maternal-Fetal Glucose Concentration Gradient?
3.2. Question 2: In Pregnant Women Who Consume a LC Diet, Is There Greater Fetal Exposure to Maternal Ketones?
3.3. Question 3: Do Pregnant Women Who Consume a LC Diet Have an Increased Risk for Micronutrient Deficiency?
3.4. Question 4: Do Pregnant Women Who Consume a LC Diet Have Higher TG or FFA, Increasing Fetal Exposure to Lipids?
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yamamoto, J.M.; Kellett, J.E.; Balsells, M.; García-Patterson, A.; Hadar, E.; Solà, I.; Gich, I.; Van Der Beek, E.M.; Castañeda-Gutiérrez, E.; Heinonen, S.; et al. Gestational diabetes mellitus and diet: A systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care 2018, 41, 1346–1361. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, T.L.; Mande, A.; Barbour, L.A. Nutrition therapy within and beyond gestational diabetes. Diabetes Res. Clin. Pract. 2018, 145, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Middleton, P.; Shepherd, E.; Van Ryswyk, E.; Crowther, C.A. Different types of dietary advice for women with gestational diabetes mellitus. Cochrane Database Syst. Rev. 2017, 2, CD009275. [Google Scholar] [CrossRef]
- Jovanovic-Peterson, L.; Peterson, C.M. Dietary manipulation as a primary treatment strategy for pregnancies complicated by diabetes. J. Am. Coll. Nutr. 1990, 9, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, T.L.; Van Pelt, R.E.; Anderson, M.A.; Daniels, L.J.; West, N.A.; Donahoo, W.; Friedman, J.E.; Barbour, L.A. A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: A randomized crossover study. Diabetes Care 2014, 37, 1254–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, T.L.; Van Pelt, R.E.; Anderson, M.A.; Reece, M.S.; Reynolds, R.M.; De La Houssaye, B.A.; Heerwagen, M.; Donahoo, W.; Daniels, L.J.; Chartier-Logan, C.; et al. Women with gestational diabetes mellitus randomized to a higher–complex carbohydrate/low-fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: A pilot study. Diabetes Care 2016, 39, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Asemi, Z.; Tabassi, Z.; Samimi, M.; Fahiminejad, T.; Esmaillzadeh, A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: A randomised clinical trial. Br. J. Nutr. 2013, 109, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Esmaillzadeh, A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: A randomized controlled clinical trial. Eur. J. Clin. Nutr. 2014, 68, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Desrosiers, T.A.; Siega-Riz, A.M.; Mosley, B.S.; Meyer, R.E. National Birth Defects Prevention Study Low carbohydrate diets may increase risk of neural tube defects. Birth Defects Res. 2018, 110, 901–909. [Google Scholar] [CrossRef]
- Shaw, G.M.; Yang, W. Women’s periconceptional lowered carbohydrate intake and NTD-affected pregnancy risk in the era of prefortification with folic acid. Birth Defects Res. 2019, 111, 248–253. [Google Scholar] [CrossRef]
- Saeed, A.; Raana, T.; Saeed, A.M.; Humayun, A. Effect of antenatal depression on maternal dietary intake and neonatal outcome: A prospective cohort. Nutr. J. 2016, 15, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ásbjörnsdóttir, B.; Ronneby, H.; Vestgaard, M.; Ringholm, L.; Nichum, V.L.; Jensen, D.M.; Raben, A.; Damm, P.; Mathiesen, E.R. Lower daily carbohydrate consumption than recommended by the Institute of Medicine is common among women with type 2 diabetes in early pregnancy in Denmark. Diabetes Res. Clin. Pr. 2019, 152, 88–95. [Google Scholar] [CrossRef]
- Westman, E.C.; Yancy, W.S., Jr.; Humphreys, M. Dietary treatment of diabetes mellitus in the pre-insulin era (1914–1922). Perspect. Biol. Med. 2006, 49, 77–83. [Google Scholar] [CrossRef]
- Battaglia, F.C.; Meschia, G. An Introduction to Fetal Physiology; Academic Press, Inc.: Cambridge, MA, USA, 1986. [Google Scholar]
- Holme, A.M.; Roland, M.C.P.; Lorentzen, B.; Michelsen, T.M.; Henriksen, T. Placental glucose transfer: A human in vivo study. PLoS ONE 2015, 10, e0117084. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, T.; Metzger, B.E.; Burns, W.J.; Burns, K. Correlations between antepartum maternal metabolism and intelligence of offspring. N. Engl. J. Med. 1991, 325, 911–916. [Google Scholar] [CrossRef]
- Shubert, P.J.; Gordon, M.C.; Landon, M.B.; Gabbe, S.G.; Kniss, D.A. Ketoacids attenuate glucose uptake in human trophoblasts isolated from first-trimester chorionic villi. Am. J. Obstet. Gynecol. 1996, 175, 56–62. [Google Scholar] [CrossRef]
- Metzger, B.E.; Freinkel, N. Accelerated starvation in pregnancy: Implications for dietary treatment of obesity and gestational diabetes mellitus. Neonatology 1987, 51, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Magee, M.S.; Knopp, R.H.; Benedetti, T.J. Metabolic effects of 1200-kcal diet in obese pregnant women with gestational diabetes. Diabetes 1990, 39, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Magee, M.S.; Raisys, V.; Benedetti, T. Metabolic effects of hypocaloric diets in management of gestational diabetes. Diabetes 1991, 40 (Suppl. 2), 165–171. [Google Scholar] [CrossRef] [PubMed]
- Churuangsuk, C.; Kherouf, M.; Combet, E.; Lean, M. Low-carbohydrate diets for overweight and obesity: A systematic review of the systematic reviews. Obes. Rev. 2018, 19, 1700–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Sim, K.; Gordon, A.; Brand-Miller, J. The Effects of Nutrition and Micronutrients on Reproductive Success; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Schaefer-Graf, U.M.; Graf, K.; Kulbacka, I.; Kjos, S.L.; Dudenhausen, J.; Vetter, K.; Herrera, E. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008, 31, 1858–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; De Leiva, A.; Dunger, P.D.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007, 30 (Suppl. 2), S251–S260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merzouk, H.; Meghelli-Bouchenak, M.; Loukidi, B.; Prost, J.; Belleville, J. Impaired serum lipids and lipoproteins in fetal macrosomia related to maternal obesity. Biol. Neonate 2000, 77, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Misra, V.K.; Trudeau, S.; Perni, U. Maternal serum lipids during pregnancy and infant birth weight: The influence of prepregnancy BMI. Obesity 2011, 19, 1476–1481. [Google Scholar] [CrossRef] [Green Version]
- Bomba-Opon, D.; Wielgos, M.; Szymanska, M.; Bablok, L. Effects of free fatty acids on the course of gestational diabetes mellitus. Neuro Endocrinol. Lett. 2006, 27, 277–280. [Google Scholar]
- Welsh, J.; Lu, Y.; Dhruva, S.S.; Bikdeli, B.; Desai, N.R.; Benchetrit, L.; Zimmerman, C.O.; Mu, L.; Ross, J.; Krumholz, H.M. Age of data at the time of publication of contemporary clinical trials. JAMA Netw. Open 2018, 1, e181065. [Google Scholar] [CrossRef] [Green Version]
- Eshak, E.S.; Okada, C.; Baba, S.; Kimura, T.; Ikehara, S.; Sato, T.; Shirai, K.; Iso, H.; For the Japan Environment and Children’s Study Group. Maternal total energy, macronutrient and vitamin intakes during pregnancy associated with the offspring’s birth size in the Japan Environment and Children’s Study. Br. J. Nutr. 2020, 124, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Fahey, C.A.; Chevrier, J.; Crause, M.; Obida, M.; Bornman, R.; Eskenazi, B. Seasonality of antenatal care attendance, maternal dietary intake, and fetal growth in the VHEMBE birth cohort, South Africa. PLoS ONE 2019, 14, e0222888. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Thomas, T.; Bosch, R.J.; Dwarkanath, P.; Thomas, A.; Duggan, C.P.; Kurpad, A.V. Fetal sex modifies the effect of maternal macronutrient intake on the incidence of small-for-gestational-age births: A prospective observational cohort study. Am. J. Clin. Nutr. 2018, 108, 814–820. [Google Scholar] [CrossRef]
- Hjertholm, K.G.; Iversen, P.O.; Holmboe-Ottesen, G.; Mdala, I.; Munthali, A.; Maleta, K.; Shi, Z.; Ferguson, E.; Kamudoni, P. Maternal dietary intake during pregnancy and its association to birth size in rural Malawi: A cross-sectional study. Matern. Child Nutr. 2018, 14, e12433. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Cheng, Y.; Mi, B.; Zeng, L.; Qu, P.; Li, S.; Zhang, R.; Qi, Q.; Wu, C.; Gao, X.; et al. Maternal dietary patterns during pregnancy derived by reduced-rank regression and birth weight in the Chinese population. Br. J. Nutr. 2020, 123, 1176–1186. [Google Scholar] [CrossRef]
- Powell, C.D.; Wilson, W.M.; Olesaningo, G.; Manyama, M.; Jamniczky, H.; Spritz, R.; Cross, J.C.; Lukowiak, K.; Hallgrimsson, B.; Gonzalez, P.N. Lack of head sparing following third-trimester caloric restriction among Tanzanian Maasai. PLoS ONE 2020, 15, e0237700. [Google Scholar] [CrossRef]
- Morisaki, N.; Nagata, C.; Yasuo, S.; Morokuma, S.; Kato, K.; Sanefuji, M.; Shibata, E.; Tsuji, M.; Senju, A.; Kawamoto, T.; et al. Optimal protein intake during pregnancy for reducing the risk of fetal growth restriction: The Japan Environment and Children’s Study. Br. J. Nutr. 2018, 120, 1432–1440. [Google Scholar] [CrossRef]
- Saunders, C.M.; Rehbinder, E.M.; Carlsen, K.C.L.; Gudbrandsgard, M.; Carlsen, K.-H.; Haugen, G.; Hedlin, G.; Jonassen, C.M.; Sjøborg, K.D.; Landrø, L.; et al. Food and nutrient intake and adherence to dietary recommendations during pregnancy: A Nordic mother–child population-based cohort. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Looman, M.; Geelen, A.; Samlal, R.A.K.; Heijligenberg, R.; Gunnewiek, J.M.T.K.; Balvers, M.G.J.; Wijnberger, L.D.E.; Brouwer-Brolsma, E.M.; Feskens, E.J.M. Changes in micronutrient intake and status, diet quality and glucose tolerance from preconception to the second trimester of pregnancy. Nutrients 2019, 11, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijatovic, J.; Louie, J.C.Y.; Buso, M.E.C.; Atkinson, F.S.; Ross, G.P.; Markovic, T.P.; Brand-Miller, J.C. Effects of a modestly lower carbohydrate diet in gestational diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 112, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Harreiter, J.; Simmons, D.; Desoye, G.; Corcoy, R.; Adelantado, J.M.; Devlieger, R.; Galjaard, S.; Damm, P.; Mathiesen, E.R.; Jensen, D.M.; et al. Nutritional lifestyle intervention in obese pregnant women, including lower carbohydrate intake, is associated with increased maternal free fatty acids, 3-β-hydroxybutyrate, and fasting glucose concentrations: A secondary factorial analysis of the European multicenter, randomized controlled DALI lifestyle intervention trial. Diabetes Care 2019, 42, 1380–1389. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [Green Version]
- Marconi, A.M.; Paolini, C.; Buscaglia, M.; Zerbe, G.; Battaglia, F.C.; Pardi, G. The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference. Obstet. Gynecol. 1996, 87, 937–942. [Google Scholar] [CrossRef]
- Harding, J.E.; Johnston, B.M. Nutrition and fetal growth. Reprod. Fertil. Dev. 1995, 7, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.; Sferruzzi-Perri, A.N. Developmental programming of offspring adipose tissue biology. Int. J. Obes. 2021, 45, 1170–1192. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Gluckman, P.D. Early development conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef]
- Barker, D.J.; Thornburg, K.L. The obstetric origins of health for a lifetime. Clin. Obstet. Gynecol. 2013, 56, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Goletzke, J.; Buyken, A.E.; Louie, J.C.Y.; Moses, R.G.; Brand-Miller, J.C. Dietary micronutrient intake during pregnancy is a function of carbohydrate quality. Am. J. Clin. Nutr. 2015, 102, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.E.; Maki, K.C. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: A scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689.e1–711.e1. [Google Scholar]
- Paterson, P.; Sheath, J.; Taft, P.; Wood, C. Maternal and foetal ketone concentrations in plasma and urine. Lancet 1967, 1, 862–865. [Google Scholar] [CrossRef]
- Knopp, R.H.; Magee, M.S.; Raisys, V.; Benedetti, T.; Bonet, B. Hypocaloric diets and ketogenesis in the management of obese gestational diabetic women. J. Am. Coll. Nutr. 1991, 10, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Stewart, C.P. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J. Nutr. 2010, 140, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity; Nord; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar] [CrossRef]
- Wang, J.; Moore, D.; Subramanian, A.; Cheng, K.K.; Toulis, K.A.; Qiu, X.; Saravanan, P.; Price, M.J.; Nirantharakumar, K. Gestational dyslipidaemia and adverse birthweight outcomes: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1256–1268. [Google Scholar] [CrossRef]
- Barbour, L.A.; Farabi, S.S.; Friedman, J.E.; Hirsch, M.N.M.; Reece, M.S.; Van Pelt, R.E.; Hernandez, T.L. Postprandial triglycerides predict newborn fat more strongly than glucose in women with obesity in early pregnancy. Obesity 2018, 26, 1347–1356. [Google Scholar] [CrossRef] [Green Version]
- Desoye, G.; Herrera, E. Adipose tissue development and lipid metabolism in the human fetus: The 2020 perspective focusing on maternal diabetes and obesity. Prog. Lipid Res. 2021, 81, 101082. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.; Hanson, P.; Kabisch, S.; Pfeiffer, A.; Weickert, M. The low-carbohydrate diet: Short-term metabolic efficacy versus longer-term limitations. Nutrients 2021, 13, 1187. [Google Scholar] [CrossRef]
- Barbour, L.A.; Hernandez, T.L. Maternal lipids and fetal overgrowth: Making fat from fat. Clin. Ther. 2018, 40, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Duarte, S.; Carvajal, L.; Garchitorena, M.J.; Subiabre, M.; Fuenzalida, B.; Cantin, C.; Farías, M.; Leiva, A. Gestational diabetes mellitus treatment schemes modify maternal plasma cholesterol levels dependent to women’s weight: Possible impact on feto-placental vascular function. Nutrients 2020, 12, 506. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.E. Developmental programming of obesity and diabetes in mouse, monkey, and man in 2018: Where are we headed? Diabetes 2018, 67, 2137–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumbaugh, D.E.; Friedman, J.E. Developmental origins of nonalcoholic fatty liver disease. Pediatr. Res. 2014, 75, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, T.L.; Farabi, S.S.; Hirsch, N.; Dunn, E.Z.; Haugen, E.A.; Brumbaugh, D.; Brown, M.S.; Friedman, J.E.; Barbour, L.A. 352-OR: Maternal triglycerides in gestational diabetes are strongly associated with increased newborn hepatic fat independent of subcutaneous fat. Diabetes 2019, 68 (Suppl. 1), 352. [Google Scholar] [CrossRef]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef]
- Bell, J.D.; Margen, S.; Calloway, D.H. Ketosis, weight loss, uric acid, and nitrogen balance in obese women fed single nutrients at low caloric levels. Metabolism 1969, 18, 193–208. [Google Scholar] [CrossRef]
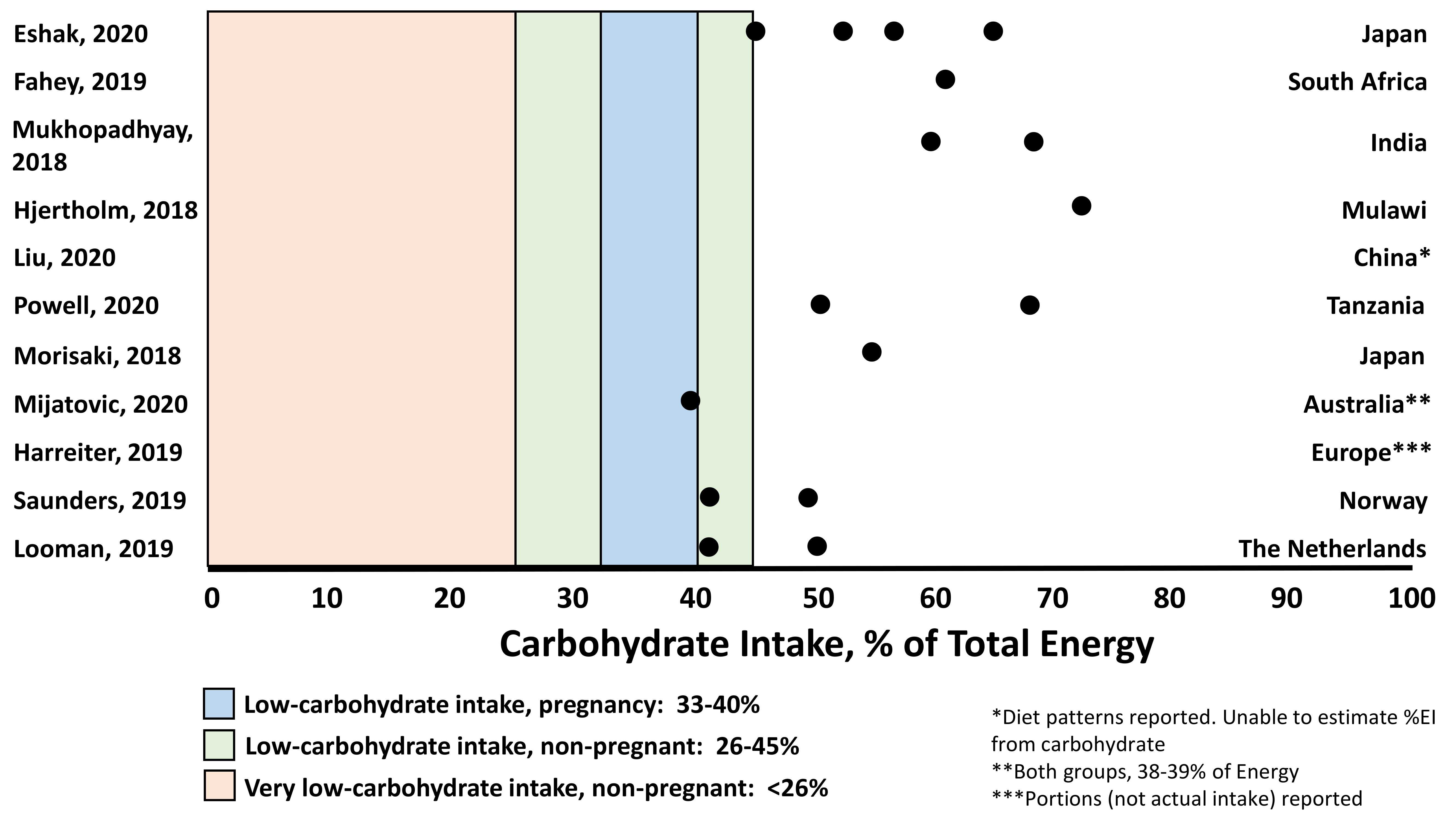
| Report | Study Design Population | Carbohydrate Intake: Measurement and Amount | Statistical Adjustment | Carbohydrate Relationship to Perinatal Concern |
|---|---|---|---|---|
| Eshak, 2020 [30] | Observational birth cohort n = 78,793 Healthy pregnant women (39.6% primiparous, 31 ± 5 years) 78% had BMI 18.5 to <25 kg/m2 Mean gestational age at delivery 38.9 ± 1.5 weeks -Mean birth weight reported in right column Japan (15 regions represented) | Food frequency questionnaire Trimester 2 Median (IQR) CHO intake 223.8 (182.6–272.4) g/d 55.3% of total energy intake 61.9% of women consumed <recommended amount of CHO/d (57.5%) Quartiles of CHO% Intake Q1: 45.1% (1075 kcal/d) Q2: 52.9% (1466 kcal/d) Q3: 57.7% (1800 kcal/d) Q4: 64.9% (2650 kcal/d) | Geometric means of nutrients adjusted for: Maternal age Height Education Household income Pre-pregnancy BMI Net weight change in pregnancy Smoking Alcohol Thyroid disease Use of folate supplement Offspring sex, gestational age at delivery | CHO and total energy intake were associated with fetal growth (fully adjusted models) Q1-3 CHO%: Increased birth weight by quartile 3030 g → 3031 g → 3037 g → 3030 g (p = 0.07) Q1-Q4 CHO%: Increased birth length and decreased ponderal index by quartile (p = 0.002, p = 0.02, respectively) Q1-Q4 kcal/d: Increased birth weight by quartile 3026 g → 3031 g → 3036 g → 3036 g (p = 0.004) * 83.9% of women consumed <2500 kcal/d (recommended amount) Birth weight < 2500 g by energy intake quartile 8.4% → 7.6% → 7.2% → 7.1% (p < 0.001) -Fat intake was inversely associated with ponderal index (p = 0.05) -Protein intake was not associated with fetal growth |
| Fahey, 2019 [31] | Observational birth cohort n = 752 mother/infant dyads Pregnant women (43% primiparous, 14% HIV positive, 26.4 ± 6.3 years) Mean gestational age at delivery 39.3 ± 2.3 weeks Mean birth weight 3125 ± 452 g Vehmbe District, Limpopo Provence, South Africa | Food frequency questionnaire at delivery, to account for intake 1 month before delivery: 61 ± 10% CHO 24 ± 8.2% Fat 13 ± 2.9% Protein Rainfall: November–February Harvest: March–June Gardening: July–October Lean: November–February, ↑food insecurity | Models of dietary intake adjusted for: Maternal parity HIV status Height Education Marital status Household income Duration of pregnancy Models of birth size z-score adjusted for: Maternal parity HIV status Height Education Marital status Household income | CHO intake highest in lean season (64%, January) and lowest at end of Harvest (56%, June) Fat intake was lowest in lean (21%, January) and highest in Harvest season (28%, June) Birth size z-scores (weight, length, head circumference) peaked at lean season onset (November), declined, and were lowest at gardening season onset. Birth size scores tracked with seasonal CHO intake, where higher CHO intake was associated with higher birth size scores and vice versa |
| Mukhopadhyay, 2018 [32] | Observational birth cohort n = 1837 Healthy pregnant women (59% primiparous, 24.4 ± 3.8 years, BMI ~22 ± 4 kg/m2) Gestational age at delivery 38.6 ± 1.5 weeks Mean birth weight 2875 ± 450 g (28% SGA rate) -Women with an SGA infant were younger (0.5 year), shorter (0.1 m), weighed ~3 kg less and were more often primiparous) Bangalore, India | Food frequency questionnaire Trimester 1 Total energy: 1910 ± 517 kcal CHO: 64.6 ± 5.1% Fat: 23.9 ± 4.4% Protein: 11.5 ± 1.1% No differences in macronutrient intake between those with AGA vs. SGA infant Categories for low, adequate, high macronutrients: CHO: low < 60%, high > 70% Fat: low < 20%, high > 25% Protein: low < 10%, high > 20% | Macronutrient intakes adjusted for total energy intake (nutrient density method) AORs accounted for: Maternal age Education Parity Height Weight at recruitment Fetal sex Total energy intake | Male births only: Risk of SGA was higher with higher CHO intake (aOR per 5% energy: 1.15 [1.01–1.32]) Risk of SGA was lower with lower fat intake (aOR per 5% energy: 0.83 [0.71–0.97]) Categorical analysis In women with high CHO intake (≥334 g/d): 29% SGA rate aOR for SGA: 1.67 [1.002–2.780], p = 0.049 In women with high fat intake: 26% SGA rate aOR for SGA: 0.61 [0.41–0.90], p = 0.01 -This was only true for male infants |
| Hjertholm, 2018 [33] | Cross-sectional with random sampling n = 132 Maternal characteristics not reported Mean birth weight 3104 ± 401 g (6% had ‘low birth weight’) Nankumba Traditional Authority, Mangochi District, Malawi | Over a 10 d period: 3-d repeated interactive multi-pass 24-h recall Collected during post-harvest season, 28–35 weeks’ gestation (mean week of collection not reported) Median (IQR) intake: Energy: 2096.5 kcal (1778.1, 2570.6) CHO: 377 g (306, 454), ~72% Fat: 37.5 g (21.9, 51.7), ~16% Protein: 55 g (46, 67), ~10% ~1% of women consumed <135 g CHO/d and 60.6% consumed <59 g protein/d (both the estimated average requirement for pregnancy) | Associations adjusted for: Maternal age Weight Height Gestational age Literacy Marital status Household assets Parity Maternal energy intake Newborn gender | With each 1%↑ in fat intake, there was a 0.1 cm increase in birth length and abdominal circumference With each 1% ↑ in CHO intake, there was a 0.1 cm decrease in birth length and abdominal circumference CHO intake was negatively associated with head circumference (β ≤ −0.01, p = 0.04) [small effect] Adjusted for energy intake -Most CHO intake in this region is accounted for by nisma (porridge made from maize) |
| Liu, 2020 [34] | Cross-sectional n = 7194 Pregnant women (56.7% ages 25–34 years, 76.6% rural, 60.4% primiparous) Mean gestational age 39 ± 1 weeks Mean birth weight 3253.9 ± 448.3 g (z-score −0.07 [SD 1.15], SGA rate 13.2% Shaanxi Province, China | 107-item semi-quantitative food frequency questionnaire -represented intake during all of gestation Response variables in relation to birth weight were: protein and CHO density (g/4184 kJ), ratio PUFA + MUFA: SFA, haem Fe density (mg/4184 kJ) 2 diet patterns (DP) identified explained 63.1% of variation in response variables): DP1: higher protein and haem Fe, lower CHO and higher fat density. ↑legumes, soyabean, vegetables, meat, dairy, eggs, fish; ↓wheat, oils (Explained 40.1% of total response variables; 13.1% of response variables explained by CHO) DP2: Lower protein, higher CHO, lower fat and haem Fe. ↑wheat, rice, potatoes, vegetables, fruit; ↓nuts, red meat, oils (Explained 23.0% of total response variables; 65.6% of response variables explained by CHO) | Model 1: Unadjusted Model 2: Total energy intake Maternal age Education Residence Per captia annual household income Model 3: Model 2 + Parity Smoking Passive smoking Alcohol Pregnancy consultation Number of antenatal visits Folic acid/Fe/ multiple-micronutrient supplementation Models for BW and LBW were also adjusted for sex, gestational age | Across low, medium, and high adherence to DP1: SGA incidence 15.1% → 13.0% → 11.7% (p = 0.002) Birth weight 3225 g → 3261 g → 3276 g (p < 0.001) Birth weight z-score −0.15 → −0.05 → −0.01 (p < 0.001) These associations were significant across fully adjusted models |
| Powell, 2020 [35] | Observational birth cohort n = 141 mother/infant dyads Maasai pastoralist women n = 102 neonates from Mwanza (urban/peri-urban center) Included for BW comparisons only. Women were not instructed to reduce EI, but intake not measured All births during the dry season (June–September) Gestational age at delivery not reported Ngorongoro Conservation Area (NCA), Northern Tanzania | Food frequency questionnaire developed/validated for this Maasai cohort Administered 2−3 d postpartum on 2 occasions to assess early-mid (T1-2) and 3rd trimester (T3) pregnancy, respectively Intake T1-2 Total Energy: 1601 ± 734.19 kcal/d CHO intake T1-2: 276.04 g/d [95% CI: 237.72−314.37] 76% of total Fat intake: 43.83 g/d (37.67−49.99) Protein: 45.27 g/d (8.69−51.86) | Adjustments: Traditional birth attendant | Intake change from T1-2 to onset of T3: Total energy: 1601 → 799 ± 317.59 kcal/d CHO: 276 → 100.27 g/d (95% CI: 62.46−138.08) Fat: 43.83 → 23.43 g/d (17.38−29.48) Protein: 45.27 → 30.17 g/d (23.69−36.65) Reductions were: Total energy: −902.35 ± 74.94 kcal CHO: −175.78 ± 13.14 g (64% of total) Fat: −20.397 ± 2.32 g Protein: −15.099 ± 2.47 g p < 0.01 for all Birth weight and head circumference z-scores in neonates from Mwanza and NCA fell below the WHO standard Head circumference in neonates from NCA were far lower (1.7 SD) than standard (<50%tile at 36 weeks’), more so than weight (>50%tile at 36 weeks’). 31% had birth weight <2500 g (vs. 12% Mwanza), 40% were microcephalic (vs. n = 2 Mwanza). |
| Morisaki, 2018 [36] (Same cohort as Eshak) [30] | Observational birth cohort n = 91,637 Healthy pregnant women (40.3% primiparous, 31 ± 5 years) 73.6% had BMI 18.5 to <25 kg/m2 Gestational age at delivery: >28 weeks and ≤42 weeks Mean birth weight 3028 ± 406 g (6.9% SGA) Japan (15 regions represented) | Food frequency questionnaire Early pregnancy (FFQ1) to represent previous year Mid-pregnancy to represent intake during pregnancy Intake at FFQ1 Total energy: 7475.1 ± 2575.7 kJ/d CHO: 243.4 ± 80.2 g/d (55.3%) Fat: 59.9 ± 28.4 g/d (29.5%) Protein: 61.2 ± 25.6 g/d (13.5%) Intake at FFQ2 Total energy: 7184 ± 2506 kJ/d CHO: 233.7 ± 77 g/d (55.3%) Fat: 58.2 ± 27.9 g/d (29.8%) Protein: 58.9 ± 25.1 g/d (13.6%) | For models where CHO or fat were used to predict fetal growth, adjusted for: total energy intake Protein intake CHO or fat intake (appropriate to model) Confounders: Maternal age Parity Education Income Pre-pregnancy BMI Height Smoking status Infant sex Adjustments for: recruitment site Total energy intake Gestational weight gain Age | FFQ1 and FFQ2 related to birth weight: Birth weight was highest with 12% protein even when isoenergetic replacement with CHO or fat was modeled. Lower birth weight with protein >14% U-shaped association between protein density and SGA risk. Lowest SGA risk with protein at 12% even when isoenergetic replacement with CHO or fat was modeled. Higher SGA risk if protein >15% Controlled for protein, energy intake and maternal characteristics: Fat (FFQ1) Fat density of 25% associated with highest birth weight. Fat density >35% associated with lower birth weight CHO (FFQ1) CHO density of 59% (~264 g/d) had highest birth weight. CHO density <47% (~210 g/d) had lower birth weight. |
| Mijatovic, 2020 [39] | Randomized Controlled Trial n = 46 Women with gestational diabetes diagnosed at ~20 weeks’ gestation (10–14% primiparous, 33.3 ± 0.6 year, BMI 26.8 ± 0.9 kg/m2) 28.5 ± 0.4 weeks’ gestation Modestly lower CHO: 135 g/d Routine Care: 180–200 g/d Mean gestational age at delivery: 38 ± 0.2 weeks Primary outcome: difference in blood ketones between diet groups Australia | 24-h recalls 3 d food diaries Moderately lower CHO: 165 ± 7 g/d (20% achieved target) Energy intake: 7040 ± 240 kJ/d 25% insulin, 4% metformin Routine Care: 190 ± 9 g/d (65% achieved target) Energy intake: 8230 ± 320 kJ (p < 0.01) 31.8% insulin, 4.5% metformin Gestational weight gain similar (8–10 kg, p > 0.05) | Gestational weight gain Infant sex Gestational age at delivery Insulin status | No difference in birth weight, %fat, fat-free mass, LGA between groups Neonates in moderately lower CHO group had smaller head circumference (p = 0.04 after adjustment for weight gain, gestational age, infant sex) Intake differences from baseline → after 6 weeks: Moderately lower CHO: Energy: 7480 → 7040 kJ/d CHO: 167 → 165 g/d Fat: 74 → 71 g/d Protein: 100 → 85 g/d Routine care: Energy: 7510 → 8230 kJ/d CHO: 164 → 190 g/d (p = 0.04) Fat: 77 → 82 g/d (p > 0.05) Protein: 99 → 103 g/d (p < 0.01) |
| Harreiter, 2019 [40] | Randomized controlled trial Secondary analysis n = 436 Women with obesity <20 weeks’ gestation (~35% primiparous, ~32 ± 5 years, Pre-pregnancy BMI ~34 ± 4 kg/m2) Mean gestational age at delivery ~39 ± 2 weeks Mean birth weight ~3500 g Healthy eating: n = 221 No healthy eating: n = 215 ~20–22% GDM rate/group (p > 0.05) Nine European countries (86.7% of European descent) | 12-item questionnaire, frequencies (days/wk) -Only portions recorded 24–28 weeks (HE—No HE, adjusted mean difference (95%CI)) Portion size: −2.8 (−5.4, −0.1) * CHO: −2.0 (−6.4, 2.3) Fat: −1.3 (−2.3, −0.2) Protein: 1.1 (−0.2, 2.4) 35–37 weeks Portion size: −3.8 (−6.8, −0.9) ** CHO: −6.2 (−11.6, −0.9) * Fat: −1.5 (−2.8, −0.3) * Protein: 0.3 (−1.2, 1.7) * p < 0.05 ** p < 0.01 | Baseline level of outcome variable Or Baseline level of outcome variable + age + BMI at assessment date + gestational age + HOMA-IR + self-reported physical activity + self-reported food intake + smoking Gestational weight gain analyses adjusted for baseline BMI Dietary, physical activity analyses adjusted for baseline level | No differences in birth weight, LGA or SGA No difference in physical activity Weight gain (HE vs. No HE) 24–28 weeks’ gestation: 3.3 ± 2.7 vs. 4.3 ± 2.8 kg (p < 0.001) 35–37 weeks’ gestation: 7.0 ± 4.4 vs. 8.5 ± 4.7 kg (p < 0.01) |
| Report | Study Design Population | Carbohydrate Intake: Measurement and Amount | Statistical Adjustment | Carbohydrate Relationship to Perinatal Concern |
|---|---|---|---|---|
| Mijatovic, 2020 [39] | Randomized Controlled Trial n = 46 Women with gestational diabetes diagnosed at ~20 weeks’ gestation (10–14% primiparous, 33.3 ± 0.6 year, BMI 26.8 ± 0.9 kg/m2) 28.5 ± 0.4 weeks’ gestation Modestly lower CHO: 135 g/d Routine Care: 180–200 g/d Mean gestational age at delivery: 38 ± 0.2 weeks Primary outcome: difference in blood ketones between diet groups Australia | 24-h recalls 3 d food diaries Moderately lower CHO: 165 ± 7 g/d (20% achieved target) Energy intake: 7040 ± 240 kJ/d 25% insulin, 4% metformin Routine Care: 190 ± 9 g/d (65% achieved target) Energy intake: 8230 ± 320 kJ (p < 0.01) 31.8% insulin, 4.5% metformin Gestational weight gain similar (8–10 kg, p > 0.05) | Gestational weight gain Infant sex Gestational age at delivery Insulin status | Moderately lower CHO vs. Routine Care (ketones < 0.5 mmol/L = normal) Average of fasting blood, pre-prandial lunch, dinner Baseline 0.1 ± 00 vs. 0.2 ± 00 mmol/L (p > 0.05) 6 weeks later 0.1 ± 0.0 vs. 0.1 ± 0.0 mmol/L (p > 0.05) |
| Harreiter, 2019 [40] | Randomized controlled trial Secondary analysis n = 436 Women with obesity <20 weeks’ gestation (~35% primiparous, ~32 ± 5 years, Pre-pregnancy BMI ~34 ± 4 kg/m2) Mean gestational age at delivery ~39 ± 2 weeks Mean birth weight ~3500 g Healthy eating (HE): n = 221 No healthy eating: n = 215 ~20–22% GDM rate/group (p > 0.05) Nine European countries (86.7% of European descent) | 12-item questionnaire, frequencies (days/wk) -Only portions recorded 24–28 weeks (HE—No HE, adjusted mean difference (95%CI)) Portion size: −2.8 (−5.4, −0.1) * CHO: −2.0 (−6.4, 2.3) Fat: −1.3 (−2.3, −0.2) Protein: 1.1 (−0.2, 2.4) 35–37 weeks’ gestation Portion size: −3.8 (−6.8, −0.9) ** CHO: −6.2 (−11.6, −0.9) * Fat: −1.5 (−2.8, −0.3) * Protein: 0.3 (−1.2, 1.7) * p < 0.05 ** p < 0.01 | Baseline level of outcome variable Or Baseline level of outcome variable + age + BMI at assessment date + gestational age + HOMA-IR + self-reported physical activity + self-reported food intake + smoking Gestational weight gain analyses adjusted for baseline BMI Dietary, physical activity analyses adjusted for baseline level | HE vs. No HE 24–28 weeks’ gestation Fasting blood beta-hydroxybutyrate: 0.082 ± 0.065 vs. 0.068 ± 0.067 (p < 0.05) 35–37 weeks’ gestation Fasting blood beta-hydroxybutyrate: 0.107 ± 0.071 vs. 0.101 ± 0.092 |
| Report | Study Design Population | Carbohydrate Intake: Measurement and Amount | Statistical Adjustment | Carbohydrate Relationship to Perinatal Concern |
|---|---|---|---|---|
| Eshak, 2020 [30] | Observational birth cohort n = 78,793 Healthy pregnant women (39.6% primiparous, 31 ± 5 years) 78% had BMI 18.5 to < 25 kg/m2 Mean gestational age at delivery 38.9 ± 1.5 weeks -Mean birth weight reported in right column Japan (15 regions represented) | Food frequency questionnaire Trimester 2 Median (IQR) CHO intake 223.8 (182.6–272.4) g/d 55.3% of total energy intake 61.9% of women consumed <recommended amount of CHO/d (57.5%) Quartiles of CHO% Intake Q1: 45.1% (1075 kcal/d) Q2: 52.9% (1466 kcal/d) Q3: 57.7% (1800 kcal/d) Q4: 64.9% (2650 kcal/d) Proportion of women consumed < recommended amount of micronutrients Vitamin A 63% Vitamin K 48% Vitamin E 61% Vitamin D 87% Vitamin C 67%, Vitamin B6 73% Folate 88% Vitamin B12 26% | Geometric means of nutrients adjusted for: Maternal age Height Education Household income Pre-pregnancy BMI Net weight change in pregnancy Smoking Alcohol Thyroid disease Use of folate supplement Offspring sex, gestational age at delivery | Increasing quartiles of micronutrients: Vitamin C and folate intake associated with birthweight; Vitamins C, D, K, B6, B12 and folate associated with birth length Vitamins A, E and D associated with head circumference; Vitamins A, C and D associated with chest circumference Vitamin K inversely associated with the ponderal index in the offspring |
| Saunders, 2019 [37] | Observational birth cohort n = 1674 Healthy pregnant women (62.9% primiparous, 32.5 ± 4.1 years) BMI 24.6 ± 3.5 kg/m2 Recruited between 16–22 weeks’ gestation Norway | Food frequency questionnaire First half of pregnancy Total energy: 10,082 (4139) kJ CHO: 45.7 (42.3–49.2) % Fat: 34.5 (31.2–37.8) % Protein: 16.5 (15.1–18.1) % Below and above Recommended Intake Range for macronutrients: CHO: Below 43.9%, above 0.5% Fat: Below 2.9%, above 14.0% Protein: Below 0.2%, above 6.9% Micronutrients: Vit A: Below 9.6%, above 90.4% Vit C: Below 4.4%, above 95.6% Vit D: Below 28.7%, above 71.3% Vit B12: Below 0.3%, above 99.7% Iodine: Below 24.4%, above 75.6% Folate: Below 54.4, above 45.6% Zinc: Below 10.2, above 89.8% Ca: Below 36.2%, above 63.8% Selenium: Below 41.3%, above 58.7% Iron: Below 41.3%, above 58.7% Median (IQR) Based on Nordic Nutrition Recommendations, 2012 [52] | Educational level (post-hoc analysis) | No association between educational levels and micronutrient intake |
| Looman, 2019 [38] | Observational Birth Cohort n = 105 Preconception to <24 weeks’ gestation (32yo, 93% multiparous, median BMI preconception 24.4 kg/m2 The Netherlands | FFQ 75 g 2-h OGTT At pre-conception, 12 and 24 weeks’ gestation Energy intake increased during pregnancy from 8583 (6713; 9462) kJ at preconception to 9189 (7432; 10,541) kJ at 24 weeks’ gestation Median CHO 46.5% (43.2; 49.7) Preconception 45.4% (42.3; 48.6) TM1 46.5% (45.2; 50.3) TM2 48.1% (44.8; 50.3) | Covariates: Age Education Ethnicity Parity Smoking Nausea in pregnancy Vomiting in pregnancy Season of blood collection Physical Activity Energy intake Alcohol Time between measurements Hx of GDM BMI Adjusted for supplement intake | Iron intake inversely associated with fasting glucose and HbA1c Folate, vitamin B6 and vitamin D levels significantly changed through pregnancy, accounted for by intake of supplements |
| Mijatovic, 2020 [39] | Randomized Controlled Trial n = 46 Women with gestational diabetes diagnosed at ~20 weeks’ gestation (10–14% primiparous, 33.3 ± 0.6 years, BMI 26.8 ± 0.9 kg/m2) 28.5 ± 0.4 weeks’ gestation Modestly lower CHO: 135 g/d Routine Care: 180–200 g/d Mean gestational age at delivery: 38 ± 0.2 weeks’ Primary outcome: difference in blood ketones between diet groups Australia | 24-h recalls 3d food diaries Moderately lower CHO: 165 ± 7 g/d (20% achieved target) Energy intake: 7040 ± 240 kJ/d 25% insulin, 4% metformin Routine Care: 190 ± 9 g/d (65% achieved target) Energy intake: 8230 ± 320 kJ (p < 0.01) 31.8% insulin, 4.5% metformin Gestational weight gain similar (8–10 kg, p > 0.05) | Gestational weight gain Infant sex Gestational age at delivery Insulin status | Moderately lower CHO: lower Fe, iodine |
| Report | Study Design Population | Carbohydrate Intake: Measurement and Amount | Statistical Adjustment | Carbohydrate Relationship to Perinatal Concern |
|---|---|---|---|---|
| Harreiter, 2019 [40] | Randomized controlled trial Secondary analysis n = 436 Women with obesity <20 weeks’ gestation (~35% primiparous, ~32 ± 5 years, pre-pregnancy BMI ~34 ± 4 kg/m2) Mean gestational age at delivery ~39 ± 2 weeks Mean birth weight ~3500 g Healthy eating: n = 221 No healthy eating: n = 215 ~20–22% GDM rate/group (p > 0.05) Nine European countries (86.7% of European descent) | 12-item questionnaire, frequencies (days/wk) -Only portions recorded 24–28 weeks’ gestation (HE—No HE, adjusted mean difference (95%CI)) Portion size: −2.8 (−5.4, −0.1) * CHO: −2.0 (−6.4, 2.3) Fat: −1.3 (−2.3, −0.2) Protein: 1.1 (−0.2, 2.4) 35–37 weeks’ gestation Portion size: −3.8 (−6.8, −0.9) ** CHO: −6.2 (−11.6, −0.9) * Fat: −1.5 (−2.8, −0.3) * Protein: 0.3 (−1.2, 1.7) * p < 0.05 ** p < 0.01 | Baseline level of outcome variable Or Baseline level of outcome variable + age + BMI at assessment date + gestational age + HOMA-IR + self-reported physical activity + self-reported food intake + smoking Gestational weight gain analyses adjusted for baseline BMI Dietary, physical activity analyses adjusted for baseline level | HE vs. No HE 24–28 weeks’ gestation TG: 1.88 ± 0.63 vs. 1.85 ± 0.68 mmol/L FFA: 0.60 ± 0.19 vs. 0.55 ± 0.17 mmol/L (p < 0.01) Fasting glucose: 4.8 ± 0.4 vs. 4.6 ± 0.4 mmol/L (p < 0.05) 35–37 weeks’ gestation TG 2.42 ± 0.8 vs. 2.27 ± 0.8 mmol/L FFA: 0.64 ± 0.23 vs. 0.59 ± 0.21 (p < 0.05) Fasting glucose: 4.6 ± 0.5 vs. 4.5 ± 0.4 mmol/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sweeting, A.; Mijatovic, J.; Brinkworth, G.D.; Markovic, T.P.; Ross, G.P.; Brand-Miller, J.; Hernandez, T.L. The Carbohydrate Threshold in Pregnancy and Gestational Diabetes: How Low Can We Go? Nutrients 2021, 13, 2599. https://doi.org/10.3390/nu13082599
Sweeting A, Mijatovic J, Brinkworth GD, Markovic TP, Ross GP, Brand-Miller J, Hernandez TL. The Carbohydrate Threshold in Pregnancy and Gestational Diabetes: How Low Can We Go? Nutrients. 2021; 13(8):2599. https://doi.org/10.3390/nu13082599
Chicago/Turabian StyleSweeting, Arianne, Jovana Mijatovic, Grant D. Brinkworth, Tania P. Markovic, Glynis P. Ross, Jennie Brand-Miller, and Teri L. Hernandez. 2021. "The Carbohydrate Threshold in Pregnancy and Gestational Diabetes: How Low Can We Go?" Nutrients 13, no. 8: 2599. https://doi.org/10.3390/nu13082599
APA StyleSweeting, A., Mijatovic, J., Brinkworth, G. D., Markovic, T. P., Ross, G. P., Brand-Miller, J., & Hernandez, T. L. (2021). The Carbohydrate Threshold in Pregnancy and Gestational Diabetes: How Low Can We Go? Nutrients, 13(8), 2599. https://doi.org/10.3390/nu13082599







