An Updated Overview of Almond Allergens
Abstract
:1. Introduction
Methods
2. Food Allergy
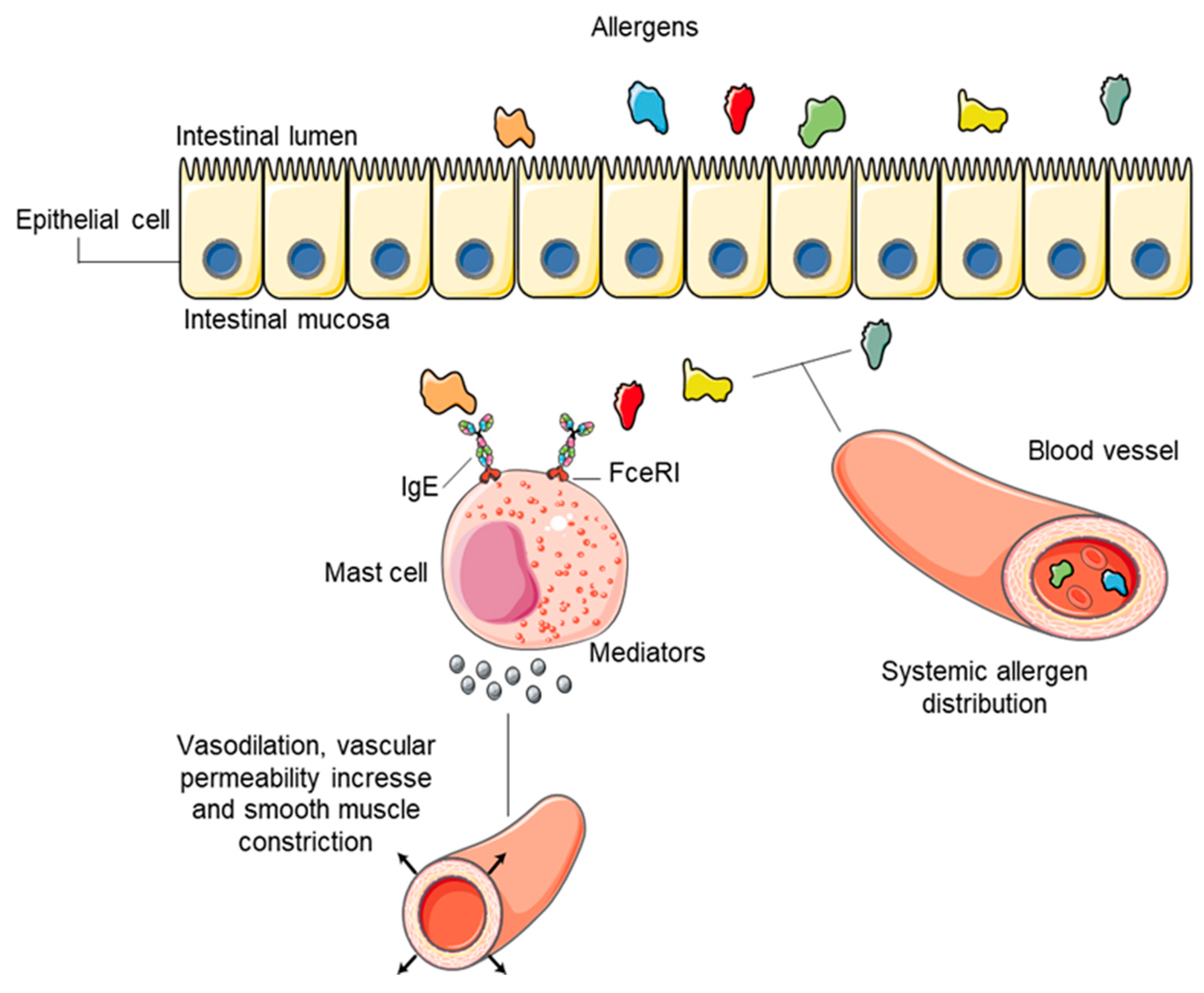
2.1. Molecular Pathway of Immunoglobulin E-Mediated Food Reaction
2.2. Legal Framework
3. Almond
3.1. Almond Allergy
3.2. Almond Allergens
3.2.1. WHO/IUIS Designated Almond Allergens
Pru du 6 (Amandin)
Pru du 5 (60S Acidic Ribossomal Protein P2)
Pru du 3 (nsLTP)
Pru du 4 (Profilins)
Pru du 8
Pru du 10
3.2.2. Allergens Not Included in the WHO/IUIS Allergen List
Pru du γ-Conglutin
Pru du 1-PR-10 Protein (Pathogenesis Related-10 Protein)
Pru du 2 (PR-5/Thaumatin-Like Protein)
Pru du 2S Albumin
3.3. Methods for Almond Allergens Detection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The Prevalence, Severity, and Distribution of Childhood Food Allergy in the United States. Pediatrics 2011, 128, e9–e17. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prev-alence and severity of food allergies among US adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Kumfer, A.M.; Commins, S.P. Primary prevention of food allergy. Curr. Allergy Asthma Rep. 2019, 19, 7. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, L.E.; Stewart, P.H.; Deshazo, R.D. Food Allergy: What We Know Now. Am. J. Med. Sci. 2017, 353, 353–366. [Google Scholar] [CrossRef]
- Sánchez-García, S.; Del Río, P.R.; Escudero, C.; Martínez-Gómez, M.J.; Ibáñez, M.D. Possible eosin-ophilic esophagitis induced by milk oral immunotherapy. J. Allergy Clin. Immunol. 2012, 129, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Steele, P.H.; Vickery, B.P.; Bird, J.A.; Thyagarajan, A.; Scurlock, A.M.; Perry, T.T.; Jones, S.M.; Burks, A.W. Adverse reactions during peanut oral immunotherapy home dosing. J. Allergy Clin. Immunol. 2009, 124, 1351–1352. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jin, T. Almond allergens: Update and perspective on identification and characterization. J. Sci. Food Agric. 2020, 100, 4657–4663. [Google Scholar] [CrossRef]
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, B. Almond Allergens: Molecular Characterization, Detection, and Clinical Relevance. J. Agric. Food Chem. 2012, 60, 1337–1349. [Google Scholar] [CrossRef]
- Mandalari, G.; Mackie, A.R. Almond Allergy: An Overview on Prevalence, Thresholds, Regulations and Allergen Detection. Nutrients 2018, 10, 1706. [Google Scholar] [CrossRef] [Green Version]
- Boyce, J.A.; Assa’Ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J. Am. Acad. Dermatol. 2011, 64, 175–192. [Google Scholar] [CrossRef] [Green Version]
- Green, P.H.; Lebwohl, B.; Greywoode, R. Celiac disease. J. Allergy Clin. Immunol. 2015, 135, 1099–1106. [Google Scholar] [CrossRef]
- Prescott, S.; Allen, K. Food allergy: Riding the second wave of the allergy epidemic. Pediatr. Allergy Immunol. 2011, 22, 155–160. [Google Scholar] [CrossRef]
- Panjari, M.; Koplin, J.; Dharmage, S.; Peters, R.; Gurrin, L.; Sawyer, S.; McWilliam, V.; Eckert, J.; Vicendese, D.; Erbas, B. Nut allergy prevalence and differences between Asian-born children and Australian-born children of A sian descent: A state-wide survey of children at primary school entry in Victoria, Australia. Clin. Exp. Allergy 2016, 46, 602–609. [Google Scholar] [CrossRef]
- Du Toit, G.; Tsakok, T.; Lack, S.; Lack, G. Prevention of food allergy. J. Allergy Clin. Immunol. 2016, 137, 998–1010. [Google Scholar] [CrossRef] [Green Version]
- Sicherer, S.H.; Allen, K.; Lack, G.; Taylor, S.L.; Donovan, S.M.; Oria, M. Critical Issues in Food Allergy: A National Academies Consensus Report. Pediatrics 2017, 140, e20170194. [Google Scholar] [CrossRef] [Green Version]
- Alasalvar, C.; Shahidi, F. Tree Nuts: Composition, Phytochemicals, and Health Effects; Informa UK Limited: London, UK, 2008. [Google Scholar]
- Panel, N.-S.E. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126, S1–S58. [Google Scholar]
- Sampson, H.A.; Mendelson, L.; Rosen, J.P. Fatal and Near-Fatal Anaphylactic Reactions to Food in Children and Adolescents. N. Engl. J. Med. 1992, 327, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Yunginger, J.W.; Sweeney, K.G.; Sturner, W.Q.; Giannandrea, L.A.; Teigland, J.D.; Bray, M.; Benson, P.A.; York, J.A.; Biedrzycki, L.; Squillace, D.L.; et al. Fatal Food-Induced Anaphylaxis. JAMA 1988, 260, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Luyt, D.K.; Vaughan, D.; Oyewole, E.; Stiefel, G. Ethnic differences in prevalence of cashew nut, pistachio nut and almond allergy. Pediatr. Allergy Immunol. 2016, 27, 651–654. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Burton, O.T.; Darling, A.R.; Zhou, J.S.; Noval-Rivas, M.; Jones, T.G.; Gurish, M.F.; Chatila, T.A.; Oettgen, H.C. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013, 6, 740–750. [Google Scholar] [CrossRef] [Green Version]
- Perrier, C.; Corthésy, B. Gut permeability and food allergies. Clin. Exp. Allergy 2010, 41, 20–28. [Google Scholar] [CrossRef]
- Vadas, P.; Gold, M.; Perelman, B.; Liss, G.M.; Lack, G.; Blyth, T.; Simons, F.E.R.; Simons, K.J.; Cass, D.; Yeung, J. Platelet-Activating Factor, PAF Acetylhydrolase, and Severe Anaphylaxis. N. Engl. J. Med. 2008, 358, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.W.; Sharma, H.P. Anaphylaxis and Urticaria. Immunol. Allergy Clin. N. Am. 2015, 35, 199–219. [Google Scholar] [CrossRef]
- Mishra, A.; Schlotman, J.; Wang, M.; Rothenberg, M.E. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J. Leukoc. Biol. 2006, 81, 916–924. [Google Scholar] [CrossRef]
- Clayton, F.; Fang, J.C.; Gleich, G.J.; Lucendo, A.J.; Olalla, J.M.; Vinson, L.A.; Lowichik, A.; Chen, X.; Emerson, L.; Cox, K.; et al. Eosinophilic Esophagitis in Adults Is Associated With IgG4 and Not Mediated by IgE. Gastroenterology 2014, 147, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Gendel, S.M. Comparison of international food allergen labeling regulations. Regul. Toxicol. Pharmacol. 2012, 63, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Derr, L.E. When food is poison: The history, consequences, and limitations of the Food Allergen Labeling and Consumer Protection Act of 2004. Food Drug Law J. 2006, 61, 65–165. [Google Scholar]
- Rehm, C.D.; Drewnowski, A. Replacing American snacks with tree nuts increases consumption of key nutrients among US children and adults: Results of an NHANES modeling study. Nutr. J. 2017, 16, 17. [Google Scholar] [CrossRef] [Green Version]
- Bottone, A.; Montoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolomics and antioxidant activity of the leaves of Prunus dulcis Mill. (Italian cvs. Toritto and Avola). J. Pharm. Biomed. Anal. 2018, 158, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Nanos, G.D.; Kazantzis, I.; Kefalas, P.; Petrakis, C.; Stavroulakis, G.G. Irrigation and harvest time affect almond kernel quality and composition. Sci. Hortic. 2002, 96, 249–256. [Google Scholar] [CrossRef]
- Piscopo, A.; Romeo, F.; Petrovicova, B.; Poiana, M. Effect of the harvest time on kernel quality of several almond varieties (Prunus dulcis (Mill.) D.A. Webb). Sci. Hortic. 2010, 125, 41–46. [Google Scholar] [CrossRef]
- de Giorgio, D.; Leo, L.; Zacheo, G.; Lamascese, N. Evaluation of 52 almond (Prunus amygdalusBatsch) cultivars from the Apulia region in Southern Italy. J. Hortic. Sci. Biotechnol. 2007, 82, 541–546. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#home (accessed on 10 October 2020).
- Griel, A.E.; Kris-Etherton, P.M. Tree nuts and the lipid profile: A review of clinical studies. Br. J. Nutr. 2006, 96, S68–S78. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.A.; Hu, F.B.; Tapsell, L.C.; Josse, A.; Kendall, C.W.C. Possible Benefit of Nuts in Type 2 Diabetes. J. Nutr. 2008, 138, 1752S–1756S. [Google Scholar] [CrossRef] [Green Version]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Turco, V.L.; Cacciola, F.; Rich, G.; Bisignano, G.; Saija, A.; Dugo, P.; et al. Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Richardson, D.P.; Astrup, A.; Cocaul, A.; Ellis, P. The nutritional and health benefits of almonds: A healthy food choice. Food Sci. Technol. Bull. Funct. Foods 2009, 6, 41–50. [Google Scholar] [CrossRef]
- E Berryman, C.; Preston, A.G.; Karmally, W.; Deckelbaum, R.J.; Kris-Etherton, P. Effects of almond consumption on the reduction of LDL-cholesterol: A discussion of potential mechanisms and future research directions. Nutr. Rev. 2011, 69, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids--food sources and health benefits. Roczniki Państwowego Zakładu Higieny 2014, 65, 65. [Google Scholar]
- Mandalari, G.; Genovese, T.; Bisignano, C.; Mazzon, E.; Wickham, M.; Di Paola, R.; Bisignano, G.; Cuzzocrea, S. Neuroprotective effects of almond skins in experimental spinal cord injury. Clin. Nutr. 2011, 30, 221–233. [Google Scholar] [CrossRef]
- Seo, K.; Lee, D.; Kang, H.; Kim, H.; Kim, Y.; Baek, N.; Lee, D. Hepatoprotective and neuroprotective tocopherol analogues isolated from the peels of Citrus unshiuMarcovich. Nat. Prod. Res. 2014, 29, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, H.; Zhan, P.; Tian, F. Composition and antioxidant and antimicrobial activities of white apricot almond (Amygdalus communis L.) oil. Eur. J. Lipid Sci. Technol. 2011, 113, 1138–1144. [Google Scholar] [CrossRef]
- Cabrita, L.; Apostolova, E.; Neves, A.; Marreiros, A.; Leitão, J. Genetic diversity assessment of the almond (Prunus dulcis (Mill.) D.A. Webb) traditional germplasm of Algarve, Portugal, using molecular markers. Plant Genet. Resour. 2014, 12, S164–S167. [Google Scholar] [CrossRef]
- Monastra, F.; Raparelli, E. Inventory of almond research, germplasm and references. In REUR Technical Series; FAO: Rome, Italy, 1997. [Google Scholar]
- Bargman, T.R.J.; Rupnow, J.O.H.; Taylor, S.T.L. IgE-binding proteins in almonds (Prunus amygdalus); identi-fication by immunoblotting with sera from almond-allergic adults. J. Food Sci. 1992, 57, 717–720. [Google Scholar] [CrossRef]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.-Y.O. Polyphenol content and antioxidant activity of California almonds depend on cultivar and harvest year. Food Chem. 2010, 122, 819–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summo, C.; Palasciano, M.; De Angelis, D.; Paradiso, V.M.; Caponio, F.; Pasqualone, A. Evaluation of the chemical and nu-tritional characteristics of almonds (Prunus dulcis (Mill). DA Webb) as influenced by harvest time and cultivar. J. Sci. Food Agric. 2018, 98, 5647–5655. [Google Scholar] [CrossRef] [Green Version]
- Ewan, P.W. Clinical study of peanut and nut allergy in 62 consecutive patients: New features and associations. BMJ 1996, 312, 1074–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicherer, S.H.; Muñoz-Furlong, A.; A Sampson, H. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: A 5-year follow-up study. J. Allergy Clin. Immunol. 2003, 112, 1203–1207. [Google Scholar] [CrossRef]
- Geiselhart, S.; Hoffmann-Sommergruber, K.; Bublin, M. Tree nut allergens. Mol. Immunol. 2018, 100, 71–81. [Google Scholar] [CrossRef]
- Weinberger, T.; Sicherer, S. Current perspectives on tree nut allergy: A review. J. Asthma Allergy 2018, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.R.; Park, H.J.; Park, K.H.; Lee, J.-H.; Park, J.-W. IgE Sensitization Patterns to Commonly Consumed Foods Determined by Skin Prick Test in Korean Adults. J. Korean Med. Sci. 2016, 31, 1197–1201. [Google Scholar] [CrossRef] [Green Version]
- McWilliam, V.; Koplin, J.; Lodge, C.; Tang, M.; Dharmage, S.; Allen, K.J. The Prevalence of Tree Nut Allergy: A Systematic Review. Curr. Allergy Asthma Rep. 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Segura, L.R.; Pérez, E.F.; Nowak-Wegrzyn, A.; Siepmann, T.; Larenas-Linnemann, D. Food allergen sensitization pat-terns in a large allergic population in Mexico. Allergol. Immunopathol. 2020, 48, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Rentzos, G.; Johanson, L.; Goksör, E.; Telemo, E.; Lundbäck, B.; Ekerljung, L. Prevalence of food hypersensitivity in relation to IgE sensitisation to common food allergens among the general adult population in West Sweden. Clin. Transl. Allergy 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, G.; Anagnostou, K.; Boyle, R.; Brathwaite, N.; Ewan, P.; Fox, A.T.; Huber, P.; Luyt, D.; Till, S.J.; Venter, C.; et al. BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin. Exp. Allergy 2017, 47, 719–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.-W.; Lin, Y.-C.; Nong, B.-R.; Liu, P.-Y.; Huang, Y.-F.; Lu, L.-Y.; Lee, H.-S. Nut sensitization profile in Southern Taiwan. Immunol. Infect. 2020, 53, 791–796. [Google Scholar] [CrossRef]
- Ben Kayale, L.; Ling, J.; Henderson, E.; Carter, N. The influence of cultural attitudes to nut exposure on reported nut allergy: A pilot cross sectional study. PLoS ONE 2020, 15, e0234846. [Google Scholar] [CrossRef]
- Byrne, A.M.; Malka-Rais, J.; Burks, A.W.; Fleischer, D.M. How do we know when peanut and tree nut allergy have resolved, and how do we keep it resolved? Clin. Exp. Allergy 2010, 40, 1303–1311. [Google Scholar] [CrossRef]
- Albin, S.; Nowak-Węgrzyn, A.J.I.; Clinics, A. Potential treatments for food allergy. Immunol. Allergy Clin. 2015, 35, 77–100. [Google Scholar] [CrossRef]
- Kulis, M.; Vickery, B.; Burks, A.W. Pioneering immunotherapy for food allergy: Clinical outcomes and modulation of the immune response. Immunol. Res. 2011, 49, 216–226. [Google Scholar] [CrossRef]
- Egger, M.; Hauser, M.; Mari, A.; Ferreira, F.; Gadermaier, G. The Role of Lipid Transfer Proteins in Allergic Diseases. Curr. Allergy Asthma Rep. 2010, 10, 326–335. [Google Scholar] [CrossRef]
- Noble, K.A.; Liu, C.; Sathe, S.K.; Roux, K.H. A Cherry Seed-Derived Spice, Mahleb, is Recognized by Anti-Almond Antibodies Including Almond-Allergic Patient IgE. J. Food Sci. 2017, 82, 1786–1791. [Google Scholar] [CrossRef]
- Lee, S.-H.; Benmoussa, M.; Sathe, S.K.; Roux, K.H.; Teuber, S.S.; Hamaker, B.R. A 50 kDa Maize γ-Zein Has Marked Cross-Reactivity with the Almond Major Protein. J. Agric. Food Chem. 2005, 53, 7965–7970. [Google Scholar] [CrossRef]
- Tawde, P.; Venkatesh, Y.P.; Wang, F.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Cloning and characterization of profilin (Pru du 4), a cross-reactive almond (Prunus dulcis) allergen. J. Allergy Clin. Immunol. 2006, 118, 915–922. [Google Scholar] [CrossRef]
- de Leon, M.; Drew, A.; Glaspole, I.; Suphioglu, C.; O’Hehir, R.; Rolland, J. IgE cross-reactivity between the major peanut allergen Ara h 2 and tree nut allergens. Mol. Immunol. 2007, 44, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Teuber, S.S.; Cheng, H.; Chen, D.; Comstock, S.S.; Ruan, S.; Schein, C.H. Computationally predicted IgE epitopes of walnut allergens contribute to cross-reactivity with peanuts. Allergy 2011, 66, 1522–1529. [Google Scholar] [CrossRef] [Green Version]
- Wallowitz, M.; Teuber, S.; Beyer, K.; Sampson, H.; Roux, K.; Sathe, S.; Wang, F.; Robotham, J. Cross-reactivity of walnut, cashew, and hazelnut legumin proteins in tree nut allergic patients. J. Allergy Clin. Immunol. 2004, 113, S156. [Google Scholar] [CrossRef]
- Flinterman, A.E.; Hoekstra, M.O.; Meijer, Y.; Van Ree, R.; Akkerdaas, J.H.; Bruijnzeel-Koomen, C.A.; Knulst, A.C.; Pasmans, S.G. Clinical reactivity to hazelnut in children: Association with sensitization to birch pollen or nuts? J. Allergy Clin. Immunol. 2006, 118, 1186–1189. [Google Scholar] [CrossRef]
- Vieths, S.; Scheurer, S.; BALLMER-WEBER, B. Current understanding of cross-reactivity of food allergens and pollen. Ann. N. Y. Acad. Sci. 2002, 964, 47–68. [Google Scholar] [CrossRef]
- Hasegawa, M.; Inomata, N.; Yamazaki, H.; Morita, A.; Kirino, M.; Ikezawa, Z. Clinical Features of Four Cases with Cashew Nut Allergy and Cross-Reactivity between Cashew Nut and Pistachio. Allergol. Int. 2009, 58, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Noorbakhsh, R.; Mortazavi, S.A.; Sankian, M.; Shahidi, F.; Tehrani, M.; Azad, F.J.; Behmanesh, F.; Varasteh, A. Pistachio Allergy-Prevalence and In vitro Cross-Reactivity with Other Nuts. Allergol. Int. 2011, 60, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Asero, R. 7 Lipid Transfer Protein Cross-reactivity Assessed In Vivo and In Vitro in the Office: Pros and Cons. J. Investig. Allergol. Clin. Immunol. 2011, 21, 129. [Google Scholar] [PubMed]
- KewalRamani, A.; Maleki, S.; Cheng, H.; Teuber, S. Cross-Reactivity Among Almond, Peanut and Other Tree Nuts in Almond Allergic Patients. J. Allergy Clin. Immunol. 2006, 117, S32. [Google Scholar] [CrossRef]
- Albillos, S.M.; Jin, T.; Howard, A.; Zhang, Y.; Kothary, M.H.; Fu, T.-J. Purification, Crystallization and Preliminary X-ray Characterization of Prunin-1, a Major Component of the Almond (Prunus dulcis) Allergen Amandin. J. Agric. Food Chem. 2008, 56, 5352–5358. [Google Scholar] [CrossRef]
- Albillos, S.M.; Menhart, N.; Fu, T.-J. Structural Stability of Amandin, a Major Allergen from Almond (Prunus dulcis), and Its Acidic and Basic Polypeptides. J. Agric. Food Chem. 2009, 57, 4698–4705. [Google Scholar] [CrossRef]
- Roux, K.H.; Teuber, S.S.; Robotham, J.M.; Sathe, S.K. Detection and Stability of the Major Almond Allergen in Foods. J. Agric. Food Chem. 2001, 49, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.K.; Wolf, W.J.; Roux, K.H.; Teuber, S.S.; Venkatachalam, M.; Sze-Tao, K.W.C. Biochemical Characterization of Amandin, the Major Storage Protein in Almond (Prunus dulcis L.). J. Agric. Food Chem. 2002, 50, 4333–4341. [Google Scholar] [CrossRef]
- Willison, L.N.; Tripathi, P.; Sharma, G.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Cloning, Expression and Patient IgE Reactivity of Recombinant Pru du 6, an 11S Globulin from Almond. Int. Arch. Allergy Immunol. 2011, 156, 267–281. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Effects of Roasting, Blanching, Autoclaving, and Microwave Heating on Antigenicity of Almond (Prunus dulcis L.) Proteins. J. Agric. Food Chem. 2002, 50, 3544–3548. [Google Scholar] [CrossRef]
- Mandalari, G.; Rigby, N.M.; Bisignano, C.; Curto, R.B.L.; Mulholland, F.; Su, M.; Venkatachalam, M.; Robotham, J.M.; Willison, L.N.; Lapsley, K.; et al. Effect of food matrix and processing on release of almond protein during simulated digestion. LWT 2014, 59, 439–447. [Google Scholar] [CrossRef]
- Holden, L.; Sletten, G.B.; Lindvik, H.; Fæste, C.K.; Dooper, M.M. Characterization of IgE binding to lupin, peanut and al-mond with sera from lupin-allergic patients. Int. Arch. Allergy Immunol. 2008, 146, 267–276. [Google Scholar] [CrossRef]
- Abou Alhasani, M.; Roux, K.H. cDNA Cloning, expression and characterization of an allergenic 60s ribosomal protein of almond (Prunus dulcis). Iran. J. Allergy Asthma Immunol. 2009, 8, 77–84. [Google Scholar]
- Yao, L.; Li, H.; Yang, J.; Li, C.; Shen, Y. Purification and characterization of a hydroxynitrile lyase from Amygdalus pedunculata Pall. Int. J. Biol. Macromol. 2018, 118, 189–194. [Google Scholar] [CrossRef]
- Mills, E.C.; Sancho, A.; Moreno, J.; Kostyra, H. The effects of food processing on allergens. In Managing Allergens in Food; Elsevier: Amsterdam, The Netherlands, 2007; pp. 117–133. [Google Scholar]
- De Angelis, E.; Bavaro, S.L.; Forte, G.; Pilolli, R.; Monaci, L. Heat and Pressure Treatments on Almond Protein Stability and Change in Immunoreactivity after Simulated Human Digestion. Nutrients 2018, 10, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, H.; Zhang, Y.; Jiang, S.; Jin, T.; Lyu, S.-C.; Nadeau, K.C.; McHugh, T. Almond (Prunus dulcis) Allergen Pru du 8, the First Member of a New Family of Food Allergens. J. Agric. Food Chem. 2019, 67, 8626–8631. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.P.; Green, J.L.; Goulter, K.C.; Manners, J.M. A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels. Plant J. 1999, 19, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Poltronieri, P.; Cappello, M.; Dohmae, N.; Conti, A.; Fortunato, D.; Pastorello, E.; Ortolani, C.; Zacheo, G. Identification and characterisation of the IgE-binding proteins 2S albumin and conglutin γ in almond (Prunus dulcis) seeds. Int. Arch. Allergy Immunol. 2002, 128, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Garino, C.; De Paolis, A.; Coïsson, J.D.; Arlorio, M. Pru du 2S albumin or Pru du vicilin? Comput. Biol. Chem. 2015, 56, 30–32. [Google Scholar] [CrossRef]
- Kolivas, S.; Gayler, K.R. Structure of the cDNA coding for conglutin γ, a sulphur-rich protein from Lupinus angusti-folius. Plant Mol. Biol. 1993, 21, 397–401. [Google Scholar] [CrossRef]
- Burks, A.W.; Williams, L.W.; Helm, R.M.; Connaughton, C.; Cockrell, G.; O’Brien, T. Identification of a major peanut allergen, Ara h I, in patients with atopic dermatitis and positive peanut challenges. J. Allergy Clin. Immunol. 1991, 88, 172–179. [Google Scholar] [CrossRef]
- Burks, A.W., Jr.; Brooks, J.R.; Sampson, H.A. Allergenicity of major component proteins of soybean determined by enzyme-linked immunosorbent assay (ELISA) and immunoblotting in children with atopic dermatitis and positive soy challenges. J. Allergy Clin. Immunol. 1988, 81, 1135–1142. [Google Scholar] [CrossRef]
- Wang, F.; Robotham, J.M.; Teuber, S.S.; Tawde, P.; Sathe, S.K.; Roux, K.H. Ana o 1, a cashew (Ana-cardium occidental) allergen of the vicilin seed storage protein family. J. Allergy Clin. Immunol. 2002, 110, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Scarafoni, A.; Consonni, A.; Pessina, S.; Balzaretti, S.; Capraro, J.; Galanti, E.; Duranti, M. Structural basis of the lack of endoglucanase inhibitory activity of Lupinus albus γ-conglutin. Plant Physiol. Biochem. 2016, 99, 79–85. [Google Scholar] [CrossRef]
- Fernandes, H.; Michalska, K.; Sikorski, M.; Jaskolski, M. Structural and functional aspects of PR-10 proteins. FEBS J. 2013, 280, 1169–1199. [Google Scholar] [CrossRef]
- Mittag, D.; Akkerdaas, J.; Ballmer-Weber, B.K.; Vogel, L.; Wensing, M.; Becker, W.-M.; Koppelman, S.J.; Knulst, A.C.; Helbling, A.; Hefle, S.L.; et al. Ara h 8, a Bet v 1–homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J. Allergy Clin. Immunol. 2004, 114, 1410–1417. [Google Scholar] [CrossRef]
- Scala, E.; Alessandri, C.; Palazzo, P.; Pomponi, D.; Liso, M.; Bernardi, M.L.; Ferrara, R.; Zennaro, D.; Santoro, M.; Rasi, C. IgE recognition patterns of profilin, PR-10, and tropomyosin panallergens tested in 3,113 allergic patients by allergen microar-ray-based technology. PLoS ONE 2011, 6, e24912. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhang, S.; Illa, E.; Song, L.; Wu, S.; Howad, W.; Arús, P.; Van de Weg, E.; Chen, K.; Gao, Z. Genomic characterization of putative allergen genes in peach/almond and their synteny with apple. BMC Genom. 2008, 9, 543. [Google Scholar] [CrossRef] [Green Version]
- Palacin, A.; Tordesillas, L.; Gamboa, P.; Sanchez-Monge, R.; Cuesta-Herranz, J.; Sanz, M.; Barber, D.; Salcedo, G.; Díaz-Perales, A. Characterization of peach thaumatin-like proteins and their identification as major peach allergens. Clin. Exp. Allergy 2010, 40, 1422–1430. [Google Scholar] [CrossRef]
- Roux, K.H.; Teuber, S.S.; Sathe, S.K. Tree Nut Allergens. Int. Arch. Allergy Immunol. 2003, 131, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945. [Google Scholar] [PubMed] [Green Version]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, F.J.; Clemente, A. 2S Albumin Storage Proteins: What Makes them Food Allergens? Open Biochem. J. 2008, 2, 16–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, A.; Chambers, S.J.; Lodi, F.; Nicoletti, C.; Brett, G.M. Use of the indirect competitive ELISA for the detection of Brazil nut in food products. Food Control. 2004, 15, 65–69. [Google Scholar] [CrossRef]
- Koppelman, S.J.; Wensing, M.; Ertmann, M.; Knulst, A.C.; Knol, E. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin. Exp. Allergy 2004, 34, 583–590. [Google Scholar] [CrossRef]
- Nicolaou, N.; Poorafshar, M.; Murray, C.; Simpson, A.; Winell, H.; Kerry, G.; Härlin, A.; Woodcock, A.; Ahlstedt, S.; Custovic, A. Allergy or tolerance in children sensitized to peanut: Prevalence and differentiation using component-resolved diagnostics. J. Allergy Clin. Immunol. 2010, 125, 191–197.e113. [Google Scholar] [CrossRef]
- Palmer, G.W.; Dibbern, D.A., Jr.; Burks, A.W.; Bannon, G.A.; Bock, S.A.; Porterfield, H.S.; McDermott, R.A.; Dreskin, S.C. Comparative potency of Ara h 1 and Ara h 2 in immunochemical and functional assays of allergenicity. Clin. Immunol. 2005, 115, 302–312. [Google Scholar] [CrossRef]
- Buhler, S.; Tedeschi, T.; Faccini, A.; Garino, C.; Arlorio, M.; Dossena, A.; Sforza, S. Isolation and full characterisation of a potentially allergenic lipid transfer protein (LTP) in almond. Food Addit. Contam. Part A 2015, 32, 648–656. [Google Scholar]
- Liu, J.-J.; Sturrock, R.; Ekramoddoullah, A.K.M. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef]
- Jackson, L.S.; Al-Taher, F.M.; Moorman, M.; DeVRIES, J.W.; Tippett, R.; Swanson, K.M.J.; Fu, T.-J.; Salter, R.; Dunaif, G.; Estes, S.; et al. Cleaning and Other Control and Validation Strategies To Prevent Allergen Cross-Contact in Food-Processing Operations. J. Food Prot. 2008, 71, 445–458. [Google Scholar] [CrossRef]
- Wang, X.; Young, O.; Karl, D. Evaluation of Cleaning Procedures for Allergen Control in a Food Industry Environment. J. Food Sci. 2010, 75, T149–T155. [Google Scholar] [CrossRef]
- Pafundo, S.; Gulli, M.; Marmiroli, N. SYBR® GreenER™ Real-Time PCR to detect almond in traces in processed food. Food Chem. 2009, 116, 811–815. [Google Scholar] [CrossRef]
- Van Hengel, A.J. Food allergen detection methods and the challenge to protect food-allergic consumers. Anal. Bioanal. Chem. 2007, 389, 111–118. [Google Scholar] [CrossRef]
- Hoffmann-Sommergruber, K. Proteomics and its impact on food allergy diagnosis. EuPA Open Proteom. 2016, 12, 10–12. [Google Scholar] [CrossRef] [Green Version]
- Piras, C.; Roncada, P.; Rodrigues, P.; Bonizzi, L.; Soggiu, A. Proteomics in food: Quality, safety, microbes, and allergens. Proteomics 2015, 16, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.E.; Baumgartner, S.; Aldick, T.; Bessant, C.; Giosafatto, V.; Heick, J.; Mamone, G.; O’connor, G.; Poms, R.; Pop-ping, B. Current perspectives and recommendations for the development of mass spectrometry methods for the determination of allergens in foods. J. AOAC Int. 2011, 94, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Monaci, L.; De Angelis, E.; Montemurro, N.; Pilolli, R. Comprehensive overview and recent advances in proteomics MS based methods for food allergens analysis. TrAC Trends Anal. Chem. 2018, 106, 21–36. [Google Scholar] [CrossRef]
- Bignardi, C.; Elviri, L.; Penna, A.; Careri, M.; Mangia, A. Particle-packed column versus silica-based monolithic column for liquid chromatography–electrospray-linear ion trap-tandem mass spectrometry multiallergen trace analysis in foods. J. Chromatogr. A 2010, 1217, 7579–7585. [Google Scholar] [CrossRef] [PubMed]
- Lupinek, C.; Wollmann, E.; Baar, A.; Banerjee, S.; Breiteneder, H.; Broecker, B.M.; Bublin, M.; Curin, M.; Flicker, S.; Garmatiuk, T.; et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods 2014, 66, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.F.; Lack, G. Basophil activation test: Food challenge in a test tube or specialist research tool? Clin. Transl. Allergy 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ebo, D.; Bridts, C.H.; Hagendorens, M.; Aerts, N.E.; De Clerck, L.S.; Stevens, W.J. Basophil activation test by flow cytometry: Present and future applications in allergology. Cytom. Part B Clin. Cytom. 2008, 74, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Celik, A.; Hoang, J.A.; Schmidthaler, K.; So, D.; Yin, X.; Ditlof, C.M.; Ponce, M.; Upton, J.E.; Lee, J.; et al. Basophil activation test shows high accuracy in the diagnosis of peanut and tree nut allergy: The Markers of Nut Allergy Study. Allergy 2021, 76, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
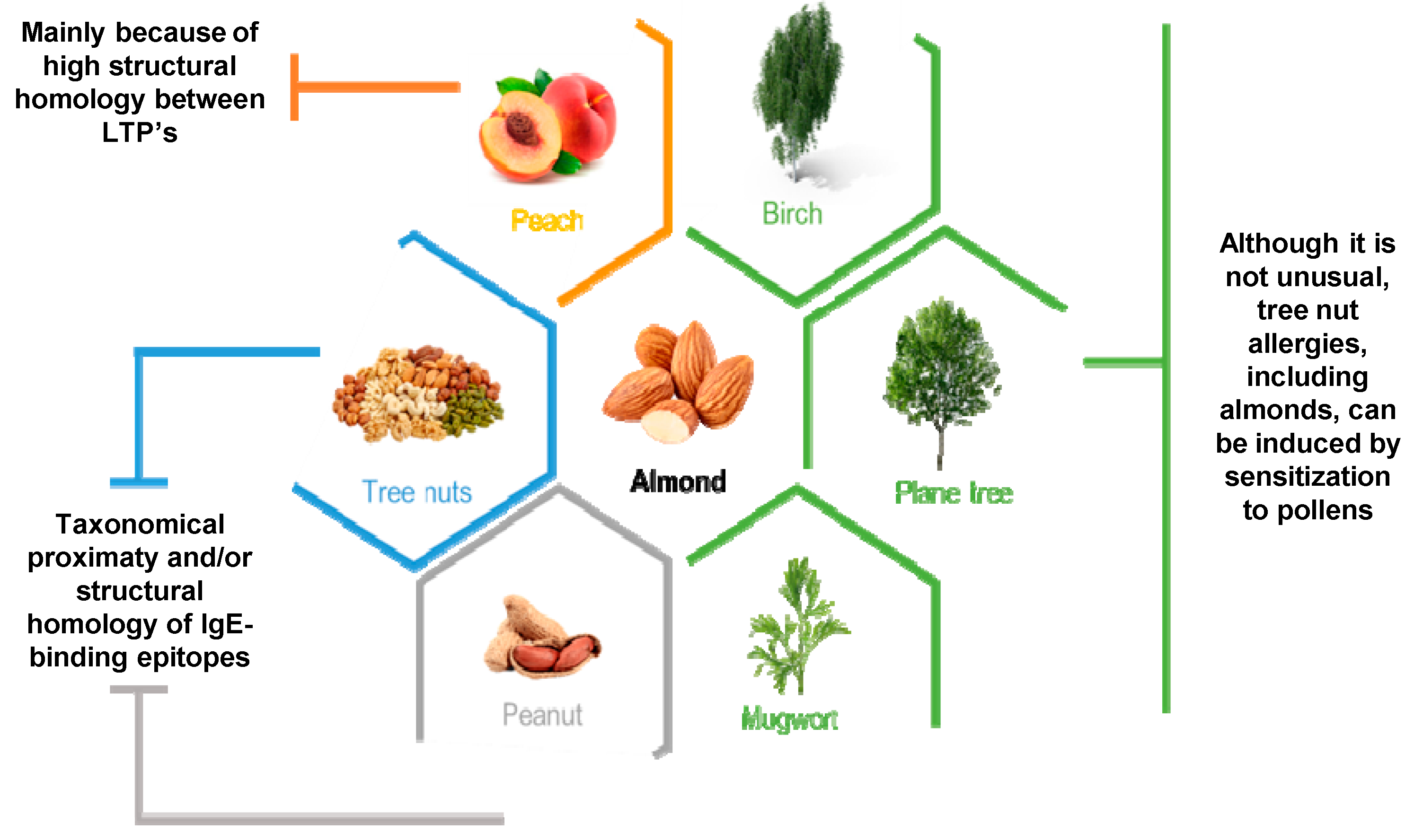
| Possible Cross-Reaction Source | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | Allergen | Mahleb | Peanut | Chestnut | Hazelnut | Walnut | Peach | Pollen | Profilin-Containing Plants | Maze |
| Almond | Pru du 3 | [65] | ||||||||
| Pru du 6 | [66] | [67] | ||||||||
| Pru du 1 | [59] | |||||||||
| Pru du 4 | [68] | |||||||||
| Pru du γ-conglutin | [69] | |||||||||
| Allergen | Biochemical Name | WHO-IUIS | Isoallergen and Variants | GenBank Nucleotide | UniProt | Biological Function | MW (kDa) | Processing | Clinical Relevance | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Pru du 3 | non-specific Lipid Transfer Protein 1 nsLTP1 | Yes (2009) | Pru du 3.0101 | FJ652103 | C0L0I5 | Non-specific lipid transfer protein (nslTP1) and plant defense proteins against pathogens | 9 | Very resistant to pH, thermal and enzyme treatments | Systemic and life-threatening symptoms; cross reactivity among Rosaceae fruit | [112] |
| Pru du 4 | Profilin | Yes (2006) | Pru du 4.0101 Pru du 4.0102 | AY081850 AY081852 | Q8GSL5 Q8GSL5 | Actin-binding protein for cellular function | 14 | Unstable during heat processing | Mild symptoms and mainly in oral cavity | [68] |
| Pru du 5 | 60S acidic ribosomal protein P2 | Yes (2007) | Pru du 5.0101 | DQ836316 | Q8H2B9 | Protein synthesis | 10 | Unknown | Unknown | [86] |
| Pru du 6 | Amandin, 11S globulin legumin-like protein | Yes (2010) | Pru du 6.0101 Pru du 6.0201 | GU059260 GU059261 | E3SH28 E3SH29 | Major storage protein | 360 | Stable to dry heat but can be denatured by boiling | Severe IgE allergic reactions | [82] |
| Pru du 8 | Antimicrobial seed storage protein | Yes (2018) | Pru du 8.0101 | MH922028 | A0A516F3L2 | Antimicrobial and seed storage function | 31 | Unknown | Unknown | [90] |
| Pru du 10 | Mandelonitrile lyase 2 | Yes (2019) | Pru du 10.0101 | AF412329.1 | Q945K2 | Highly efficient catalytical enzyme | 60 | Resistant to enzyme digestion | Unknown | [87,89] |
| Pru du γ-conglutin | Cupin superfamily | No | _______ | _______ | _______ | 7S vicilin storage protein | 45 for each subunit | Unknown | Unknown | [92] |
| Pru du 1 | PR-10 protein | No | _______ | _______ | _______ | Plant pathogenic and stress response | 17 | Wet heat processing reduces IgE reactivity | Unknown | [99] |
| Pru du 2 | PR-5/thaumatin-like protein | No | _______ | _______ | _______ | Pathogenic response | 23–27 | Resistant to protease, pH or heat treatment | Unknown | [113] |
| Pru 2S albumin | Prolamin super family | No | _______ | _______ | _______ | Seed storage protein | 12 | Stable to heat treatment | Unknown | [92] |
| Kit 1 | Assay Time | Assay Type | LOD (ppm) | LOQ (ppm) | Company |
|---|---|---|---|---|---|
| ELISA-based | |||||
| MonoTrace ELISA kit | 40 min | Monoclonal antibody-based ELISA | 0.15 | 1 | BioFront Technologies, Tallahassee, FL, USA |
| SENSISpec ELISA almond | 75 min | Sandwich enzyme immunoassay | 0.2 | 0.4 | Eurofins Technologies, Budapest, Hungary |
| RIDASCREEN FAST Mandel/Almond | 50 min | Polyclonal antibody specifically for almond protein detection, sandwich ELISA | 0.1 | 2.5 | R-Biopharm AG, Madrid, Spain |
| AgraQuant® Plus Almond | 30 min | Sandwich enzyme-linked immunosorbent assay | 0.5 | 1 | Romer Labs®, Getzersdorf, Austria |
| LFD-based | |||||
| AgraStrip® Almond | 11 min | Lateral flow device | 2 | __________ | Romer Labs®, Getzersdorf, Austria |
| Reveal 3-D Almond Test | 10 min | Lateral flow device | 5 | __________ | Neogen Corp., Lansing, MI, USA |
| Lateral Flow Almond incl. Hook Line 2 | 10 min | Lateral flow device | 1 | __________ | R-Biopharm AG, Madrid, Spain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezerra, M.; Ribeiro, M.; Igrejas, G. An Updated Overview of Almond Allergens. Nutrients 2021, 13, 2578. https://doi.org/10.3390/nu13082578
Bezerra M, Ribeiro M, Igrejas G. An Updated Overview of Almond Allergens. Nutrients. 2021; 13(8):2578. https://doi.org/10.3390/nu13082578
Chicago/Turabian StyleBezerra, Mário, Miguel Ribeiro, and Gilberto Igrejas. 2021. "An Updated Overview of Almond Allergens" Nutrients 13, no. 8: 2578. https://doi.org/10.3390/nu13082578
APA StyleBezerra, M., Ribeiro, M., & Igrejas, G. (2021). An Updated Overview of Almond Allergens. Nutrients, 13(8), 2578. https://doi.org/10.3390/nu13082578







