Association of Prenatal Sugar Consumption with Newborn Brain Tissue Organization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Dietary Intake of Mothers
2.4. MRI Scanning of Infants
2.4.1. MRI Scanning Procedures
2.4.2. MRI Data Processing
2.5. Statistical Analysis
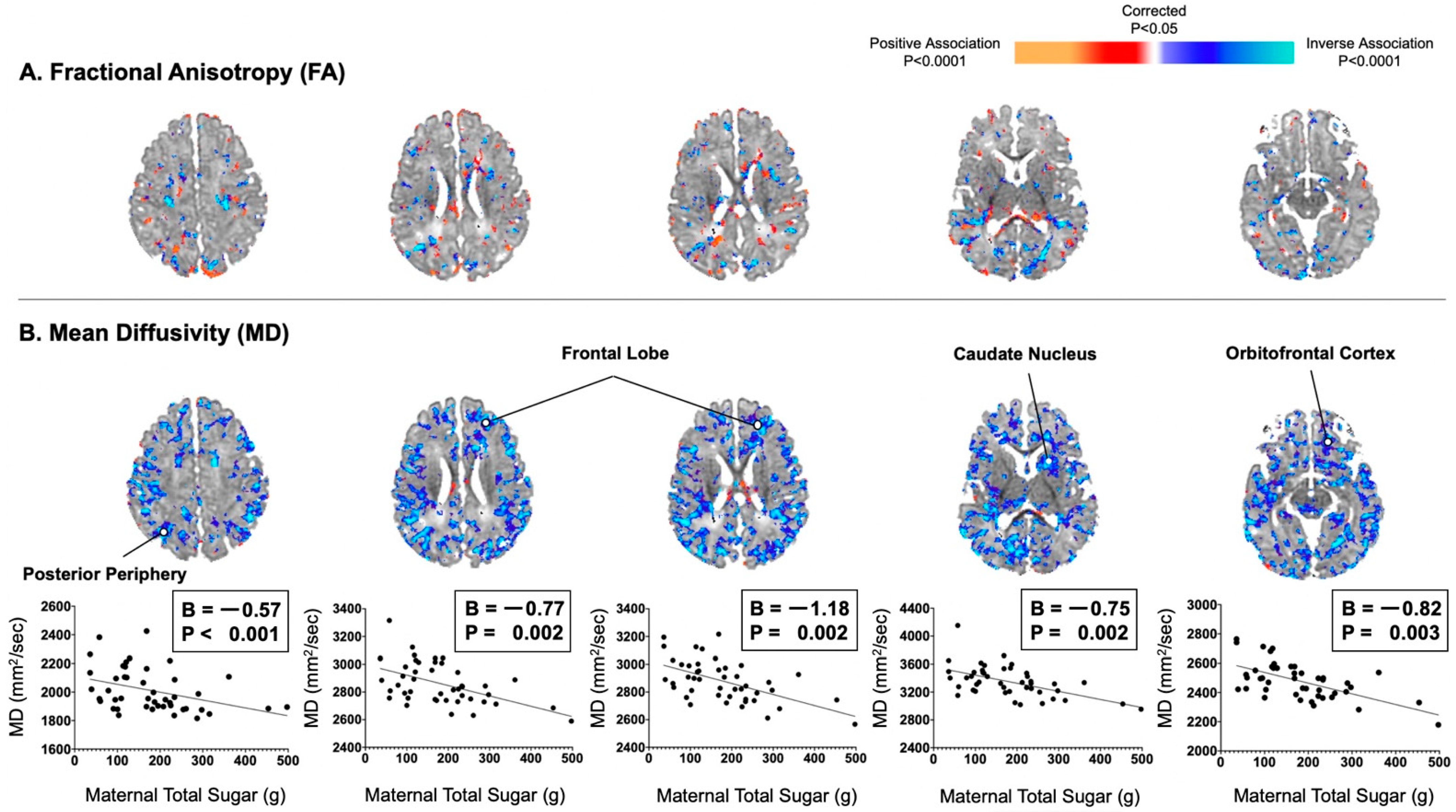
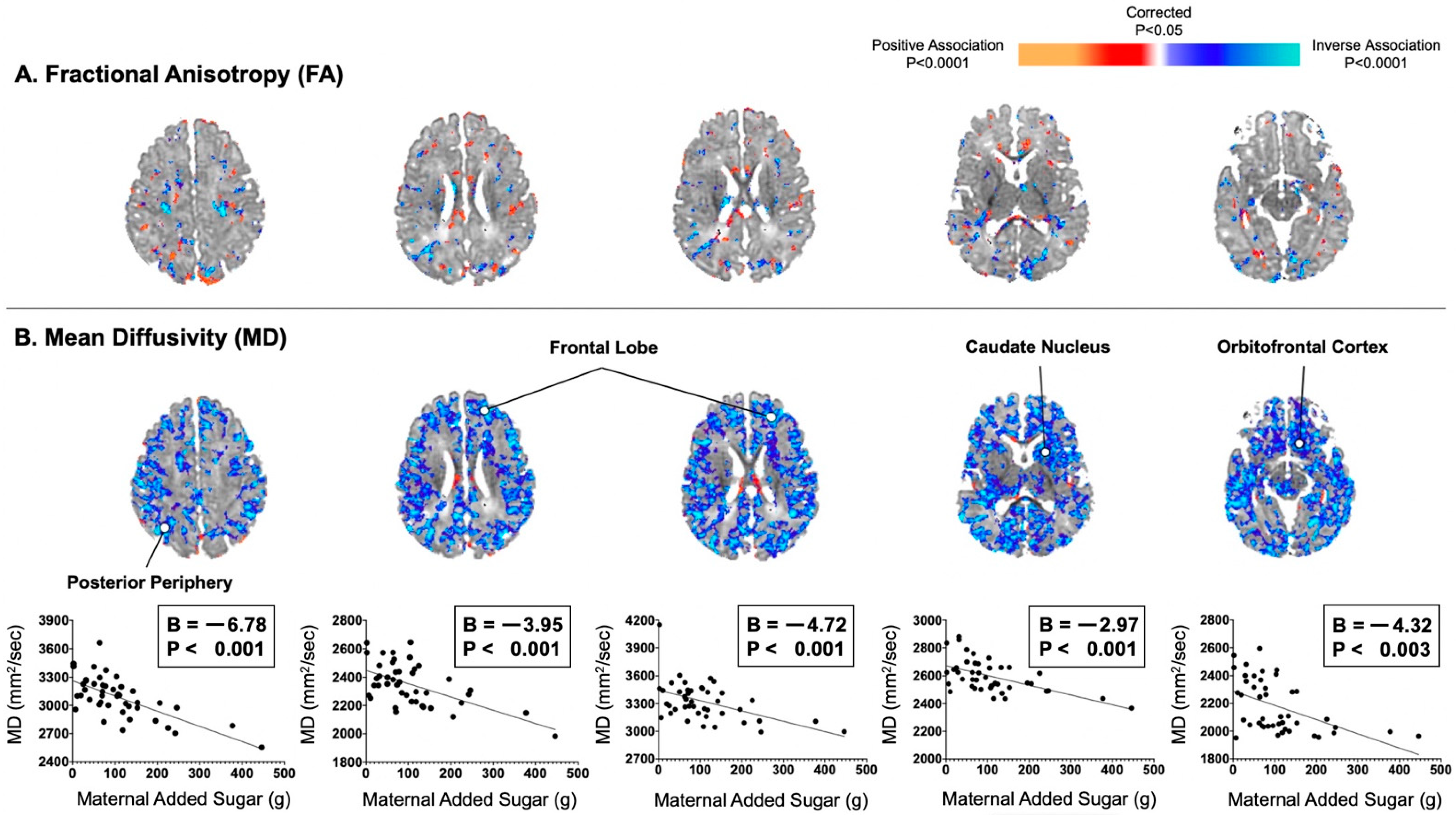
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.C.; Bleich, S.N.; Gortmaker, S.L. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics 2008, 121, e1604–e1614. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Calorie-sweetened beverages and fructose: What have we learned 10 years later. Pediatr. Obes. 2013, 8, 242–248. [Google Scholar] [CrossRef] [PubMed]
- An, R. Beverage Consumption in Relation to Discretionary Food Intake and Diet Quality among US Adults, 2003 to 2012. J. Acad. Nutr. Diet. 2016, 116, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Cragg, M.; deFonseka, J.; Hite, A.; Rosenberg, M.; Zhou, B. Statistical review of US macronutrient consumption data, 1965–2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition 2015, 31, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Steffen, L.M.; Zhou, X.; Harnack, L.; Luepker, R.V. Consistency between increasing trends in added-sugar intake and body mass index among adults: The Minnesota Heart Survey, 1980–1982 to 2007–2009. Am. J. Public Health 2013, 103, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Rawana, S.; Clark, K.; Zhong, S.; Buison, A.; Chackunkal, S.; Jen, K.L. Low dose fructose ingestion during gestation and lactation affects carbohydrate metabolism in rat dams and their offspring. J. Nutr. 1993, 123, 2158–2165. [Google Scholar] [CrossRef]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef]
- Jen, K.L.; Rochon, C.; Zhong, S.B.; Whitcomb, L. Fructose and sucrose feeding during pregnancy and lactation in rats changes maternal and pup fuel metabolism. J. Nutr. 1991, 121, 1999–2005. [Google Scholar] [CrossRef] [Green Version]
- Micha, R.; Penalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [Green Version]
- Ghusain-Choueiri, A.A.; Rath, E.A. Effect of carbohydrate source on lipid metabolism in lactating mice and on pup development. Br. J. Nutr. 1995, 74, 821–831. [Google Scholar]
- Swithers, S.E.; Laboy, A.F.; Clark, K.; Cooper, S.; Davidson, T.L. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav. Brain Res. 2012, 233, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, A.; Hu, F.B. Effects of carbohydrates on satiety: Differences between liquid and solid food. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; Konanur, V.R.; Taing, L.; Usui, R.; Kayser, B.D.; Goran, M.I.; Kanoski, S.E. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus 2015, 25, 227–239. [Google Scholar] [CrossRef]
- Noble, E.E.; Hsu, T.M.; Liang, J.; Kanoski, S.E. Early-life sugar consumption has long-term negative effects on memory function in male rats. Nutr. Neurosci. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.W.; Rifas-Shiman, S.L.; Young, J.; Oken, E. Associations of Prenatal and Child Sugar Intake with Child Cognition. Am. J. Prev. Med. 2018, 54, 727–735. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal development of brain circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef] [Green Version]
- Berger, P.K.; Fields, D.A.; Demerath, E.W.; Fujiwara, H.; Goran, M.I. High-fructose corn syrup-sweetened beverage intake increases 5-hour breast milk fructose concentrations in lactating women. J. Nutr. 2018, 10, 669. [Google Scholar] [CrossRef] [Green Version]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Rios, C.; Pickering, T.A.; Fields, D.A.; Bode, L.; Peterson, B.S.; Goran, M.I. Associations of maternal fructose and sugar-sweetened beverage and juice intake during lactation with infant neurodevelopmental outcomes at 24 months. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Goran, M.I.; Martin, A.A.; Alderete, T.L.; Fujiwara, H.; Fields, D.A. Fructose in Breast Milk Is Positively Associated with Infant Body Composition at 6 Months of Age. Nutrients 2017, 9, 146. [Google Scholar] [CrossRef] [Green Version]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human milk oligosaccharide 2′-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS ONE 2020, 15, e0228323. [Google Scholar] [CrossRef]
- Bukhari, S.H.F.; Clark, O.E.; Williamson, L.L. Maternal high fructose diet and neonatal immune challenge alter offspring anxiety-like behavior and inflammation across the lifespan. Life Sci. 2018, 197, 114–121. [Google Scholar] [CrossRef]
- Sanguesa, G.; Cascales, M.; Grinan, C.; Sanchez, R.M.; Roglans, N.; Pallas, M.; Laguna, J.C.; Alegret, M. Impairment of Novel Object Recognition Memory and Brain Insulin Signaling in Fructose-but Not Glucose-Drinking Female Rats. Mol. Neurobiol. 2018, 55, 6984–6999. [Google Scholar] [CrossRef]
- Agrawal, R.; Noble, E.; Vergnes, L.; Ying, Z.; Reue, K.; Gomez-Pinilla, F. Dietary fructose aggravates the pathobiology of traumatic brain injury by influencing energy homeostasis and plasticity. J. Cereb. Blood Flow Metab. 2016, 36, 941–953. [Google Scholar] [CrossRef]
- Guthrie, J.F.; Morton, J.F. Food sources of added sweeteners in the diets of Americans. J. Am. Diet Assoc. 2000, 100, 43–51. [Google Scholar] [CrossRef]
- Ervin, R.B.; Kit, B.K.; Carroll, M.D.; Ogden, C.L. Consumption of Added Sugar among U.S. Children and Adolescents, 2005–2008; NCHS Data Briefs: Atlanta, GA, USA, 2012; pp. 1–8. [Google Scholar]
- Spann, M.N.; Monk, C.; Scheinost, D.; Peterson, B.S. Maternal Immune Activation During the Third Trimester Is Associated with Neonatal Functional Connectivity of the Salience Network and Fetal to Toddler Behavior. J. Neurosci. 2018, 38, 2877–2886. [Google Scholar] [CrossRef] [PubMed]
- Monk, C.; Georgieff, M.K.; Xu, D.; Hao, X.; Bansal, R.; Gustafsson, H.; Spicer, J.; Peterson, B.S. Maternal prenatal iron status and tissue organization in the neonatal brain. Pediatr. Res. 2016, 79, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The Automated Self-Administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet 2012, 112, 1134–1137. [Google Scholar] [CrossRef] [Green Version]
- Theytaz, F.; de Giorgi, S.; Hodson, L.; Stefanoni, N.; Rey, V.; Schneiter, P.; Giusti, V.; Tappy, L. Metabolic fate of fructose ingested with and without glucose in a mixed meal. Nutrients 2014, 6, 2632–2649. [Google Scholar] [CrossRef] [Green Version]
- Kupis, J.; Johnson, S.; Hallihan, G.; Olstad, D.L. Assessing the Usability of the Automated Self-Administered Dietary Assessment Tool (ASA24) among Low-Income Adults. Nutrients 2019, 11, 132. [Google Scholar] [CrossRef] [Green Version]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [Green Version]
- Provenzale, J.M.; Liang, L.; DeLong, D.; White, L.E. Diffusion tensor imaging assessment of brain white matter maturation during the first postnatal year. AJR Am. J. Roentgenol. 2007, 189, 476–486. [Google Scholar] [CrossRef] [Green Version]
- Lebel, C.; Walker, L.; Leemans, A.; Phillips, L.; Beaulieu, C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 2008, 40, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Woolrich, M.W.; Jbabdi, S.; Patenaude, B.; Chappell, M.; Makni, S.; Behrens, T.; Beckmann, C.; Jenkinson, M.; Smith, S.M. Bayesian analysis of neuroimaging data in FSL. Neuroimage 2009, 45, S173–S186. [Google Scholar] [CrossRef]
- Alexander, D.C.; Barker, G.J. Optimal imaging parameters for fiber-orientation estimation in diffusion MRI. Neuroimage 2005, 27, 357–367. [Google Scholar] [CrossRef]
- Christensen, G.E.; Rabbitt, R.D.; Miller, M.I. 3D brain mapping using a deformable neuroanatomy. Phys. Med. Biol. 1994, 39, 609–618. [Google Scholar] [CrossRef]
- Koay, C.G.; Chang, L.C.; Carew, J.D.; Pierpaoli, C.; Basser, P.J. A unifying theoretical and algorithmic framework for least squares methods of estimation in diffusion tensor imaging. J. Magn. Reson. 2006, 182, 115–125. [Google Scholar] [CrossRef]
- Engle, W.A.; American Academy of Pediatrics Committee on Fetus and Newborn. Age terminology during the perinatal period. Pediatrics 2004, 114, 1362–1364. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Hadi, A.S. Regression Analysis by Example, 5th ed.; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Sotardi, S.; Gollub, R.L.; Bates, S.V.; Weiss, R.; Murphy, S.N.; Grant, P.E.; Ou, Y. Voxelwise and Regional Brain Apparent Diffusion Coefficient Changes on MRI from Birth to 6 Years of Age. Radiology 2021, 298, 415–424. [Google Scholar] [CrossRef]
- Schneider, J.; Fischer Fumeaux, C.J.; Duerden, E.G.; Guo, T.; Foong, J.; Graz, M.B.; Hagmann, P.; Chakravarty, M.M.; Huppi, P.S.; Beauport, L.; et al. Nutrient Intake in the First Two Weeks of Life and Brain Growth in Preterm Neonates. Pediatrics 2018, 141. [Google Scholar] [CrossRef] [Green Version]
- Altman, D.G.; Royston, P. The cost of dichotomising continuous variables. BMJ 2006, 332, 1080. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Yekutieli, D. False Discovery Rate: Adjusted Multiple Confidence Intervals for Selected Parameters. J. Am. Stat. Assoc. 2005, 100, 71–81. [Google Scholar] [CrossRef]
- Chong, C.P.; Shahar, S.; Haron, H.; Din, N.C. Habitual sugar intake and cognitive impairment among multi-ethnic Malaysian older adults. Clin. Interv. Aging 2019, 14, 1331–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuppel, A.; Shipley, M.J.; Llewellyn, C.H.; Brunner, E.J. Sugar intake from sweet food and beverages, common mental disorder and depression: Prospective findings from the Whitehall II study. Sci. Rep. 2017, 7, 6287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lien, L.; Lien, N.; Heyerdahl, S.; Thoresen, M.; Bjertness, E. Consumption of soft drinks and hyperactivity, mental distress, and conduct problems among adolescents in Oslo, Norway. Am. J. Public Health 2006, 96, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- McKinstry, R.C.; Mathur, A.; Miller, J.H.; Ozcan, A.; Snyder, A.Z.; Schefft, G.L.; Almli, C.R.; Shiran, S.I.; Conturo, T.E.; Neil, J.J. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb. Cortex 2002, 12, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.D.; Van Essen, D.C.; Inder, T.E.; Rees, S.; Bretthorst, G.L.; Neil, J.J. Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J. Neurosci. 2007, 27, 12506–12515. [Google Scholar] [CrossRef] [Green Version]
- Matsuzawa, J.; Matsui, M.; Konishi, T.; Noguchi, K.; Gur, R.C.; Bilker, W.; Miyawaki, T. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cereb. Cortex 2001, 11, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Jespersen, S.N.; Kroenke, C.D.; Ostergaard, L.; Ackerman, J.J.; Yablonskiy, D.A. Modeling dendrite density from magnetic resonance diffusion measurements. Neuroimage 2007, 34, 1473–1486. [Google Scholar] [CrossRef]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gomez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef] [Green Version]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Diet-Induced Cognitive Deficits: The Role of Fat and Sugar, Potential Mechanisms and Nutritional Interventions. Nutrients 2015, 7, 6719–6738. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, R.M.; Sprunger, D.B.; Campeau, S.; Watkins, L.R.; Rudy, J.W.; Maier, S.F. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J. Neuroimmunol. 2004, 155, 119–126. [Google Scholar] [CrossRef]
- Kwon, M.; Fernandez, J.R.; Zegarek, G.F.; Lo, S.B.; Firestein, B.L. BDNF-promoted increases in proximal dendrites occur via CREB-dependent transcriptional regulation of cypin. J. Neurosci. 2011, 31, 9735–9745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagasia, R.; Steib, K.; Englberger, E.; Herold, S.; Faus-Kessler, T.; Saxe, M.; Gage, F.H.; Song, H.; Lie, D.C. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J. Neurosci. 2009, 29, 7966–7977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednarek, E.; Caroni, P. beta-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron 2011, 69, 1132–1146. [Google Scholar] [CrossRef] [Green Version]
- Porro, F.; Rosato-Siri, M.; Leone, E.; Costessi, L.; Iaconcig, A.; Tongiorgi, E.; Muro, A.F. beta-adducin (Add2) KO mice show synaptic plasticity, motor coordination and behavioral deficits accompanied by changes in the expression and phosphorylation levels of the alpha- and gamma-adducin subunits. Genes Brain Behav. 2010, 9, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.K.; Loken, E.; Mitchell, D.C.; Smiciklas-Wright, H.; Birch, L.L. Understanding reporting bias in the dietary recall data of 11-year-old girls. Obesity 2006, 14, 1073–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, A.P.; Clegg, D.J. JAMA Patient Page. Dietary Guidelines for Americans—Eat Less Sugar. JAMA 2016, 315, 1196. [Google Scholar] [CrossRef]
| V | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Mothers | ||||
| Age at delivery (years) | 18.2 | 1.37 | 14.0 | 20.0 |
| Prepregnancy BMI (kg/m2) | 25.2 | 6.37 | 14.4 | 41.2 |
| Vaginal delivery (%) | 83 | |||
| Hispanic Ethnicity (%) | 95 | |||
| Total energy per day, second trimester (kcals) | 2549 | 1151 | 851 | 6695 |
| Added sugar per day, second trimester (g) | 106 | 91.5 | 2.03 | 446 |
| Total sugar per day, second trimester (g) | 170 | 102 | 36.0 | 498 |
| Infants | ||||
| Male (%) | 61 | |||
| Postmenstrual age (weeks) | 42.6 | 1.7 | 38.7 | 47.0 |
| Chronological age (days) | 23.9 | 13.2 | 4.00 | 94.0 |
| Birth weight (g) | 3204 | 456 | 2466 | 4380 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, P.K.; Monk, C.; Bansal, R.; Sawardekar, S.; Goran, M.I.; Peterson, B.S. Association of Prenatal Sugar Consumption with Newborn Brain Tissue Organization. Nutrients 2021, 13, 2435. https://doi.org/10.3390/nu13072435
Berger PK, Monk C, Bansal R, Sawardekar S, Goran MI, Peterson BS. Association of Prenatal Sugar Consumption with Newborn Brain Tissue Organization. Nutrients. 2021; 13(7):2435. https://doi.org/10.3390/nu13072435
Chicago/Turabian StyleBerger, Paige K., Catherine Monk, Ravi Bansal, Siddhant Sawardekar, Michael I. Goran, and Bradley S. Peterson. 2021. "Association of Prenatal Sugar Consumption with Newborn Brain Tissue Organization" Nutrients 13, no. 7: 2435. https://doi.org/10.3390/nu13072435
APA StyleBerger, P. K., Monk, C., Bansal, R., Sawardekar, S., Goran, M. I., & Peterson, B. S. (2021). Association of Prenatal Sugar Consumption with Newborn Brain Tissue Organization. Nutrients, 13(7), 2435. https://doi.org/10.3390/nu13072435






