Lactobacillus plantarum HAC01 Supplementation Improves Glycemic Control in Prediabetic Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
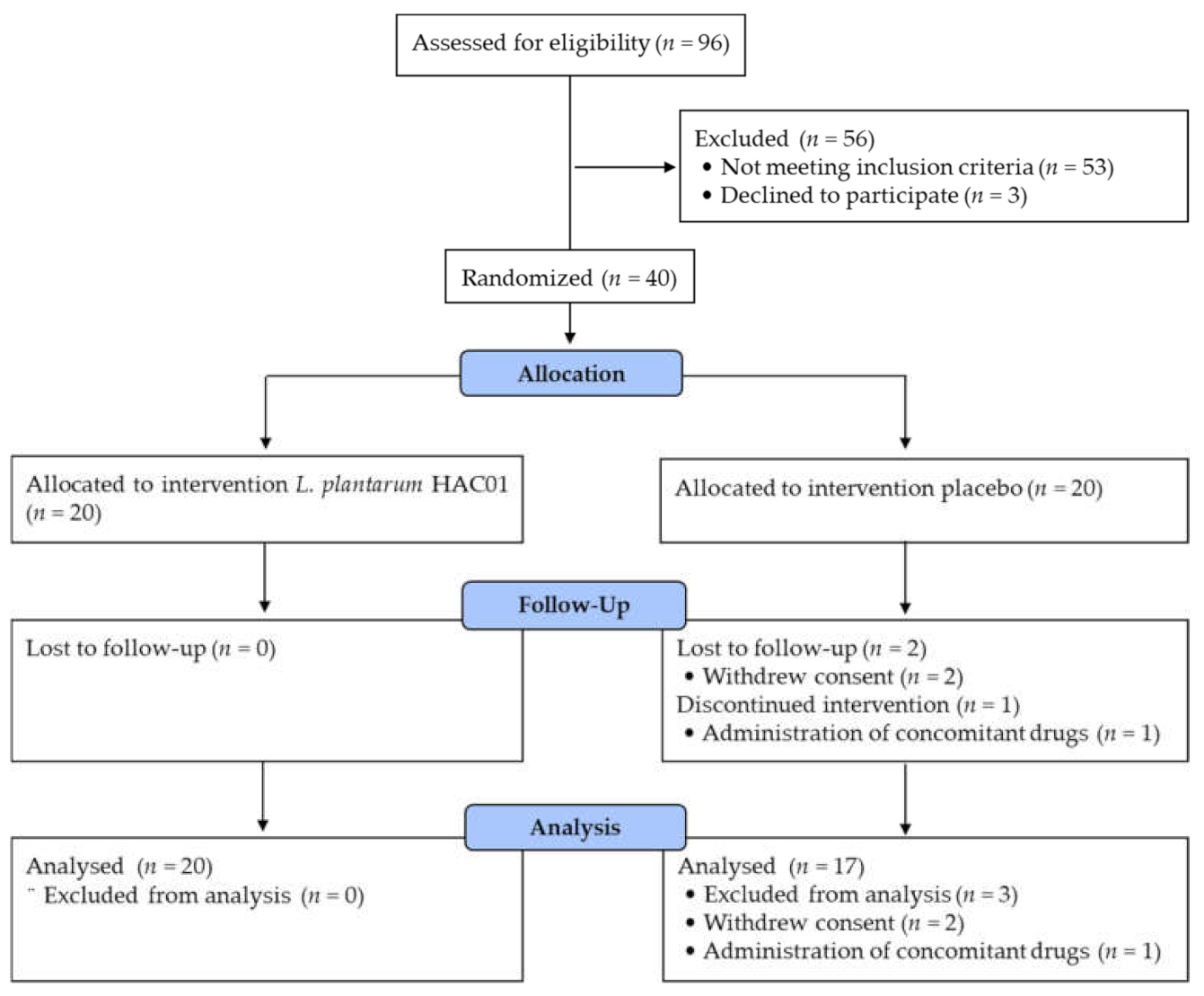
2. Materials and Methods
2.1. Ethics
2.2. Subjects
2.3. Study Design
2.4. Biochemical Measurements
2.5. Analysis of Fecal Microbiota
2.6. Analysis of Fecal Short-Chain Fatty Acid
2.7. Sample Size and Statistical Analysis
3. Results
3.1. Subjects Characteristics
3.2. Parameters of Glucose Metabolism
3.3. Lipid Profiles, Adiponectin, and Leptin
3.4. Fecal Microbiota Composition and SCFAs
3.5. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haddad, J.A.; Haddad, A.N. The past decade in type 2 diabetes and future challenges. Hormones 2018, 17, 451–459. [Google Scholar] [CrossRef]
- Thomas, R.L.; Halim, S.; Gurudas, S.; Sivaprasad, S.; Owens, D.R. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res. Clin. Pract. 2019, 157, 107840. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Wang, C.Y.; Neil, D.L.; Home, P. 2020 vision—An overview of prospects for diabetes management and prevention in the next decade. Diabetes Res. Clin. Pract. 2018, 143, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, T.; Molnar, B.; Nemeth, D.; Hegyi, P.; Szakacs, Z.; Balint, A.; Garami, A.; Soos, A.; Marta, K.; Solymar, M. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: A meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 11787. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Toshimitsu, T.; Gotou, A.; Sashihara, T.; Hachimura, S.; Shioya, N.; Suzuki, S.; Asami, Y. Effects of 12-week ingestion of yogurt containing Lactobacillus plantarum OLL2712 on glucose metabolism and chronic inflammation in prediabetic adults: A randomized placebo-controlled trial. Nutrients 2020, 12, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobini, R.; Tremaroli, V.; Stahlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Berteus Forslund, H.; Perkins, R.; Backhed, F.; et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Efficacy of UB0316, a multi-strain probiotic formulation in patients with type 2 diabetes mellitus: A double blind, randomized, placebo controlled study. PLoS ONE 2019, 14, e0225168. [Google Scholar] [CrossRef]
- Park, S.; Ji, Y.; Jung, H.Y.; Park, H.; Kang, J.; Choi, S.H.; Shin, H.; Hyun, C.K.; Kim, K.T.; Holzapfel, W.H. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, D.; Park, G.S.; Ko, S.H.; Park, J.; Lee, Y.K.; Kang, J. Lactobacillus plantarum HAC01 ameliorates type 2 diabetes in high-fat diet and streptozotocin-induced diabetic mice in association with modulating the gut microbiota. Food Funct. 2021, in press. [Google Scholar] [CrossRef]
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2010, 5, 169. [Google Scholar] [CrossRef] [Green Version]
- Asemi, Z.; Alizadeh, S.A.; Ahmad, K.; Goli, M.; Esmaillzadeh, A. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2016, 35, 819–825. [Google Scholar] [CrossRef]
- Oh, M.R.; Park, S.H.; Kim, S.Y.; Back, H.I.; Kim, M.G.; Jeon, J.Y.; Ha, K.C.; Na, W.T.; Cha, Y.S.; Park, B.H.; et al. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern Med. 2014, 14, 237. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
- Tuomilehto, J.; Lindstrom, J.; Eriksson, J.G.; Valle, T.T.; Hamalainen, H.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Gillies, C.L.; Abrams, K.R.; Lambert, P.C.; Cooper, N.J.; Sutton, A.J.; Hsu, R.T.; Khunti, K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: Systematic review and meta-analysis. BMJ 2007, 334, 299. [Google Scholar] [CrossRef] [Green Version]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Oh, M.R.; Jung, S.J.; Bae, E.J.; Park, B.H.; Chae, S.W. Clinical characteristics and associated risk factors of prediabetes in the southwestern region of Korea from 2010–2019. J. Clin. Med. 2020, 9, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faerch, K.; Borch-Johnsen, K.; Holst, J.J.; Vaag, A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: Does it matter for prevention and treatment of type 2 diabetes? Diabetologia 2009, 52, 1714–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perraudeau, F.; McMurdie, P.; Bullard, J.; Cheng, A.; Cutcliffe, C.; Deo, A.; Eid, J.; Gines, J.; Iyer, M.; Justice, N.; et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: A multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res. Care 2020, 8, e001319. [Google Scholar] [CrossRef]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M.Y. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2017, 56, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Khalili, L.; Alipour, B.; Asghari Jafar-Abadi, M.; Faraji, I.; Hassanalilou, T.; Mesgari Abbasi, M.; Vaghef-Mehrabany, E.; Alizadeh Sani, M. The effects of Lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-A levels in patients with type 2 diabetes mellitus: A randomized controlled trial. Iran. Biomed. J. 2019, 23, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, B.; Delgado, S.; Blanco-Miguez, A.; Lourenco, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [Green Version]
- Ott, S.J.; Musfeldt, M.; Timmis, K.N.; Hampe, J.; Wenderoth, D.F.; Schreiber, S. In vitro alterations of intestinal bacterial microbiota in fecal samples during storage. Diagn. Microbiol. Infect. Dis. 2004, 50, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Rochet, V.; Rigottier-Gois, L.; Rabot, S.; Dore, J. Validation of fluorescent in situ hybridization combined with flow cytometry for assessing interindividual variation in the composition of human fecal microflora during long-term storage of samples. J. Microbiol. Methods 2004, 59, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, S.C.; Northrop, W.; Melton, P.E.; Ellison, G.C.; Newsholme, P.; Mamotte, C.D.S. Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol. Res. 2020, 160, 105174. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oufir, L.E.; Barry, J.L.; Flourie, B.; Cherbut, C.; Cloarec, D.; Bornet, F.; Galmiche, J.P. Relationships between transit time in man and in vitro fermentation of dietary fiber by fecal bacteria. Eur. J. Clin. Nutr. 2000, 54, 603–609. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, J.R.; Fitch, M.D.; Mortensen, P.B.; Fleming, S.E. Absorption and metabolism of octanoate by the rat colon in vivo: Concentration dependency and influence of alternative fuels. Gut 2002, 51, 76–81. [Google Scholar] [CrossRef]
- Willing, B.P.; Dicksved, J.; Halfvarson, J.; Andersson, A.F.; Lucio, M.; Zheng, Z.; Jarnerot, G.; Tysk, C.; Jansson, J.K.; Engstrand, L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 2010, 139, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
| Placebo (n = 20) | L. plantarum HAC01 (n = 20) | p-Value | |
|---|---|---|---|
| Age, years | 53.55 ± 10.18 | 56.40 ± 11.57 | 0.413 |
| Sex, female, n (%) | 17 (85.0) | 14 (70.0) | 0.451 |
| Height, cm | 159.90 ± 6.07 | 161.60 ± 8.74 | 0.479 |
| Weight, kg | 63.99 ± 5.68 | 66.53 ± 14.09 | 0.462 |
| BMI, kg/m2 | 25.03 ± 1.92 | 25.25 ± 3.14 | 0.793 |
| WHR | |||
| Female | 0.91 ± 0.05 | 0.93 ± 0.04 | 0.149 |
| Male | 0.91 ± 0.03 | 0.95 ± 0.02 | 0.066 |
| Current smoker, n (%) | 2 (10.0) | 0 (0.0) | 0.487 |
| Alcohol consumption, unit/day | 6.81 ± 5.16 | 7.32 ± 12.02 | 0.922 |
| Physical activity, MET-min/week | 1940 (830–2720) | 980 (440–3060) | 0.762 |
| FPG, mg/dL | 101.65 ± 8.21 | 99.00 ± 5.90 | 0.249 |
| 2h-PPG, mg/dL | 161.95 ± 14.69 | 172.15 ± 19.09 | 0.066 |
| HbA1c, % | 5.94 ± 0.38 | 5.93 ± 0.33 | 0.930 |
| Placebo (n = 17) | L. plantarum HAC01 (n = 20) | p-Value (1) | ||
|---|---|---|---|---|
| FPG, mg/dL | Baseline | 101.65 ± 8.44 | 99.00 ± 5.90 | 0.271 |
| 8 weeks | 101.47 ± 10.36 | 97.45 ± 8.48 | 0.625 | |
| Change value | −0.18 ± 10.09 | −1.55 ± 5.89 | ||
| p-value (2) | 0.943 | 0.254 | ||
| 0.5h-PPG, mg/dL | Baseline | 172.71 ± 32.64 | 166.15 ± 22.77 | 0.478 |
| 8 weeks | 166.24 ± 25.36 | 160.25 ± 28.80 | 0.946 | |
| Change value | −6.47 ± 27.22 | −5.90 ± 23.69 | ||
| p-value (2) | 0.342 | 0.279 | ||
| 1h-PPG, mg/dL | Baseline | 194.18 ± 37.84 | 191.10 ± 25.91 | 0.772 |
| 8 weeks | 192.88 ± 23.51 | 187.10 ± 29.67 | 0.795 | |
| Change value | −1.29 ± 35.42 | −4.00 ± 27.28 | ||
| p-value (2) | 0.882 | 0.520 | ||
| 1.5h-PPG, mg/dL | Baseline | 181.12 ± 33.82 | 182.80 ± 27.59 | 0.869 |
| 8 weeks | 188.88 ± 32.46 | 185.00 ± 28.84 | 0.620 | |
| Change value | 7.76 ± 38.21 | 2.20 ± 29.48 | ||
| p-value (2) | 0.414 | 0.742 | ||
| 2h-PPG, mg/dL | Baseline | 163.12 ± 14.36 | 172.15 ± 19.09 | 0.118 |
| 8 weeks | 182.24 ± 30.08 | 170.30 ± 30.28 | 0.045 * | |
| Change value | 19.12 ± 35.06 | −1.85 ± 26.30 | ||
| p-value (2) | 0.039 * | 0.757 | ||
| iAUC0–2h, h·mg/dL | Baseline | 136.90 ± 40.97 | 139.81 ± 32.65 | 0.811 |
| 8 weeks | 141.99 ± 33.18 | 138.28 ± 40.47 | 0.621 | |
| Change value | 5.09 ± 43.32 | −1.53 ± 37.36 | ||
| p-value (2) | 0.635 | 0.857 | ||
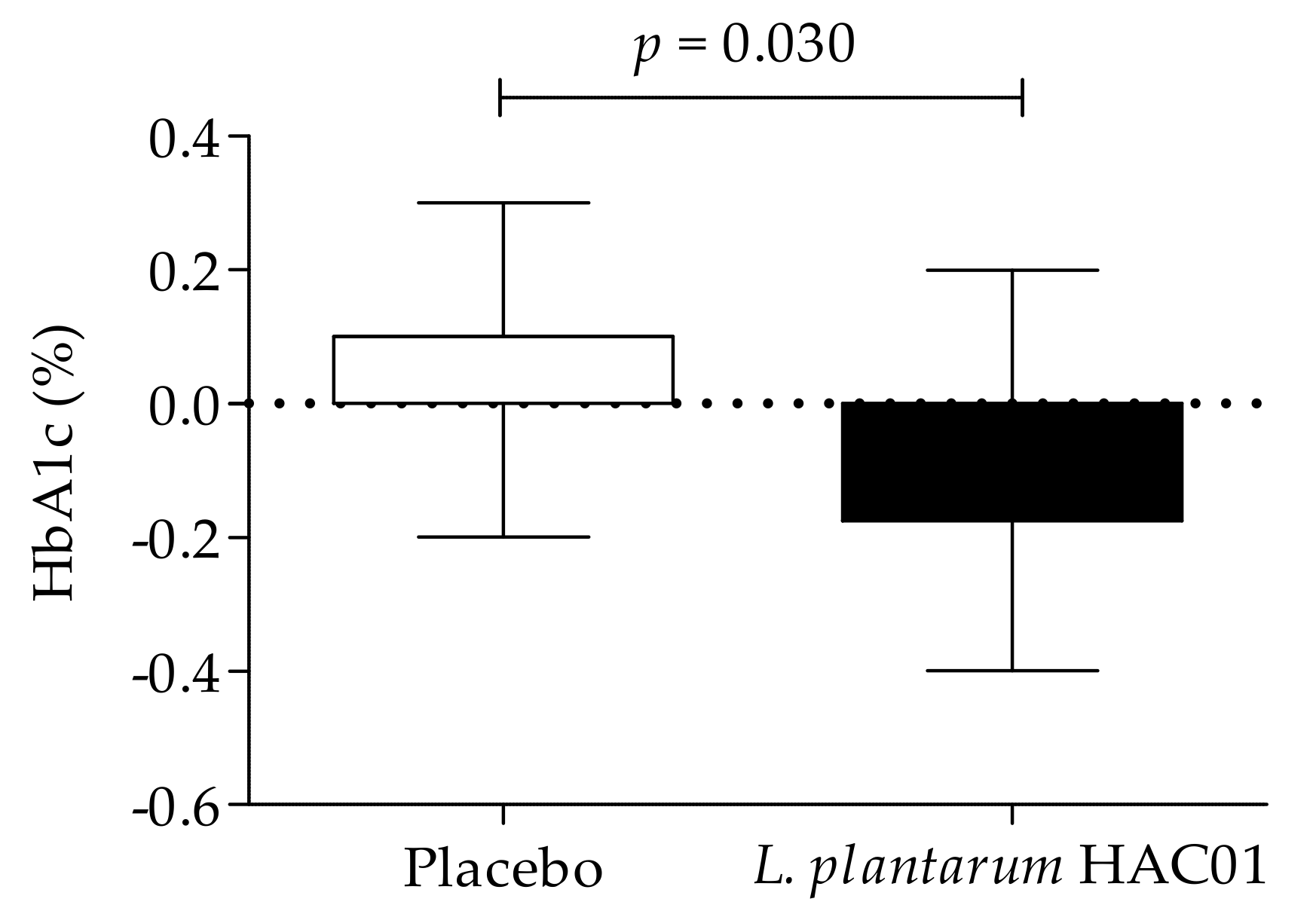
| HbA1c, % | Baseline | 5.94 ± 0.38 | 5.93 ± 0.33 | 0.931 |
| 8 weeks | 5.98 ± 0.37 | 5.84 ± 0.33 | 0.013 * | |
| Change value | 0.04 ± 0.14 | −0.09 ± 0.15 | ||
| p-value (2) | 0.248 | 0.020 * | ||
| Placebo (n = 17) | L. plantarum HAC01 (n = 20) | p-Value (1) | ||
|---|---|---|---|---|
| Insulin, μU/mL | Baseline | 11.54 ± 5.29 | 9.63 ± 4.24 | 0.230 |
| 8 weeks | 11.44 ± 6.17 | 9.53 ± 4.05 | 0.998 | |
| Change value | −0.10 ± 5.34 | −0.09 ± 3.06 | ||
| p-value (2) | 0.941 | 0.892 | ||
| HOMA-IR | Baseline | 2.93 ± 1.42 | 2.36 ± 1.06 | 0.166 |
| 8 weeks | 2.91 ± 1.60 | 2.35 ± 1.10 | 0.958 | |
| Change value | −0.02 ± 1.56 | 0.00 ± 0.86 | ||
| p-value (2) | 0.949 | 0.992 | ||
| QUICKI | Baseline | 0.33 ± 0.03 | 0.34 ± 0.03 | 0.268 |
| 8 weeks | 0.34 ± 0.04 | 0.34 ± 0.03 | 0.761 | |
| Change value | 0.01 ± 0.03 | 0.00 ± 0.02 | ||
| p-value (2) | 0.515 | 0.621 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, M.-R.; Jang, H.-Y.; Lee, S.-Y.; Jung, S.-J.; Chae, S.-W.; Lee, S.-O.; Park, B.-H. Lactobacillus plantarum HAC01 Supplementation Improves Glycemic Control in Prediabetic Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2337. https://doi.org/10.3390/nu13072337
Oh M-R, Jang H-Y, Lee S-Y, Jung S-J, Chae S-W, Lee S-O, Park B-H. Lactobacillus plantarum HAC01 Supplementation Improves Glycemic Control in Prediabetic Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2021; 13(7):2337. https://doi.org/10.3390/nu13072337
Chicago/Turabian StyleOh, Mi-Ra, Hui-Yeon Jang, Si-Yeon Lee, Su-Jin Jung, Soo-Wan Chae, Seung-Ok Lee, and Byung-Hyun Park. 2021. "Lactobacillus plantarum HAC01 Supplementation Improves Glycemic Control in Prediabetic Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 13, no. 7: 2337. https://doi.org/10.3390/nu13072337
APA StyleOh, M.-R., Jang, H.-Y., Lee, S.-Y., Jung, S.-J., Chae, S.-W., Lee, S.-O., & Park, B.-H. (2021). Lactobacillus plantarum HAC01 Supplementation Improves Glycemic Control in Prediabetic Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 13(7), 2337. https://doi.org/10.3390/nu13072337







