Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Terms
2.2. Inclusion Criteria
2.3. Article Screening and Data Collection
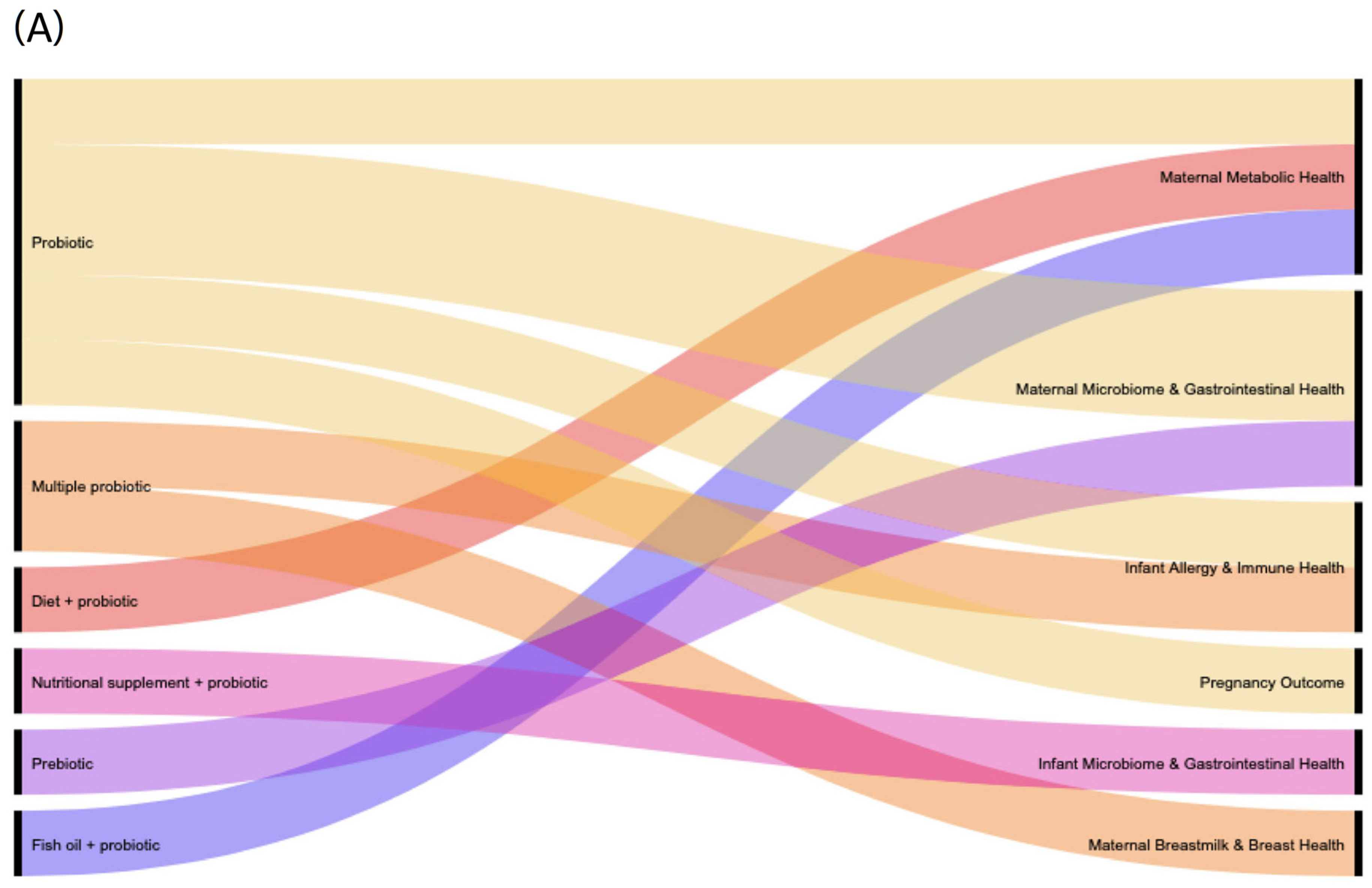
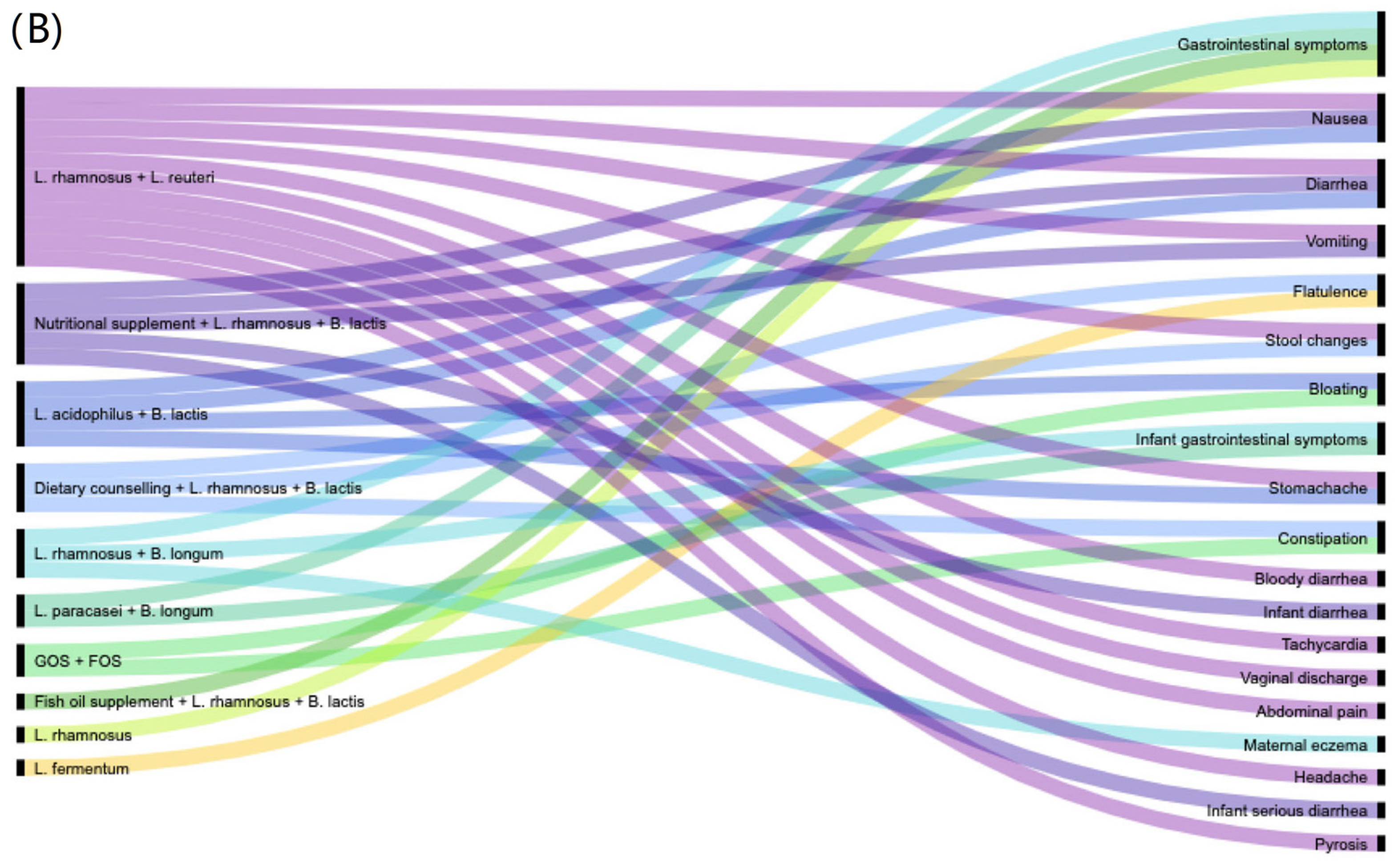
2.4. Data Synthesis and Visualisation
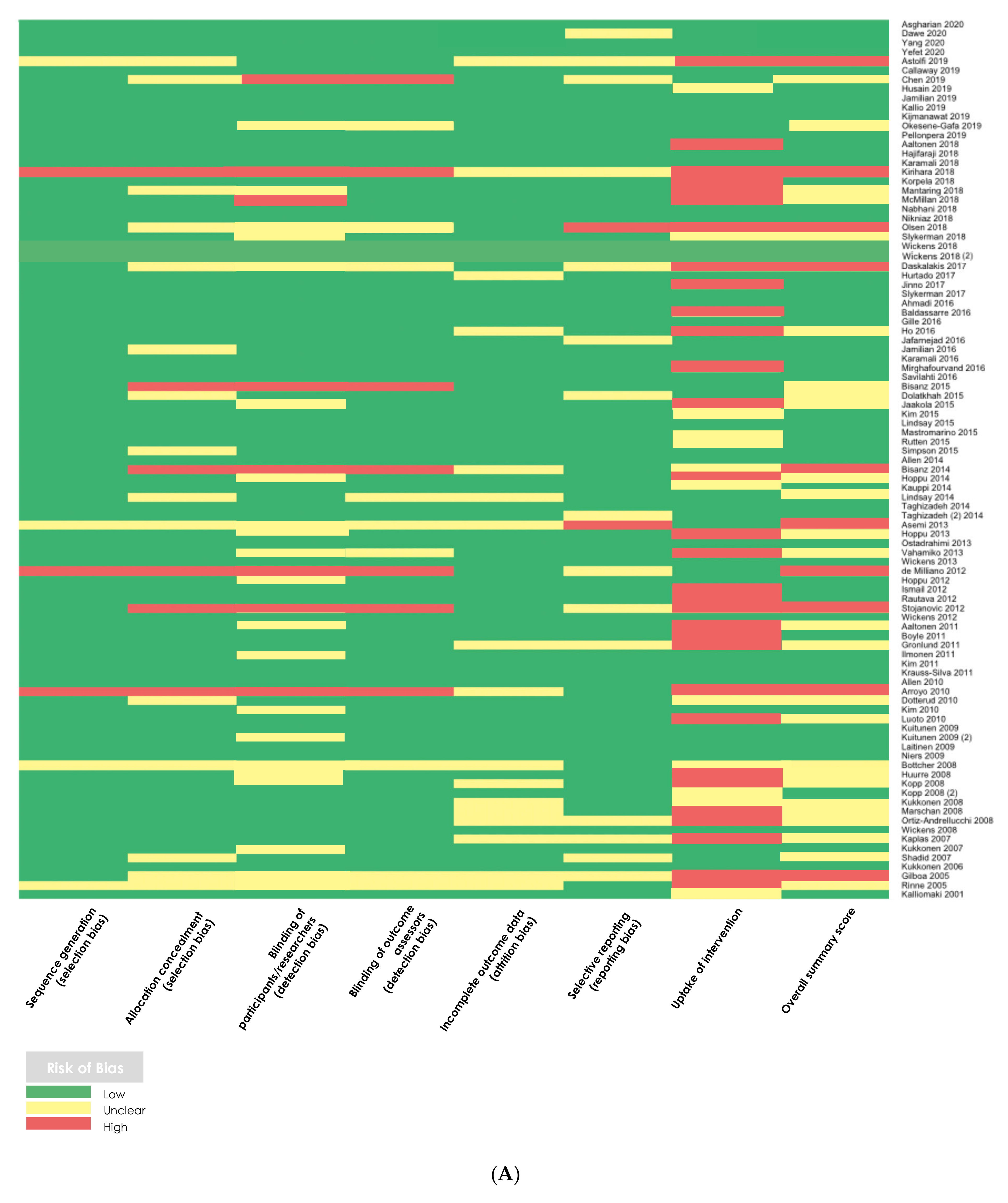
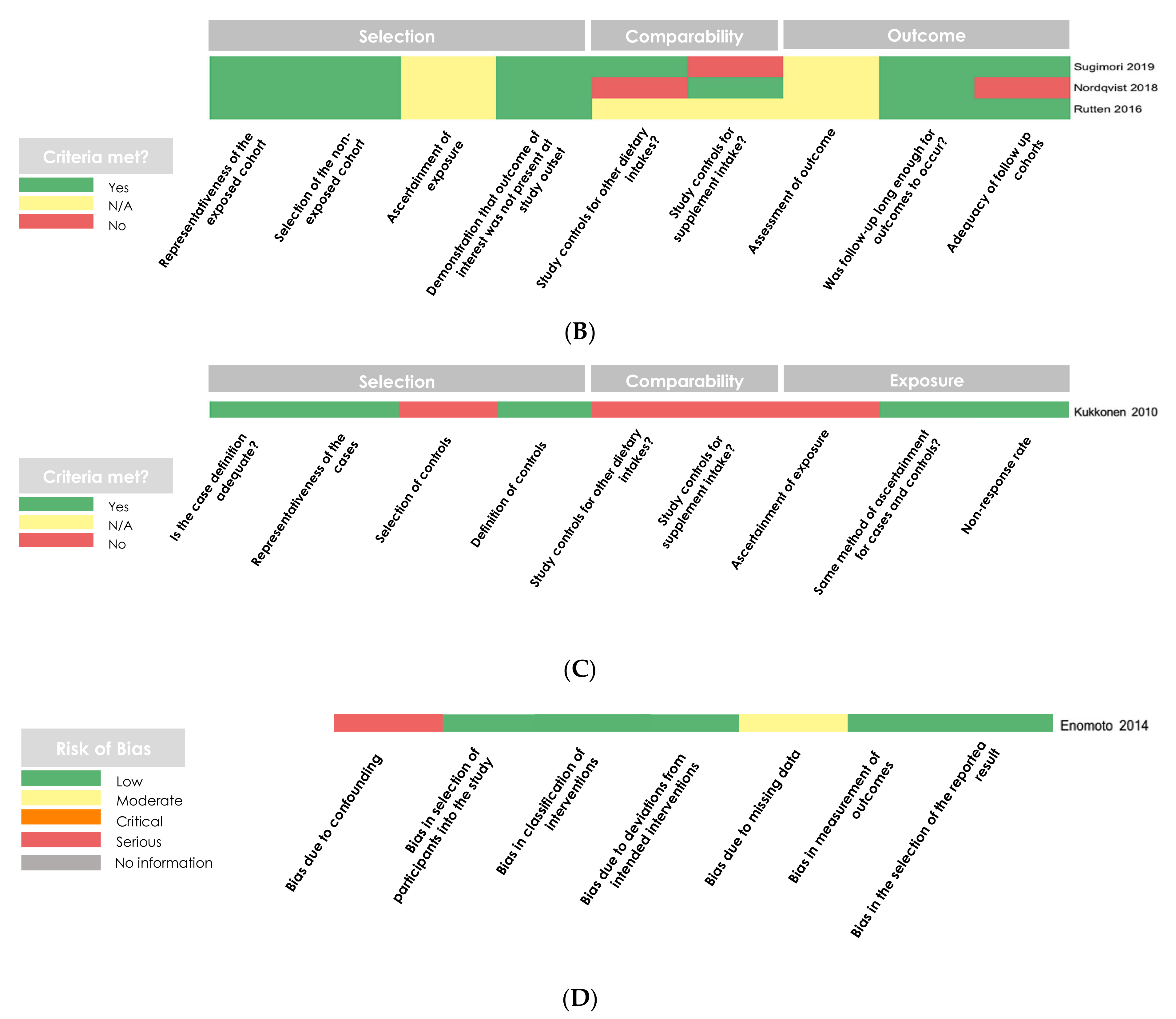
2.5. Methodological Quality Assessment
2.6. Data Analysis
3. Results
3.1. Study Location, Demographics, and Design: 100 Studies under Initial Review
3.2. Methodological Quality Assessments
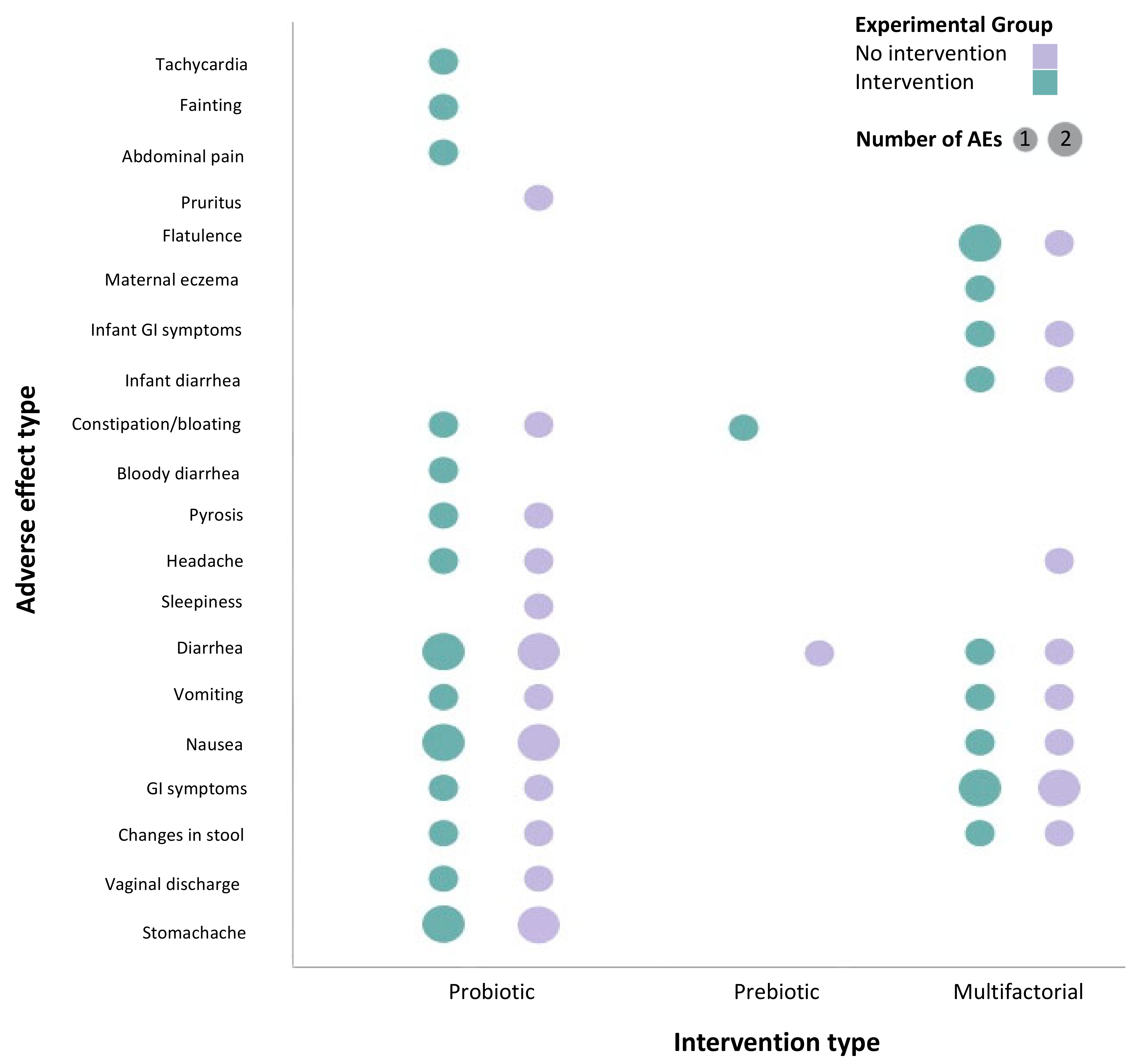
3.3. Characteristics of Studies That Reported on Adverse Effects
3.4. Probiotics Are Safe for Use during Pregnancy with Minimal Reported Adverse Effects
4. Discussion
5. Conclusions
- Researchers should provide detailed and specific safety and adverse effects reporting plans prior to conducting a study, including information and data on:
- (a)
- Intervention characteristics: strain type, dosage, duration (e.g., relative to gestational age).
- (b)
- Participant information: age, underlying health conditions, diet.
- (c)
- Adverse effects: type, timing, symptoms, severity, duration, withdrawal due to intervention.
- Results of studies, including detailed efficacy and safety data, should be published using accessible language and easy to interpret visuals that can be used by diverse audiences.
- Clinicians and consumers should refer to available scientific evidence [60] on efficacy of probiotic products and their associated adverse effects when making decisions about their use or impact on patient health, and clinicians should disseminate safety information to patients accordingly.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chin-Lee, B.; Curry, W.J.; Fetterman, J.; Graybill, M.A.; Karpa, K. Patient experience and use of probiotics in community-based health care settings. Patient Prefer. Adherence 2014, 8, 1513–1520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Betz, M.; Uzueta, A.; Rasmussen, H.; Gregoire, M.; Vanderwall, C.; Witowich, G. Knowledge, use and perceptions of probiotics and prebiotics in hospitalised patients. Nutr. Diet. 2015, 72, 261–266. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 12, Cd006095. [Google Scholar] [CrossRef] [PubMed]
- Didari, T.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J. Gastroenterol. 2015, 21, 3072–3084. [Google Scholar] [CrossRef]
- Ritchie, M.L.; Romanuk, T.N. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS ONE 2012, 7, e34938. [Google Scholar] [CrossRef]
- AlFaleh, K.; Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014, Cd005496. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, X.; Huang, X.; He, H.; Zheng, J. Association between Probiotic Yogurt Intake and Gestational Diabetes Mellitus: A Case-Control Study. Iran. J. Public Health 2019, 48, 1248–1256. [Google Scholar]
- Fernández, L.; Cárdenas, N.; Arroyo, R.; Manzano, S.; Jiménez, E.; Martín, V.; Rodríguez, J.M. Prevention of Infectious Mastitis by Oral Administration of Lactobacillus salivarius PS2 During Late Pregnancy. Clin. Infect. Dis. 2016, 62, 568–573. [Google Scholar] [CrossRef]
- Mirghafourvand, M.; Rad, A.H.; Alizadeh, S.M.; Fardiazar, Z.; Shokri, K. The Effect of Probiotic Yogurt on Constipation in Pregnant Women: A Randomized Controlled Clinical Trial. Iran. Red. Crescent Med. J. 2016, 18. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Probiotic Pregnancy Study, Grp. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. Ebiomedicine 2017, 24, 159–165. [Google Scholar] [CrossRef]
- Ho, M.; Chang, Y.Y.; Chang, W.C.; Lin, H.C.; Wang, M.H.; Lin, W.C.; Chiu, T.H. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial. Taiwan J. Obstet. Gynecol. 2016, 55, 515–518. [Google Scholar] [CrossRef]
- Parma, M.; Stella Vanni, V.; Bertini, M.; Candiani, M. Probiotics in the prevention of recurrences of bacterial vaginosis. Altern Ther. Health Med. 2014, 20 (Suppl. 1), 52–57. [Google Scholar]
- Korpela, K.; Salonen, A.; Vepsäläinen, O.; Suomalainen, M.; Kolmeder, C.; Varjosalo, M.; Miettinen, S.; Kukkonen, K.; Savi-lahti, E.; Kuitunen, M.; et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 2018, 6, 182. [Google Scholar] [CrossRef]
- Beserra, B.T.; Fernandes, R.; do Rosario, V.A.; Mocellin, M.C.; Kuntz, M.G.; Trindade, E.B. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin. Nutr. 2015, 34, 845–858. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef]
- Closa-Monasterolo, R.; Ferré, N.; Castillejo-DeVillasante, G.; Luque, V.; Gispert-Llaurado, M.; Zaragoza-Jordana, M.; Theis, S.; Escribano, J. The use of inulin-type fructans improves stool consistency in constipated children. A randomised clinical trial: Pilot study. Int. J. Food Sci. Nutr. 2017, 68, 587–594. [Google Scholar] [CrossRef]
- Christodoulides, S.; Dimidi, E.; Fragkos, K.C.; Farmer, A.D.; Whelan, K.; Scott, S.M. Systematic review with meta-analysis: Effect of fibre supplementation on chronic idiopathic constipation in adults. Aliment. Pharm. Ther. 2016, 44, 103–116. [Google Scholar] [CrossRef]
- Van Baarlen, P.; Troost, F.; van der Meer, C.; Hooiveld, G.; Boekschoten, M.; Brummer, R.J.M.; Kleerebezem, M. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Valdovinos-García, L.R.; Abreu, A.T.; Valdovinos-Díaz, M.A. Probiotic use in clinical practice: Results of a national survey of gastroenterologists and nutritionists. Rev. Gastroenterol. México 2019, 84, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Frauwallner, A.; Varga, L.; Langerholc, T.; Rogelj, I.; Lorber, M.; Lewis, P.; Povalej Bržan, P. Health Professionals’ Knowledge of Probiotics: An International Survey. Int. J. Environ. Res. Public Health 2019, 16, 3128. [Google Scholar] [CrossRef]
- Wilson, Z.; Whitehead, K. A cross sectional survey to assess healthcare professionals’ attitudes to and understanding of probiotics. Clin. Nutr. ESPEN 2019, 34, 104–109. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Quigley, E.M. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 2014, 109 (Suppl. 1), S2–S26. [Google Scholar] [CrossRef]
- Zheng, J.; Feng, Q.; Zheng, S.; Xiao, X. The effects of probiotics supplementation on metabolic health in pregnant women: An evidence based meta-analysis. PLoS ONE 2018, 13, e0197771. [Google Scholar] [CrossRef]
- He, A.; Chin, J.; Lomiguen, C.M. Benefits of Probiotic Yogurt Consumption on Maternal Health and Pregnancy Outcomes: A Systematic Review. Cureus 2020, 12, e9408. [Google Scholar] [CrossRef]
- Page, M.J.; Shamseer, L.; Altman, D.G.; Tetzlaff, J.; Sampson, M.; Tricco, A.C.; Catalá-López, F.; Li, L.; Reid, E.K.; Sarkis-Onofre, R.; et al. Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study. PLOS Med. 2016, 13, e1002028. [Google Scholar] [CrossRef]
- Lasserson, T.J.; Higgins, J.P.T. Chapter 1: Starting a review. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Higgins, T.J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Chichester, UK, 2021. [Google Scholar]
- Peryer, G.; Golder, S.; Junqueira, D.R.; Vohra, S.; Loke, Y.K. Chapter 19: Adverse effects. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.1; Higgins, T.J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Chichester, UK, 2020. [Google Scholar]
- Mammaro, A.; Carrara, S.; Cavaliere, A.; Ermito, S.; Dinatale, A.; Pappalardo, E.M.; Militello, M.; Pedata, R. Hypertensive disorders of pregnancy. J. Prenat. Med. 2009, 3, 1–5. [Google Scholar]
- De Guingand, D.L.; Palmer, K.R.; Snow, R.J.; Davies-Tuck, M.L.; Ellery, S.J. Risk of Adverse Outcomes in Females Taking Oral Creatine Monohydrate: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1780. [Google Scholar] [CrossRef]
- Stahl-Timmins, W. GOfER Guide v2.6. Available online: http://blogs.exeter.ac.uk/gofer/ (accessed on 17 November 2020).
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. RAWGraphs: A Visualisation Platform to Create Open Outputs. In Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter, Cagliari, Italy, 18 September 2017; pp. 28:21–28:25. [Google Scholar]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, comparison and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institut: Ottawa, Canada, 2011; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 17 November 2020).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savovic, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (Updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Chichester, UK, 2021. [Google Scholar]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Andrews, C.N.; Sidani, S.; Marshall, J.K. Clinical Management of the Microbiome in Irritable Bowel Syndrome. J. Can. Assoc. Gastroenterol. 2020. [Google Scholar] [CrossRef]
- Moseley, A.M.; Rahman, P.; Wells, G.A.; Zadro, J.R.; Sherrington, C.; Toupin-April, K.; Brosseau, L. Agreement between the Cochrane risk of bias tool and Physiotherapy Evidence Database (PEDro) scale: A meta-epidemiological study of randomized controlled trials of physical therapy interventions. PLoS ONE 2019, 14, e0222770. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.1 (Updated September 2020); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Chichester, UK, 2020. [Google Scholar]
- Ciorba, M.A. A Gastroenterologist’s Guide to Probiotics. Clin. Gastroenterol. Hepatol. 2012, 10, 960–968. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Eldridge, S.; Li, T. Chapter 23: Including variants on randomized trials. In Cochrane Handbook for Systematic Reviews of Interventions, version 6.1 (updated September 2020); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Chichester, UK, 2020. [Google Scholar]
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef]
- Arroyo, R.; Martín, V.; Maldonado, A.; Jiménez, E.; Fernández, L.; Rodríguez, J.M. Treatment of infectious mastitis during lactation: Antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin. Infect. Dis. 2010, 50, 1551–1558. [Google Scholar] [CrossRef]
- Martín, V.; Cárdenas, N.; Ocaña, S.; Marín, M.; Arroyo, R.; Beltrán, D.; Badiola, C.; Fernández, L.; Rodríguez, J.M. Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in Pregnant Women by Using Lactobacillus salivarius CECT 9145, A Target-specific Probiotic Strain. Nutrients 2019, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Pellonperä, O.; Mokkala, K.; Houttu, N.; Vahlberg, T.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Efficacy of Fish Oil and/or Probiotic Intervention on the Incidence of Gestational Diabetes Mellitus in an At-Risk Group of Overweight and Obese Women: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Care 2019, 42, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Krauss-Silva, L.; Moreira, M.E.L.; Alves, M.B.; Braga, A.; Camacho, K.G.; Batista, M.R.R.; Almada-Horta, A.; Rebello, M.R.; Guerra, F. A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: Preliminary results. Trials 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- Gille, C.; Boer, B.; Marschal, M.; Urschitz, M.S.; Heinecke, V.; Hund, V.; Speidel, S.; Tarnow, I.; Mylonas, I.; Franz, A.; et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am. J. Obstet. Gynecol. 2016, 215. [Google Scholar] [CrossRef]
- Body, C.; Christie, J.A. Gastrointestinal Diseases in Pregnancy: Nausea, Vomiting, Hyperemesis Gravidarum, Gastroesophageal Reflux Disease, Constipation, and Diarrhea. Gastroenterol. Clin. N. Am. 2016, 45, 267–283. [Google Scholar] [CrossRef]
- Kriss, J.L.; Ramakrishnan, U.; Beauregard, J.L.; Phadke, V.K.; Stein, A.D.; Rivera, J.A.; Omer, S.B. Yogurt consumption during pregnancy and preterm delivery in Mexican women: A prospective analysis of interaction with maternal overweight status. Matern. Child. Nutr. 2018, 14. [Google Scholar] [CrossRef]
- Celik, V.; Beken, B.; Yazicioglu, M.; Ozdemir, P.G.; Sut, N. Do traditional fermented foods protect against infantile atopic dermatitis. Pediatr. Allergy Immunol. 2019, 30, 540–546. [Google Scholar] [CrossRef]
- Asemi, Z.; Jazayeri, S.; Najafi, M.; Samimi, M.; Mofid, V.; Shidfar, F.; Shakeri, H.; Esmaillzadeh, A. Effect of Daily Consumption of Probiotic Yogurt on Oxidative Stress in Pregnant Women: A Randomized Controlled Clinical Trial. Ann. Nutr. Metab. 2012, 60, 62–68. [Google Scholar] [CrossRef]
- Othman, M.; Alfirevic, Z.; Neilson, J.P. Probiotics for preventing preterm labour. Cochrane Database Syst. Rev. 2007, Cd005941. [Google Scholar] [CrossRef]
- Pettoello-Mantovani, M.; Çokuğraş, F.; Çullu Vural, M.; Mestrovic, J.; Nigri, L.; Piazzolla, R.; Giardino, I.; Conoscitore, M.; Namazova-Baranova, L. Pilot study for the understanding and use of probiotics by different paediatric healthcare professionals working in different European countries. Ital. J. Pediatr. 2019, 45, 57. [Google Scholar] [CrossRef]
- Oliver, L.; Rasmussen, H.; Gregoire, M.B.; Chen, Y. Health Care Providers’ Knowledge, Perceptions, and Use of Probiotics and Prebiotics. Top. Clin. Nutr. 2014, 29, 139–149. [Google Scholar] [CrossRef]
- Skokovic-Sunjic, D. Clinical Guide to Probiotic Products Available in Canada. Indications, Dosage Forms, and Clinical Evidence to Date—2020 Edition. Available online: www.probioticchart.ca (accessed on 19 November 2020).
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Javdan, B.; Lopez, J.G.; Chankhamjon, P.; Lee, Y.-C.J.; Hull, R.; Wu, Q.; Wang, X.; Chatterjee, S.; Donia, M.S. Personalized Mapping of Drug Metabolism by the Human Gut Microbiome. Cell 2020, 181, 1661–1679.e22. [Google Scholar] [CrossRef]
- Bracken, M.B. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef]
- Snoy, P.J. Establishing Efficacy of Human Products Using Animals: The US Food and Drug Administration’s “Animal Rule”. Vet. Pathol. 2010, 47, 774–778. [Google Scholar] [CrossRef]
- Probiotics Market. Size, Share & Trends Analysis Report by Product (Food & Beverages, Dietary Supplements), By Ingredient (Bacteria, Yeast), By End Use, By Distribution Channel, And Segment Forecasts, 2019—2025. 2019. Available online: https://www.grandviewresearch.com/industry-analysis/probiotics-market (accessed on 12 July 2021).
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A. Assessment of socio-demographic factors, health status and the knowledge on probiotic dairy products. Food Sci. Hum. Wellness 2020, 9, 272–279. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheyholislami, H.; Connor, K.L. Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2382. https://doi.org/10.3390/nu13072382
Sheyholislami H, Connor KL. Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(7):2382. https://doi.org/10.3390/nu13072382
Chicago/Turabian StyleSheyholislami, Hauna, and Kristin L. Connor. 2021. "Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis" Nutrients 13, no. 7: 2382. https://doi.org/10.3390/nu13072382
APA StyleSheyholislami, H., & Connor, K. L. (2021). Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis. Nutrients, 13(7), 2382. https://doi.org/10.3390/nu13072382




