Vegan Diet and the Gut Microbiota Composition in Healthy Adults
Abstract
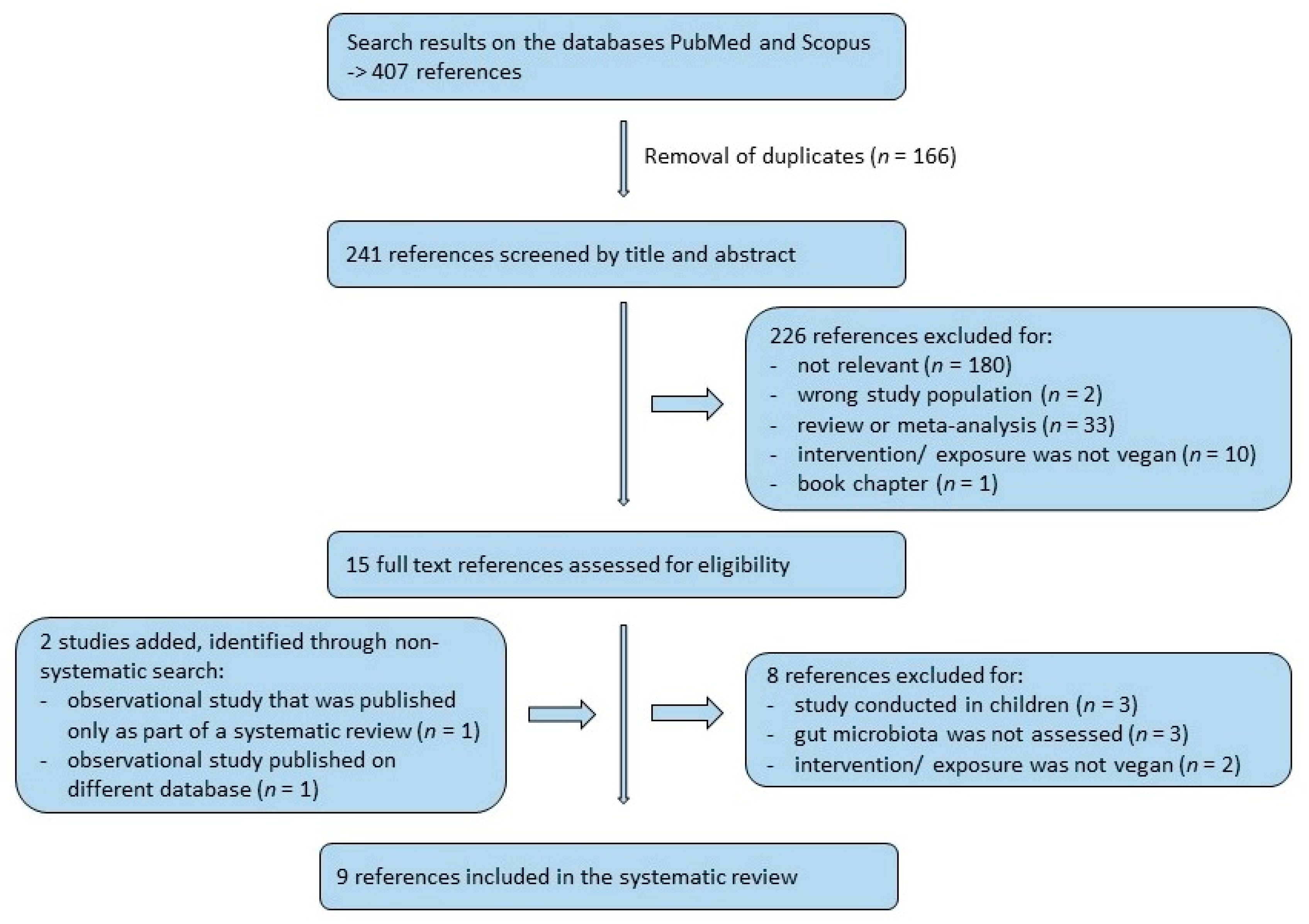
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Analysis of the Microbiota
3.2. Microbiota Composition
3.2.1. Prevotella and Bacteroides
3.2.2. Alpha Diversity
3.3. Quality of the Studies
4. Discussion
4.1. Strengths and Limitations
4.2. Directions for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, A.; Jacobi, M.; Rusch, K.; Andreas, S. Association of dietary type with fecal microbiota and short chain fatty acids in vegans and omnivores. J. Int. Soc. Microbiota 2016, 1, 1–9. Available online: https://www.researchgate.net/publication/302579749_Association_of_dietary_type_with_fecal_microbiota_and_short_chain_fatty_acids_in_vegans_and_omnivores (accessed on 1 May 2021).
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional status and the influence of the vegan diet on the gut microbiota and human health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef] [Green Version]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Orlich, M.J.; Singh, P.; Sabaté, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian dietary patterns and mortality in adventist health study 2. JAMA Intern. Med. 2013, 173, 1230–1238. [Google Scholar] [CrossRef]
- Klimenko, N.S.; Tyakht, A.V.; Popenko, A.S.; Vasiliev, A.S.; Altukhov, I.A.; Ischenko, D.S.; Shashkova, T.; Efimova, D.A.; Nikogosov, D.; Osipenko, D.A.; et al. Microbiome responses to an uncontrolled Short-Term diet intervention in the frame of the citizen science project. Nutrients 2018, 10, 576. [Google Scholar] [CrossRef] [Green Version]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Glick-Bauer, M.; Yeh, M.-C. The health advantage of a vegan diet: Exploring the gut microbiota connection. Nutrients 2014, 6, 4822–4838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: Profound implications for diet and disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, J.; Lange, B.J.; Frick, J.-S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; A Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2011, 66, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term low-calorie low-protein vegan diet and endurance exercise are associated with low cardiometabolic risk. Rejuvenation Res. 2007, 10, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davey, G.K.; A Spencer, E.; Appleby, P.N.; E Allen, N.; Knox, K.H.; Key, T.J. EPIC–Oxford:lifestyle characteristics and nutrient intakes in a cohort of 33,883 meat-eaters and 31,546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Benatar, J.R.; Stewart, R.A.H. Cardiometabolic risk factors in vegans; A meta-analysis of observational studies. PLoS ONE 2018, 13, e0209086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toohey, M.L.; Harris, M.A.; Williams, D.; Foster, G.; Schmidt, W.D.; Melby, C.L. Cardiovascular disease risk factors are lower in african-american vegans compared to Lacto-Ovo-vegetarians. J. Am. Coll. Nutr. 1998, 17, 425–434. [Google Scholar] [CrossRef]
- Medawar, E.; Huhn, S.; Villringer, A.; Witte, A.V. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Haddad, E.H.; Berk, L.S.; Kettering, J.D.; Hubbard, R.W.; Peters, W.R. Dietary intake and biochemical, hematologic and immune status of vegans compared with nonvegetarians. Am. J. Clin. Nutr. 1999, 70, 586s–593s. [Google Scholar] [CrossRef] [Green Version]
- Larsson, C.L.; Johansson, G.K. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am. J. Clin. Nutr. 2002, 76, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Federici, E.; Prete, R.; Lazzi, C.; Pellegrini, N.; Moretti, M.; Corsetti, A.; Cenci, G. Bacterial composition, genotoxicity and cytotoxicity of fecal samples from individuals consuming omnivorous or vegetarian diets. Front. Microbiol. 2017, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Hwang, S.-S.; Park, E.-J.; Bae, J.-W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ. Microbiol. Rep. 2013, 5, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G. ESH working group on CV risk in low resource settings panethnic differences in blood pressure in europe: A systematic review and Meta-Analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, M.; Ferrocino, I.; Calabrese, F.M.; De Filippis, F.; Cavallo, N.; Siragusa, S.; Rampelli, S.; Di Cagno, R.; Rantsiou, K.; Vannini, L.; et al. Diet influences the functions of the human intestinal microbiome. Sci. Rep. 2020, 10, 4247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Di Cagno, R.; De Angelis, M.; Turroni, S.; Vannini, L.; Bancalari, E.; Rantsiou, K.; Cardinali, G.; Neviani, E.; Cocolin, L. Fecal microbiota in healthy subjects following omnivore, vegetarian and vegan diets: Culturable populations and rRNA DGGE profiling. PLoS ONE 2015, 10, e0128669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LoSasso, C.; Eckert, E.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the influence of vegan, vegetarian and omnivore oriented westernized dietary styles on human gut microbiota: A cross sectional study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; A Smith, S.; Shah, R.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Trefflich, I.; Jabakhanji, A.; Menzel, J.; Blaut, M.; Michalsen, A.; Lampen, A.; Abraham, K.; Weikert, C. Is a vegan or a vegetarian diet associated with the microbiota composition in the gut? Results of a new cross-sectional study and systematic review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1–15. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Li, X.H.; Chen, W.N. Similarities and differences in gut microbiome composition correlate with dietary patterns of Indian and Chinese adults. AMB Express 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- de Moraes, A.C.; Fernandes, G.D.R.; Da Silva, I.T.; Pititto, B.D.A.; Gomes, E.P.; Pereira, A.D.C.; Ferreira, S.R.G. Enterotype may drive the dietary-associated cardiometabolic risk factors. Front. Cell. Infect. Microbiol. 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, T.; Beasley, D.; Hedenec, P.; Xiao, Z.; Zhang, S.; Li, J.; Lin, Q.; Li, X. Diet diversity is associated with beta but not alpha diversity of pika gut microbiota. Front. Microbiol. 2016, 7, 1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A David, L.; Materna, A.C.; Friedman, J.; I Campos-Baptista, M.; Blackburn, M.C.; Perrotta, A.; E Erdman, S.; Alm, E.J. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014, 15, R89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matijašić, B.B.; Obermajer, T.; Lipoglavšek, L.; Grabnar, I.; Avguštin, G.; Rogelj, I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur. J. Nutr. 2013, 53, 1051–1064. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nutrients 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.E.; Palm, N.W. Functional classification of the gut microbiota: The key to cracking the microbiota composition code. BioEssays 2017, 39, 1700032. [Google Scholar] [CrossRef]
- Langille, M.G.I. Exploring linkages between taxonomic and functional profiles of the human microbiome. mSystems 2018, 3, e00163-17. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Donoso, C.; Martínez-González, M.Á.; Martínez, J.A.; Gea, A.; Sanz-Serrano, J.; Perez-Cueto, F.J.A.; Bes-Rastrollo, M. A provegetarian food pattern emphasizing preference for healthy plant-derived foods reduces the risk of overweight/obesity in the SUN Cohort. Nutrients 2019, 11, 1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mithril, C.; Dragsted, L.O.; Meyer, C.; Blauert, E.; Holt, M.K.; Astrup, A. Guidelines for the new nordic diet. Public Health Nutr. 2012, 15, 1941–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subhan, F.B.; Chan, C.B. Review of dietary practices of the 21st century: Facts and fallacies. Can. J. Diabetes 2016, 40, 348–354. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tuomilehto, J. Lifestyle diabetes prevention. In Encyclopedia of Endocrine Diseases; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 1, pp. 148–159. [Google Scholar]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [Green Version]
| PICOS Format | Description |
|---|---|
| Population | Presumably healthy subjects. Adult humans aged 18 years or older |
| Intervention or exposure | Vegan diet, defined as a plant-based diet omitting all animal products |
| Comparisons | Control diet (omnivore, Western-type, non-vegetarians/vegans), defined as a diet consuming any type and amount of animal products such as dairy, meat, meat products, eggs, and fish |
| Outcome | Gut microbiota composition through faecal samples |
| Study design | Cross-sectional, prospective cohort studies, randomised-controlled trials of either parallel or crossover design |
| Author, Country, Year | Study Design | Vegan Sample Sample Size (n) % Female Age (Years) | Minimum Duration of Vegan Diet (Months) | Omnivore Control Group Sample Size (n) % Female Age (Years) | Method of Data Collection and Gut Microbiota Assessment | Composition of Gut Microbiota | |
|---|---|---|---|---|---|---|---|
| Significant Results | Non-Significant Results | ||||||
| De Angelis et al., Italy, 2020 | Cross-sectional ** | n = 10 (50%) 36 ± 7.0 § | >12 | n = 8 | -3 samples per person collected on the same day of three consecutive weeks and then pooled together -Shotgun sequencing of the total DNA | -Lachnospira associated with vegans -Ruminococcaceae was the most abundant family for omnivores | |
| De Filippis et al., Italy, 2015 | Cross-sectional * | n = 51 (55%) 37 ± 10 | >12 | n = 51 | -3 samples per person collected on the same day of three consecutive weeks, and then pooled together -16S rRNA gene sequences | -Bacteroidetes phylum more abundant in vegans -Firmicutes: Bacteroidetes ratio was higher in omnivores -Roseburia, Lachnospira and Prevotella was lower in omnivores -L. Ruminococcus and Streptococcus correlated positively with omnivore diets and negatively with vegan diets | -alpha diversity -increased Prevotellaceae in vegans |
| Federici et al., Italy, 2017 | Cross-sectional | n = 10 (30%) 33 ± 7 | >12 | n = 7 (42%) 41 ± 9 | -3 samples per person, over 3 weeks; analysed individually, outcomes pooled -Bacterial counting | -Staphylococci and Corynebacteria was lower in vegans than omnivores | -lower levels of Bifidobacteria, Enterobacteria and mesophilic lactobacilli in vegans -Bacteroides–Prevotella levels being higher in vegans |
| Ferrocino et al., Italy, 2015 | Cross-sectional * | n = 51 (55%) 37 ± 10 | >12 | n = 51 | -Bacterial counting -16S rRNA Denaturing Gradient Gel Electrophoresis (rRNA-DGGE) -3 samples per person collected on the same day of three consecutive weeks, and then pooled together | -Bacteroides fragilis were higher in the omnivore group -Bacteroides and Prevotella load was higher in vegans compared to omnivores -Coliforms and Bifidobacteria lower in vegans compared to omnivores -main difference was seen when comparing the sites instead of the diet | |
| Losasso et al., Italy, 2018 | Cross-sectional | n = 26 (65%) 39.4 ± 11.1 | >12 | n = 43 (73.3%) 45.0 ± 13.9 | -1 sample per person but two independent total DNA extractions -16S rDNA gene | -higher counts of Bacteroidetes on vegan diet -lower alpha diversity in omnivores compared to vegans | -no difference in Firmicutes/Bacteroidetes ratio -no difference in Prevotellaceae abundance |
| Wu et al., USA, 2016 | Cross-sectional | n = 15 | >6 | n = 16 | -1 stool sample per person -16S rRNA gene sequences | -no taxa differed significantly in presence/abundance at genus level (after correction for multiple comparisons) -measures of diversity and evenness not sig. different between groups | |
| Zimmer et al., Germany, 2011 | Cross-sectional | n = 46 (39%) 46.5 ± 12.62 # | >4 weeks | n = 46 (39%) 46.5 ± 12.26 # | -1 stool sample per person -Bacterial counting | -lower Bacteroides, Bifidobacterium and Enterobacteriaceae in vegans | -lower Escherichia Coli -Significantly lower stool pH |
| Trefflich et al., Germany, 2019 | Cross-sectional | n = 36 (50%) 37.5 (32.5–44.0) | >12 | n = 36 (50%) 38.5 (32.0–46.0) | -1 stool sample per person -16S rRNA gene sequencing | -Lachnoclostridium and Dialister invisus were significantly higher in omnivores | -Bacteroides was higher in omnivores (21.7% vs. 17.4%) -Prevotella and Faecalibacterium was higher in vegans (10.8% vs. 7.1%) |
| Schwiertz et al., Germany, 2016 | Cross-sectional | n = 9 (77.8%) | ≥6 | n = 10 (50%) | -samples from 4 time points (day 1, 4, 7, and 14) were analysed -PCR amplification, microbial DNA sequencing, detection of number of bacteria by comparison of fluorescence with standard curve of appropriate reference organism | -higher Proteobacteria and lower Verrucomicrobiota in omnivores -lower lactic acid bacteria (Lactobacillus and Lactococcus) in vegans | -lower abundance of Actinobacteria in vegans |
| Study | Representativeness of the Sample (Max. 1 Star) | Sample Size (Max. 1 Star) | Ascertainment of the Exposure (Max. 2 Stars) | Comparability (Max. 2 Stars) | Assessment of the Outcome (Max. 2 Stars) | Statistical Test (Max. 1 Star) | Total Quality Score (Max. 9 Stars) |
|---|---|---|---|---|---|---|---|
| De Angelis et al. | / | / | / | * | ** | * | 4 |
| De Filippis et al. | / | * | ** | ** | ** | * | 8 |
| Federici et al. | / | / | ** | / | ** | * | 5 |
| Ferrocino et al. | / | * | / | * | ** | * | 5 |
| Losasso et al. | / | / | ** | ** | ** | * | 7 |
| Wu et al. | / | / | * | / | ** | * | 4 |
| Schwiertz et al. | / | / | / | * | ** | * | 5 |
| Trefflich et al. | * | / | * | ** | ** | / | 5 |
| Zimmer et al. | / | / | / | ** | ** | * | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losno, E.A.; Sieferle, K.; Perez-Cueto, F.J.A.; Ritz, C. Vegan Diet and the Gut Microbiota Composition in Healthy Adults. Nutrients 2021, 13, 2402. https://doi.org/10.3390/nu13072402
Losno EA, Sieferle K, Perez-Cueto FJA, Ritz C. Vegan Diet and the Gut Microbiota Composition in Healthy Adults. Nutrients. 2021; 13(7):2402. https://doi.org/10.3390/nu13072402
Chicago/Turabian StyleLosno, Emily A., Katharina Sieferle, Federico J. Armando Perez-Cueto, and Christian Ritz. 2021. "Vegan Diet and the Gut Microbiota Composition in Healthy Adults" Nutrients 13, no. 7: 2402. https://doi.org/10.3390/nu13072402
APA StyleLosno, E. A., Sieferle, K., Perez-Cueto, F. J. A., & Ritz, C. (2021). Vegan Diet and the Gut Microbiota Composition in Healthy Adults. Nutrients, 13(7), 2402. https://doi.org/10.3390/nu13072402







