The Development of Feeding and Eating Disorders after Bariatric Surgery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Eligibility Criteria
2.1.1. Types of Studies
2.1.2. Types of Participants
2.1.3. Types of Variables/Parameters Analyzed
2.2. Exclusion Criteria
2.3. Literature Revision
2.3.1. Search Strategy
2.3.2. Data Extraction
2.3.3. Data Validation
2.4. Statistical Analysis
3. Results
3.1. Search Flow
3.2. Quality of Evidence
3.3. Study Characteristics
Types of Evaluation
4. Discussion
Study Limitations and Methodologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchwald, H.; Oien, D.M. Metabolic/bariatric surgery worldwide 2011. Obes. Surg. 2013, 23, 427–436. [Google Scholar] [CrossRef] [PubMed]
- English, W.J.; DeMaria, E.J.; Hutter, M.M.; Kothari, S.N.; Mattar, S.G.; Brethauer, S.A.; Morton, J.M. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg. Obes. Relat. Dis. 2020, 16, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.; O’Brien, P.; Brennan, L. Measurement of disordered eating following bariatric surgery: A systematic review of the literature. Obes. Surg. 2014, 24, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Opozda, M.; Chur-Hansen, A.; Wittert, G. Changes in problematic and disordered eating after gastric bypass, adjustable gastric banding and vertical sleeve gastrectomy: A systematic review of pre-post studies. Obes. Rev. 2016, 17, 770–792. [Google Scholar] [CrossRef]
- Williams-Kerver, G.A.; Steffen, K.J.; Mitchell, J.E. Eating Pathology After Bariatric Surgery: An Updated Review of the Recent Literature. Curr. Psychiatry Rep. 2019, 21, 86. [Google Scholar] [CrossRef]
- Brode, C.S.; Mitchell, J.E. Problematic Eating Behaviors and Eating Disorders Associated with Bariatric Surgery. Psychiatr. Clin. N. Am. 2019, 42, 287–297. [Google Scholar] [CrossRef]
- Meany, G.; Conceição, E.; Mitchell, J.E. Binge eating, binge eating disorder and loss of control eating: Effects on weight outcomes after bariatric surgery. Eur. Eat. Disord. Rev. 2014, 22, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- University of Illinois at Chicago’s Library of the Health Sciences at Peoria. Evidence Based Medicine—What Is the PICO Model? Available online: https://researchguides.uic.edu/c.php?g=252338&p=3954402 (accessed on 11 September 2019).
- National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 10 May 2019).
- Cochrane Methods Screening and Diagnostic Tests. Handbook for DTA Reviews. Available online: http://methods.cochrane.org/sdt/handbook-dta-reviews (accessed on 10 May 2019).
- Wilson, E.B. Probable Inference, the Law of Succession, and Statistical Inference. J. Am. Stat. Assoc. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- Mack, I.; Ölschläger, S.; Sauer, H.; von Feilitzsch, M.; Weimer, K.; Junne, F.; Peeraully, R.; Enck, P.; Zipfel, S.; Teufel, M. Does Laparoscopic Sleeve Gastrectomy Improve Depression, Stress and Eating Behavior? A 4-Year Follow-up Study. Obes. Surg. 2016, 26, 2967–2973. [Google Scholar] [CrossRef]
- Rand, C.S.; Macgregor, A.M.; Stunkard, A.J. The night eating syndrome in the general population and among postoperative obesity surgery patients. Int. J. Eat. Disord. 1997, 22, 65–69. [Google Scholar] [CrossRef]
- Larsen, J.K.; van Ramshorst, B.; Geenen, R.; Brand, N.; Stroebe, W.; van Doornen, L.J. Binge eating and its relationship to outcome after laparoscopic adjustable gastric banding. Obes. Surg. 2004, 14, 1111–1117. [Google Scholar] [CrossRef]
- Scholtz, S.; Bidlake, L.; Morgan, J.; Fiennes, A.; El-Etar, A.; Lacey, J.H.; McCluskey, S. Long-term outcomes following laparoscopic adjustable gastric banding: Postoperative psychological sequelae predict outcome at 5-year follow-up. Obes. Surg. 2007, 17, 1220–1225. [Google Scholar] [CrossRef]
- Latner, J.D.; Wetzler, S.; Goodman, E.R.; Glinski, J. Gastric bypass in a low-income, inner-city population: Eating disturbances and weight loss. Obes. Res. 2004, 12, 956–961. [Google Scholar] [CrossRef]
- Colles, S.L.; Dixon, J.B.; O’Brien, P.E. Grazing and loss of control related to eating: Two high-risk factors following bariatric surgery. Obesity 2008, 16, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.K.; Betancourt, S.; Sullivan, S.P. Eating disturbances before and after vertical banded gastroplasty: A pilot study. Int. J. Eat. Disord. 1996, 19, 23–34. [Google Scholar] [CrossRef]
- Luiz, L.B.; Brito, C.L.D.S.; Debon, L.M.; Brandalise, L.N.; Azevedo, J.T.D.; Monbach, K.D.; Heberle, L.S.; Mottin, C.C. Variation of Binge Eating One Year after Roux-en-Y Gastric Bypass and Its Relationship with Excess Weight Loss. PLoS ONE 2016, 11, e0167577. [Google Scholar] [CrossRef] [PubMed]
- Kalarchian, M.A.; King, W.C.; Devlin, M.J.; Marcus, M.D.; Garcia, L.; Chen, J.Y.; Yanovski, S.Z.; Mitchell, J.E. Psychiatric Disorders and Weight Change in a Prospective Study of Bariatric Surgery Patients: A 3-Year Follow-Up. Psychosom. Med. 2016, 78, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, P.S.; Perez, A.; Boyd, F.; Rosemurgy, A. Eating pathology before and after bariatric surgery: A prospective study. Int. J. Eat. Disord. 1999, 25, 293–300. [Google Scholar] [CrossRef]
- De Zwaan, M.; Hilbert, A.; Swan-Kremeier, L.; Simonich, H.; Lancaster, K.; Howell, L.M.; Monson, T.; Crosby, R.D.; Mitchell, J.E. Comprehensive interview assessment of eating behavior 18–35 months after gastric bypass surgery for morbid obesity. Surg. Obes. Relat. Dis. 2010, 6, 79–85. [Google Scholar] [CrossRef]
- Burgmer, R.; Grigutsch, K.; Zipfel, S.; Wolf, A.M.; de Zwaan, M.; Husemann, B.; Albus, C.; Senf, W.; Herpertz, S. The influence of eating behavior and eating pathology on weight loss after gastric restriction operations. Obes. Surg. 2005, 15, 684–691. [Google Scholar] [CrossRef]
- Smith, K.E.; Orcutt, M.; Steffen, K.J.; Crosby, R.D.; Cao, L.; Garcia, L.; Mitchell, J.E. Loss of Control Eating and Binge Eating in the 7 Years Following Bariatric Surgery. Obes. Surg. 2019, 29, 1773–1780. [Google Scholar] [CrossRef]
- Kalarchian, M.A.; King, W.C.; Devlin, M.J.; Hinerman, A.; Marcus, M.D.; Yanovski, S.Z.; Mitchell, J.E. Mental disorders and weight change in a prospective study of bariatric surgery patients: 7 years of follow-up. Surg. Obes. Relat. Dis. 2019, 15, 739–748. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Cooper, Z.; O’Connor, M.E. Eating Disorder Examination. In Cognitive Behavior Therapy and Eating Disorders, 16.0D Edition; Fairburn, C.G., Ed.; Guilford Press: New York, USA, 2008; pp. 265–308. [Google Scholar]
- Berg, K.C.; Peterson, C.B.; Frazier, P.; Crow, S.J. Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: A systematic review of the literature. Int. J. Eat. Disord. 2012, 45, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Cappelleri, J.C.; Bushmakin, A.G.; Gerber, R.A.; Leidy, N.K.; Sexton, C.C.; Lowe, M.R.; Karlsson, J. Psychometric analysis of the Three-Factor Eating Questionnaire-R21: Results from a large diverse sample of obese and non-obese participants. Int. J. Obes. 2009, 33, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Bryant, E.J.; Rehman, J.; Pepper, L.B.; Walters, E.R. Obesity and Eating Disturbance: The Role of TFEQ Restraint and Disinhibition. Curr. Obes. Rep. 2019, 8, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Ware, J.E.; Kosinski, M.; Gandek, B. SF-36 Health Survey: Manual and Interpretation Guide; Quality Metric: Lincoln, RI, USA, 2005. [Google Scholar]
- Ware, J.E.; Kosinski, M.; Keller, S.D. Physical & Mental Health Summary Scales: A User’s Manual; The Health Institute, New England Medical Center: Boston, MA, USA, 1994. [Google Scholar]
- Grupski, A.E.; Hood, M.M.; Hall, B.J.; Azarbad, L.; Fitzpatrick, S.L.; Corsica, J.A. Examining the Binge Eating Scale in screening for binge eating disorder in bariatric surgery candidates. Obes. Surg. 2013, 23, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Clausen, L.; Rosenvinge, J.H.; Friborg, O.; Rokkedal, K. Validating the Eating Disorder Inventory-3 (EDI-3): A Comparison Between 561 Female Eating Disorders Patients and 878 Females from the General Population. J. Psychopathol. Behav. Assess. 2011, 33, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013.
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th ed.; World Health Organization: Washington, DC, USA, 2019. [Google Scholar]
- Marek, R.J.; Heinberg, L.J.; Lavery, M.; Merrell Rish, J.; Ashton, K. A review of psychological assessment instruments for use in bariatric surgery evaluations. Psychol. Assess. 2016, 28, 1142–1157. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.A. Psychological assessment for bariatric surgery: Current practices. Arq. Bras Circ. Dig. 2014, 27 (Suppl. 1), 59–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PhenX Toolkit. Protocol—Eating Disorders Examination—Bariatric Surgery Interview. Available online: https://www.phenxtoolkit.org/protocols/view/230103 (accessed on 15 November 2020).
- Parker, K.; Mitchell, S.; O’Brien, P.; Brennan, L. Psychometric evaluation of disordered eating measures in bariatric surgery patients. Eat. Behav. 2015, 19, 39–48. [Google Scholar] [CrossRef]
- Parker, K.; Mitchell, S.; O’Brien, P.; Brennan, L. Psychometric Evaluation of Disordered Eating Measures in Bariatric Surgery Candidates. Obes. Surg. 2016, 26, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Indja, B.; Seco, M.; Seamark, R.; Kaplan, J.; Bannon, P.G.; Grieve, S.M.; Vallely, M.P. Neurocognitive and Psychiatric Issues Post Cardiac Surgery. Heart Lung Circ. 2017, 26, 779–785. [Google Scholar] [CrossRef]
- Bouras, G.; Markar, S.R.; Burns, E.M.; Mackenzie, H.A.; Bottle, A.; Athanasiou, T.; Hanna, G.B.; Darzi, A. Linked Hospital and Primary Care Database Analysis of the Incidence and Impact of Psychiatric Morbidity Following Gastrointestinal Cancer Surgery in England. Ann. Surg. 2016, 264, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Noh, O.K.; Oh, Y.T.; Chun, M.; Kim, L. Psychiatric comorbidities among patients undergoing liver transplantation in South Korea: A nationwide population-based study. Hepatol. Int. 2018, 12, 174–180. [Google Scholar] [CrossRef] [PubMed]
- King, W.C.; Chen, J.Y.; Courcoulas, A.P.; Dakin, G.F.; Engel, S.G.; Flum, D.R.; Hinojosa, M.W.; Kalarchian, M.A.; Mattar, S.G.; Mitchell, J.E.; et al. Alcohol and other substance use after bariatric surgery: Prospective evidence from a U.S. multicenter cohort study. Surg. Obes. Relat. Dis. 2017, 13, 1392–1402. [Google Scholar] [CrossRef]
- Lindgren, E.; Gray, K.; Miller, G.; Tyler, R.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Food addiction: A common neurobiological mechanism with drug abuse. Front. Biosci. 2018, 23, 811–836. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.M.; Hutson, P.H.; Herman, B.K.; Potenza, M.N. The neurobiological basis of binge-eating disorder. Neurosci. Biobehav. Rev. 2016, 63, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Bianciardi, E.; Fabbricatore, M.; Di Lorenzo, G.; Innamorati, M.; Tomassini, L.; Gentileschi, P.; Niolu, C.; Siracusano, A.; Imperatori, C. Prevalence of Food Addiction and Binge Eating in an Italian sample of bariatric surgery candidates and overweight/obese patients seeking low-energy-diet therapy. Riv. Psichiatr. 2019, 54, 127–130. [Google Scholar] [CrossRef]
- Imperatori, C.; Bianciardi, E.; Niolu, C.; Fabbricatore, M.; Gentileschi, P.; Di Lorenzo, G.; Siracusano, A.; Innamorati, M. The Symptom-Checklist-K-9 (SCL-K-9) Discriminates between Overweight/Obese Patients with and without Significant Binge Eating Pathology: Psychometric Properties of an Italian Version. Nutrients 2020, 12, 674. [Google Scholar] [CrossRef] [Green Version]
- Bianciardi, E.; Gentileschi, P.; Niolu, C.; Innamorati, M.; Fabbricatore, M.; Contini, L.M.; Procenesi, L.; Siracusano, A.; Imperatori, C. Assessing psychopathology in bariatric surgery candidates: Discriminant validity of the SCL-90-R and SCL-K-9 in a large sample of patients. Eat. Weight Disord. 2020. [Google Scholar] [CrossRef] [PubMed]
- Steward, T.; Menchon, J.M.; Jiménez-Murcia, S.; Soriano-Mas, C.; Fernandez-Aranda, F. Neural Network Alterations Across Eating Disorders: A Narrative Review of fMRI Studies. Curr. Neuropharmacol. 2018, 16, 1150–1163. [Google Scholar] [CrossRef] [PubMed]



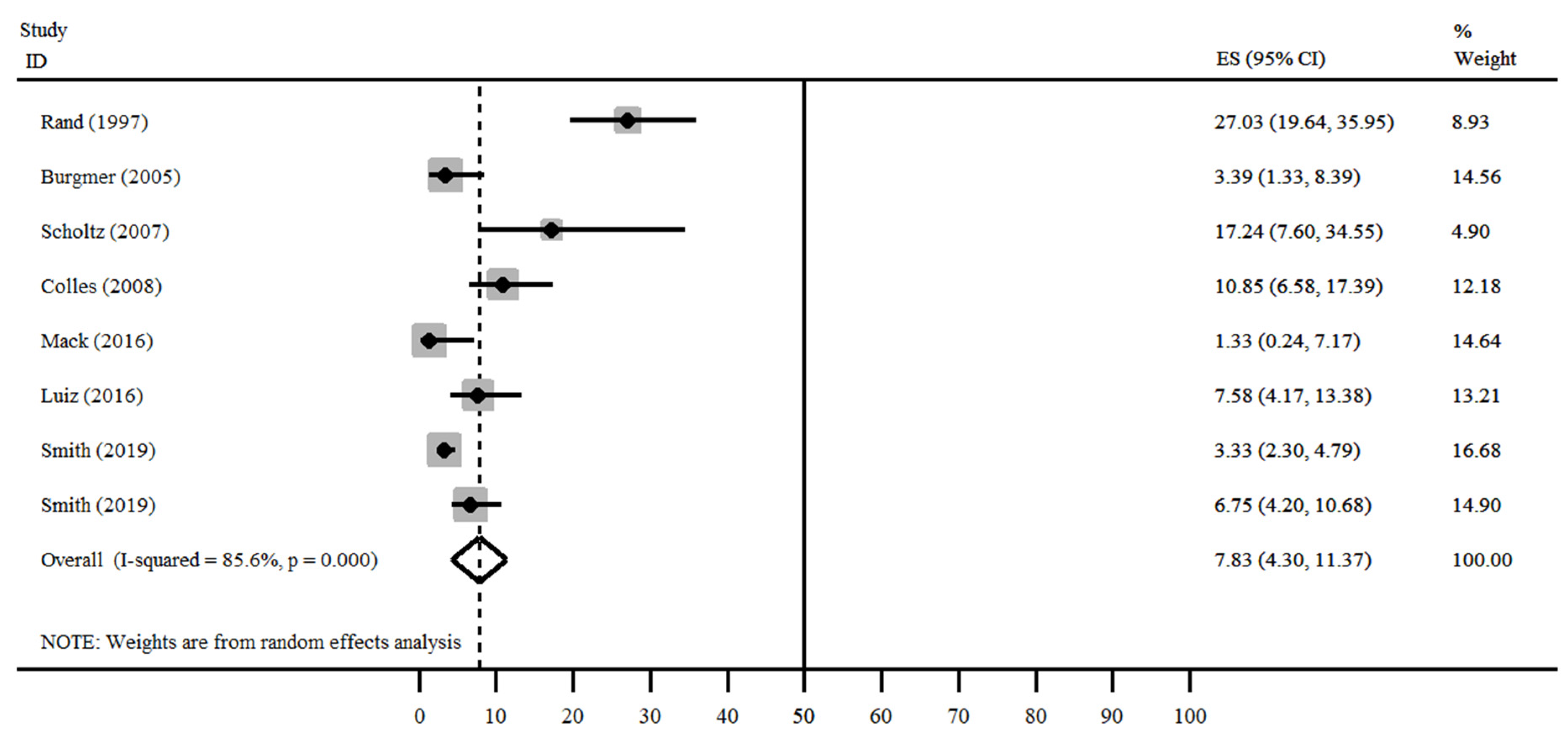

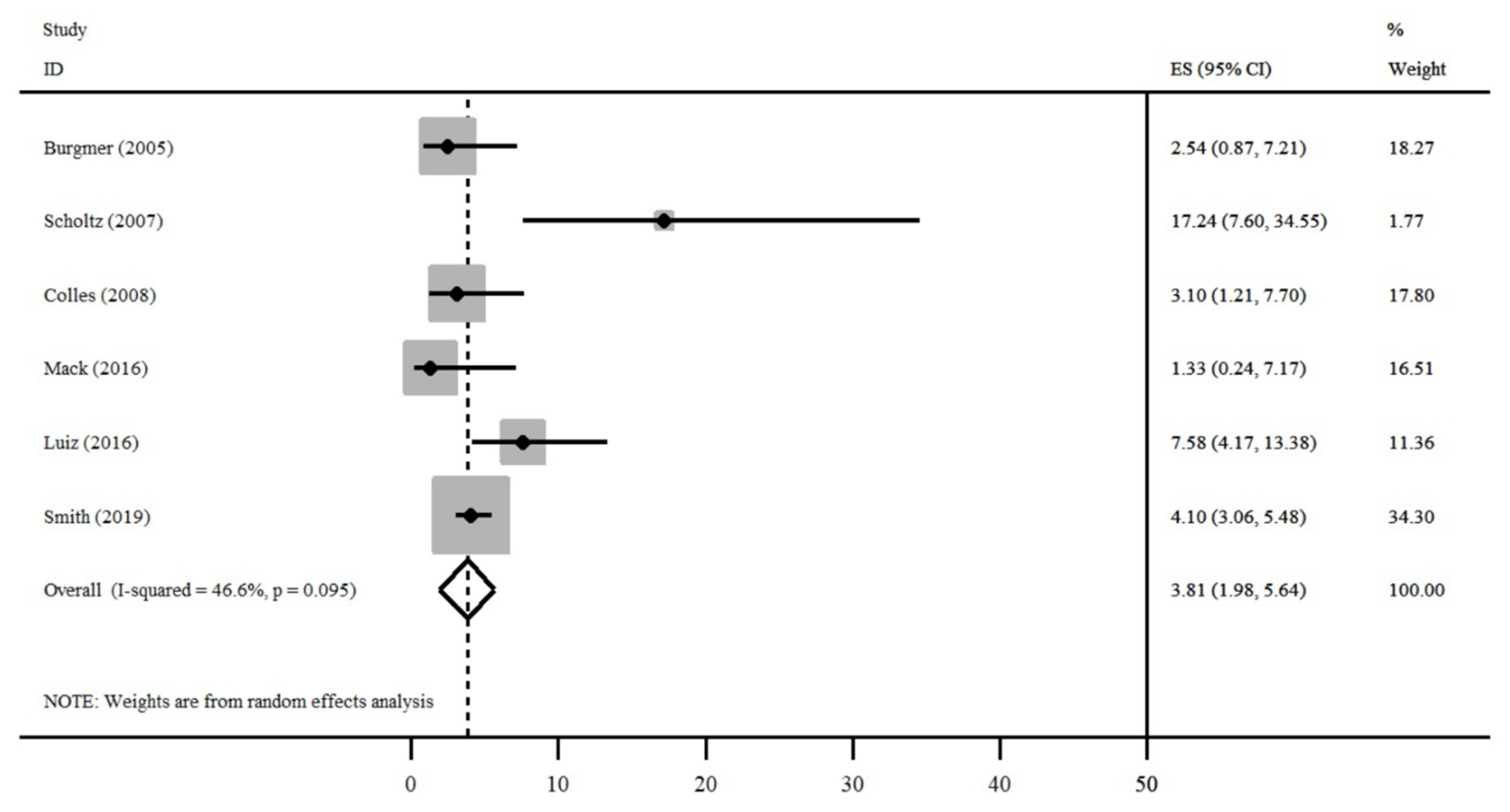
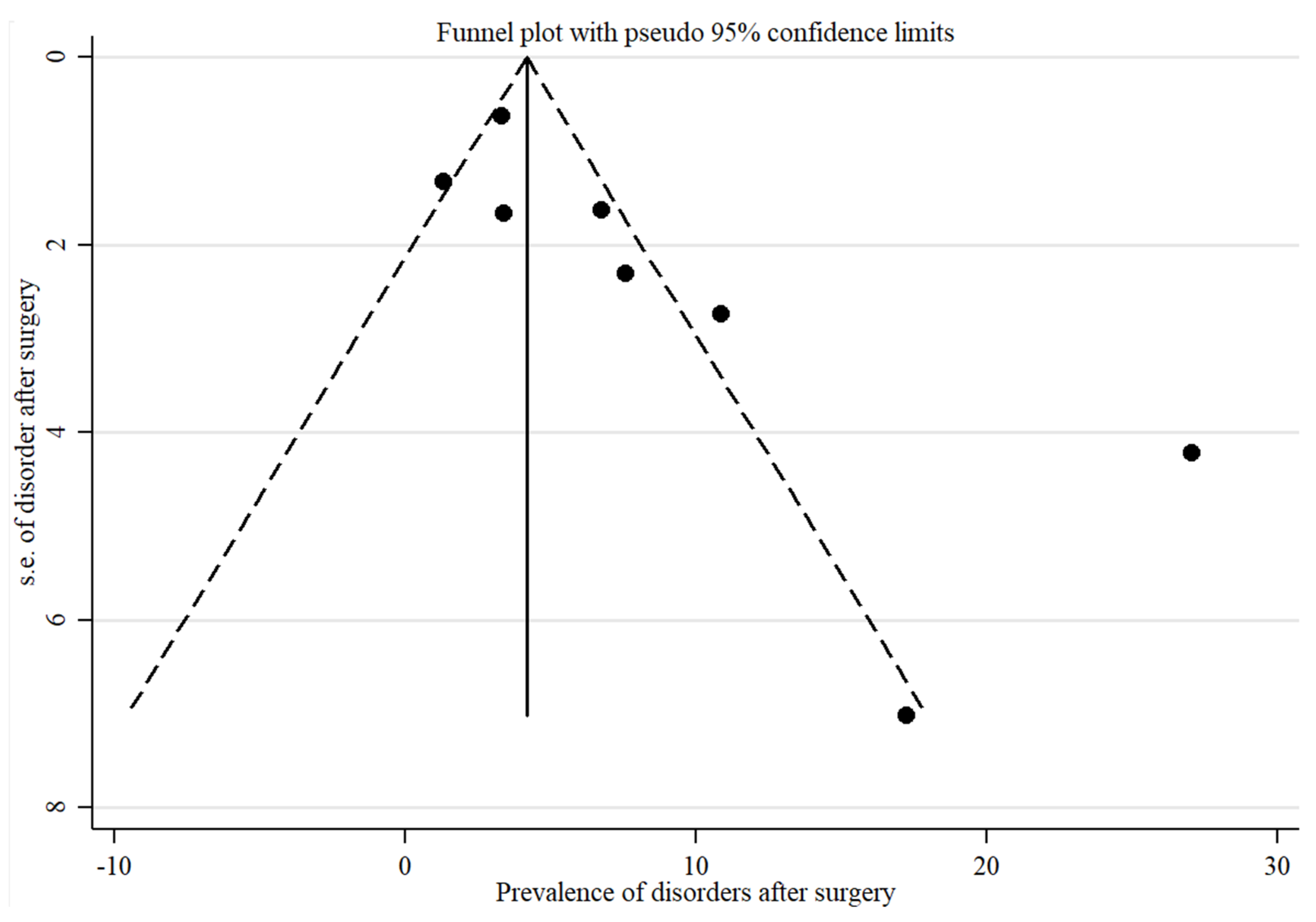
| Author, Publication Date and Country | Number of Patients | Mean Age-Years (SD) | Sex (%) | Mean BMI before and after Bariatric Surgery-kg/m2 (SD) | |
|---|---|---|---|---|---|
| Larsen JK (2004), Netherlands [15] | Total: 250 Pre-group: 93 Post-group: 157 (short-term: 48, long-term: 109) | 39.6 [range 22–61] | 29 M (72.5) 221 F (27.5) | 46.5 [range 37–67] | 45.4 [range 36–63] |
| de Zwaan M (2009), Germany [23]. | 59 | 44.5 (9.9) | 9 M (15) 50 F (85) | 51.3 (9) | 32.6 |
| Mack I (2016), Germany [13]. | 75 | 49.2 (11.6) | 27 M (36) 48 F (64) | 48.7 (8.4) | 37.1 (8.1) |
| Hsu LKG (1996), USA [19] | 24 | 39.7 (8.6) | 0 M (0) 24 F (100) | 48.8 (8.1) | 34.1 (7.7) |
| Latner JD (2004), New Zealand [17] | 65 | 39.5 [range 19–67] | 0 M (0) 65 F (100) | 54.1 (10.2) | 34.1 (8.5) |
| Colles SJ (2008), Australia [18] | 173 (initial) 129 (final) | 45.2 (11.5) | 26 M (20) (final) 103 F (80) (final) | 44.3 (6.8) | 35 (6) |
| Scholtz S (2007), UK [16] | 29 | 39 (9) | 1 M (3.5) 28 F (96.5) | 45 (7) | - |
| Smith KE (2019), USA [25] | Total: 2156 RYGB: 1640 (initial); 812 (final) AGB: 516 (initial); 237 (final) | 45.66 (11.32) | 517 M (24) 1639 F (76) | 47.06 (7.36) | Total: 35.17 (6.44) RYGB: 33.82 (6.56) AGB: 40.23 (6.47) |
| Powers PS (1999), USA [22] | 116 | 39.6 (9.3) | 20 M (17) 96 F (83) | 53.4 (10.9) | 40.7 (9.5) |
| Kalarchian MA (2019), USA [26] | Total: 173 (initial); 98 (final) RYGB: 104 AGB: 69 | RYGB: 45 (median) [IQR 34-53] 21–68 (range) AGB: 47 (median) [IQR 40–54] [range 23–67] | 31 M (18) 142 F (82) RYGB: 20 M (19.2) 84 F (80.8) AGB: 11 M (15.9) 58 F (84.1) | RYGB: 46.9 (median) [IQR 43.1–52] [range 36.1–76] AGB: 43.5 (median) [IQR 40.8–46.7] [range 33.5–65.8] | - |
| Burgmer R (2005), Germany [24] | 149 | 38.8 (10.3) | 47 M (32) 102 F (68) | 50.9 (8.1) | 38.6 (6.8) |
| Rand CSW (1997), USA [14] | Total: 2208 Control group: 2097 Experimental group: 111 | Control group: 52.8 (19.8) Experimental group: 44.6 (10.4) | Control group: 887 M (42.3) 1210 F (57.7) Experimental group: 8 M (6.9) 103 F (93.1) | - | Control group: 24.9 (4.9) Experimental group: 28.7 (6.4) |
| Luiz LB (2016), Brazil [20] | 132 | 38.27 (10.07) | 27 M (20.5) 105 F (79.5) | 48.31 (7.92) | 31.74 (5.7) |
| Kalarchian MA (2016), USA [21] | 165 | 46 (median) | 35 M (18.9) 130 F (81.1) | 44.8 (median) | - |
| Author, Publication Date and Country | Study Objectives | Study Conclusion |
|---|---|---|
| Larsen JK (2004), Netherlands [15]. | “To examine short and long-term eating behavior after laparoscopic adjustable gastric banding (LAGB) and the relationship of binge eating with weight and quality of life outcome.” | There is an improvement in short- and long-term post-bariatric disorders. The diagnosis and treatment of BED in the post is essential for a better prognosis. |
| de Zwaan M (2009), Germany [23]. | “(1) To provide a detailed description of the postoperative eating behavior of patients who had undergone RYGB and to determine which eating behaviors might be labeled non-normative or problematic; (2) to determine whether preoperative eating disorders might be associated with non-normative postoperative eating behaviors; (3) to determine the association of postoperative non-normative eating behaviors with postoperative eating-related and general psychopathology; and (4) to assess the association of preoperative and postoperative eating behaviors with the weight outcome.” | Patients with pre-bariatric disorder tend to develop BED in the postoperative period, which may be related to less weight loss. Subgroup tended to present vomiting due to weight change. The presence of these post-surgery disorders should be investigated, to identify who needs treatment. |
| Mack I (2016), Germany [13] | “To investigate the medium-term effects of LSG on mental health and eating behavior and their influence on weight loss by using a comprehensive interview-based assessment”. | After surgery, in the long term, depression, stress and eating disorders improve, BED being rare. Some patients experience disorders, along with depressive symptoms, greater stress and BMI, and less weight loss. Psychosocial improvement relates to weight loss, not surgery. |
| Hsu LKG (1996), USA [19] | “Examine what effect eating disturbances have on weight loss outcome after VBG” | AGB is not effective to change eating behavior or improve the patient’s psychiatric condition. |
| Latner JD (2004), New Zealand [17] | “To examine the prevalence of eating disturbances and psychiatric disorders among extremely obese patients before and after gastric bypass surgery and to examine the relationship between these disturbances and weight outcomes” | Presence of preoperative psychiatric disorders do not influence the outcome of bariatric surgery. More research is needed |
| Colles SJ (2008), Australia [18] | “This study prospectively assessed characteristics of BED, uncontrolled eating, NES and grazing, before, and 1 year after LAGB. We aimed to explore the nature and extent of change in these eating patterns following surgery” | More research is needed to optimize AGB results and improve postoperative psychological well-being |
| Scholtz S (2007), UK [16] | “To determine whether psychiatric profile was associated with long-term outcome” | The presence of psychiatric comorbidities should not be an impediment to performing bariatric surgery. The use of questionnaires should be considered mainly in the follow-up of patients with a psychiatric history |
| Smith KE (2019), USA [25] | “To [1] characterize LOCE and binge eating disorder (BED) over a 7-year period following bariatric surgery; [2] examine concurrent, prospective, and cumulative relationships between LOCE and weight loss; [3] assess whether these associations are moderated by surgery type; and [4] evaluate predictors of LOCE.” | LOCE and binge-eating can interfere with postoperative weight loss from bariatric surgery and must be constantly monitored |
| Powers PS (1999), USA [22] | “(1) to determine the prevalence of eating pathology in patients before bariatric surgery and at follow-up; (2) to assess the relationship of presurgical eating pathology to various measures of psychopathology; and (3) to assess the relationship between presurgical eating pathology and outcome” | There are no signs of a relation between preoperative disorders and postoperative vomiting episodes. During the first 6 months, all patients tend to lose more weight |
| Kalarchian MA (2019), USA [26] | “To report mental disorders through 7 years post surgery and examine their relationship with changes in weight and health-related quality of life” | Careful weight monitoring and post-operative mental disorders should optimize surgical results |
| Burgmer R (2005), Germany [24] | “The present study investigated the predictive value of three dimensions of eating behavior and disturbed eating on the course of weight after gastric restriction surgery” | Postoperative eating behavior influences surgery results more than preoperative behavior. |
| Rand CSW (1997), USA [14] | “To determine the prevalence of night-eating syndrome in the general population and among a new sample of obesity surgery patients” | Defined criteria, exacerbation factors and mitigation of their frequency and studies on the evolution of NES over time are needed |
| Luiz LB (2016), Brazil [20] | “To verify how the intensity of BE before the surgery and one year after the procedure, as well as the presence of BED, relate to the % EWL.” | The diagnosis of BED interferes negatively in weight loss |
| Kalarchian MA (2016), USA [21] | “To document changes in Axis I psychiatric disorders after bariatric surgery and examine their relationship with post surgery weight loss” | Preoperative disorders are not related to weight loss, unlike postoperative BED, which, although infrequent, is associated with less weight loss |
| Author, Publication Date and Country | Study Type | Evaluation Method | Type of Intervention | Eating Disorders before and after Surgery | Eating Symptoms before and after Surgery | |
|---|---|---|---|---|---|---|
| Mack I (2016), Germany [13]. | Non-randomized clinical trial | EDE TFEQ Other | SG | 9 BED | 1 BED | 6 LOCE 39% Grazing Disinhibition and Feelings of Hunger reduced |
| Rand CSW (1997), USA [14]. | Case control | Other | Other | 30.6% of patients in the experimental group experienced NES. | Control Group: 1.5% NES Experimental Group: 27% NES | - |
| Author, Publication Date and Country | Type of Study | Evaluation Method | Type of Intervention | Eating Disorders before and after Surgery | Eating Symptoms before and after Surgery | ||
|---|---|---|---|---|---|---|---|
| Larsen JK (2004), Netherlands [15]. | Cross sectional | EDE BES Other | AGB | Pre-BED group: 55.9% | Short-term BED group: 31.9% Long-term BED group: 37.4% | Pre-group: 91 Emotional Eating; 93 External Eating; 92 Restrained Eating | Short-term group: 45 Emotional Eating; 48 External Eating; 48 Restrained Eating Long-term group: 102 Emotional Eating; 108 External Eating; 108 Restrained Eating |
| de Zwaan M (2009), Germany [23] | Cross sectional | EDE TFEQ Other | RYGB | 15 BED (by EDE-BSV) 14 BED (by QEWP) 2 BN | - | 45 Plugging (76.3%) 30 Dumping (50.8%) 15 SBE or LOCE (25.4%) 19 Picking/nibbling (32.2%) 37 Not weight-related vomiting (62.7%) 7 Weight-related vomiting (11.9%) 7 Nocturnal eating (11.9%) | 15 LOCE 7 self-induced vomiting 37 vomiting for relief 7 symptoms of NES |
| Hsu LKG (1996), USA [19] | Cross sectional | EDE | AGB | 19 Eating disorders (79.2%) 9 BED (37.5%) 5 BN (20.8%) 10 NES (42%), 8 of which are also BED/BN | 5 BED (20.8%)5 BN (20.8%)8 previous BED or BN maintained the disease2 previous NES developed BED or BN | 4 Self-induced vomiting | 4 Self-induced vomiting |
| Latner JD (2004), New Zealand [17] | Prospective cohort | EDE | RYGB | BED: 48% 1 BN NES: 55% | BED: 0% BN: 0% NES: 2% | Vomiting:7% OBE: 20% | Vomiting: 5% OBE: 0% |
| Colles SJ (2008), Australia [18] | Prospective cohort | TFEQ SF-36 Other | AGB | 18 BED (14%) 22 NES (17.1%) | 4 BED (3.1%), with 2 being preoperative 10 NES (7.8%), with 4 being preoperative | LOCE: 31% | LOCE and Grazing: 20.2% Only Grazing: 5.9% Grazing had prevalence increased in 31% |
| Scholtz S (2007), UK [16] | Prospective cohort | EDE Other | AGB | 12 BED (41%) 3 BN (10%) 1 AN (3%) | 5 BED (17%) 0 BN (0%) | 11 OBE (37%) 4 Symptoms of BED (13%) | 4 Symptoms BED (13%) |
| Smith KE (2019), USA [25] | Prospective cohort | Other | RYGB AGB | BED: Total: 12.7% of 2157 RYGB: 12.1% of 1641 AGB: 14.6% of 516 | BED (1 year): Total: 2.1% of 1774 RYGB: 1.3% of 1343 AGB: 4.5% of 431 BED (7 years): Total: 4% of 1049 RYGB: 3.3% of 812 AGB: 6.6% of 237 | LOCE: Total: 35% of 2157 RYGB: 33.5% of 1641 AGB: 39.7% of 516 | LOCE (1 year): Total: 24.6% of 1774 RYGB: 21.9% of 1343 AGB: 32.9% of 431 LOCE (7 years): Total: 26.4% of 1049 RYGB: 25.6% of 812 AGB: 29.1% of 237 |
| Powers PS (1999), USA [22] | Prospective cohort | Other | Other | 19 BED (16%) 12 NES (10%) | - | 60 Symptoms BED (52%) 64 presented criteria for BED or NES (55%) | 46 Occasional vomiting (79%) 19 weekly vomiting (33%) |
| Kalarchian MA (2019), USA [26] | Prospective cohort | SF-36 Other | RYGB AGB | RYGB: 8 BED (7.7%) 2 BN (1.9%) AGB: 2 BED (3%) | 0 BED (0%) 0 BN (0%) | - | - |
| Burgmer R (2005), Germany [24] | Prospective cohort | TFEQ Other | AGB | BED: 7.4% BN: 3.4% | BED: 2%BN: 0.7% | Episodes of BED: 37.6% Grazing: 24.2% | Episodes of BED: 20.1% Grazing: 19.5% |
| Luiz LB (2016), Brazil [20] | Cross sectional | BES | RYGB | BED: 29.54% | BED: 7.58% | - | Elevation of BED symptoms: 13.63% Maintenance of BED symptoms: 6.83% Decrease in BED symptoms: 79.54% |
| Kalarchian MA (2016), USA [21] | Prospective cohort | Other | RYGB AGB | BED: 6.1% BN: 1.2% | BED: 3.1% BN: 0% | - | - |
| Author, Publication Date and Country | Reported Study Limitations |
|---|---|
| Larsen JK (2004), Netherlands [15]. | 1. Population restricted to patients undergoing LAGB, does not allow generalization of procedures 2. Cross-sectional model, with comparison between groups (limits generalization and causal relation) 3. BES questionnaire used does not access the objective consumption of quantity of food in a short period of time |
| de Zwaan M (2009), Germany [23] | 1. Small, non-consecutive sample (because there is a lot of refusal in the preoperative period to repeat the interview in the postoperative period) 2. Those who agreed to be interviewed in the postoperative period may not represent the population as a whole 3. Interview based on EDE-BSV was not conducted in the pre and postoperative period 4. Relatively short follow-up |
| Mack I (2016), Germany [13] | 1. Proportionally significant loss of follow-up (considering obese group) 2. Final sample of 66% of the initial sample 3. Depression accessed only by validated questionnaires, without a structured interview, limiting the validity of the results |
| Hsu LKG (1996), USA [19] | 1. Retrospective cross-sectional design 2. Short study duration 3. Small sample size |
| Latner JD (2004), New Zealand [17] | 1. Retrospective assessment of eating disorders 2. Absence of men in the sample 3. Use of self-reported methods, with follow-up interviews via telephone and face-to-face measurements 4. Short study duration |
| Colles SJ (2008), Australia [18] | 1. Use of self-report survey and telephone interview for assessment of postoperative eating behavior 2. Overlapping of groups and absence of agreed group definitions |
| Scholtz S (2007), UK [16] | 1. Small sample size 2. Significant number of cases excluded from the analysis due to the absence of psychiatric evaluation 3. Retrospective assessment of eating disorders |
| Smith KE (2019), USA [25] | 1. Evaluation methodology of LOCE and binge eating can interfere with the results obtained 2. Use of self-reported questionnaires 3. The proportion of AGB cases in the sample may not correspond to national averages |
| Powers PS (1999), USA [22] | No limitations reported by the authors |
| Kalarchian MA (2019), USA [26] | 1. Possible risk of attrition or self-selection bias 2. Limited statistical power for some analyses due to sample size and loss of follow-up 3. According to the methodology used, the last month of evaluation may not represent the total number of diagnoses from that period. 4. There was no justification for the prescriptions used by patients during the study |
| Burgmer R (2005), Germany [24] | 1. Pre-selection of patients 2. Exclusive results for restrictive surgeries |
| Rand CSW (1997), USA [14] | Self-selection of patients in the case group and therefore the prevalence of NES was higher |
| Luiz LB (2016), Brazil [20] | 1. The study involved only one center 2. Involvement mainly of Caucasian women, making it difficult to extrapolate the data to the general population. 3. The diagnosis of BED by BES tends to be very sensitive and unspecific, overestimating it. 4. Possibly insufficient follow-up to clearly evaluate how BED variation interferes with weight loss |
| Kalarchian MA (2016), USA [21] | 1. Possible selection bias due to self-selection to participate in the study or due to dropout 2. Limited statistical power for some analyses 3. Underestimation of disorders by applying DSM-IV criteria, which do not detect subclinical disorders 4. The evaluation period was only in 2 and 3 years after surgery, therefore, if patients develop the disorder in other periods, it will not be detected. 5. Results of RYGB and LAGB only |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taba, J.V.; Suzuki, M.O.; Nascimento, F.S.d.; Iuamoto, L.R.; Hsing, W.T.; Pipek, L.Z.; Carneiro-D’Albuquerque, L.A.; Meyer, A.; Andraus, W. The Development of Feeding and Eating Disorders after Bariatric Surgery: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2396. https://doi.org/10.3390/nu13072396
Taba JV, Suzuki MO, Nascimento FSd, Iuamoto LR, Hsing WT, Pipek LZ, Carneiro-D’Albuquerque LA, Meyer A, Andraus W. The Development of Feeding and Eating Disorders after Bariatric Surgery: A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(7):2396. https://doi.org/10.3390/nu13072396
Chicago/Turabian StyleTaba, João Victor, Milena Oliveira Suzuki, Fernanda Sayuri do Nascimento, Leandro Ryuchi Iuamoto, Wu Tu Hsing, Leonardo Zumerkorn Pipek, Luiz Augusto Carneiro-D’Albuquerque, Alberto Meyer, and Wellington Andraus. 2021. "The Development of Feeding and Eating Disorders after Bariatric Surgery: A Systematic Review and Meta-Analysis" Nutrients 13, no. 7: 2396. https://doi.org/10.3390/nu13072396
APA StyleTaba, J. V., Suzuki, M. O., Nascimento, F. S. d., Iuamoto, L. R., Hsing, W. T., Pipek, L. Z., Carneiro-D’Albuquerque, L. A., Meyer, A., & Andraus, W. (2021). The Development of Feeding and Eating Disorders after Bariatric Surgery: A Systematic Review and Meta-Analysis. Nutrients, 13(7), 2396. https://doi.org/10.3390/nu13072396







