The Relationship of Tree Nuts and Peanuts with Adiposity Parameters: A Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Categorization of Available Evidence
2.4. Data Extraction and Quality Assessment
2.5. Grading the Quality of Evidence
2.6. Dealing with Missing Data
2.7. Data Synthesis
3. Results
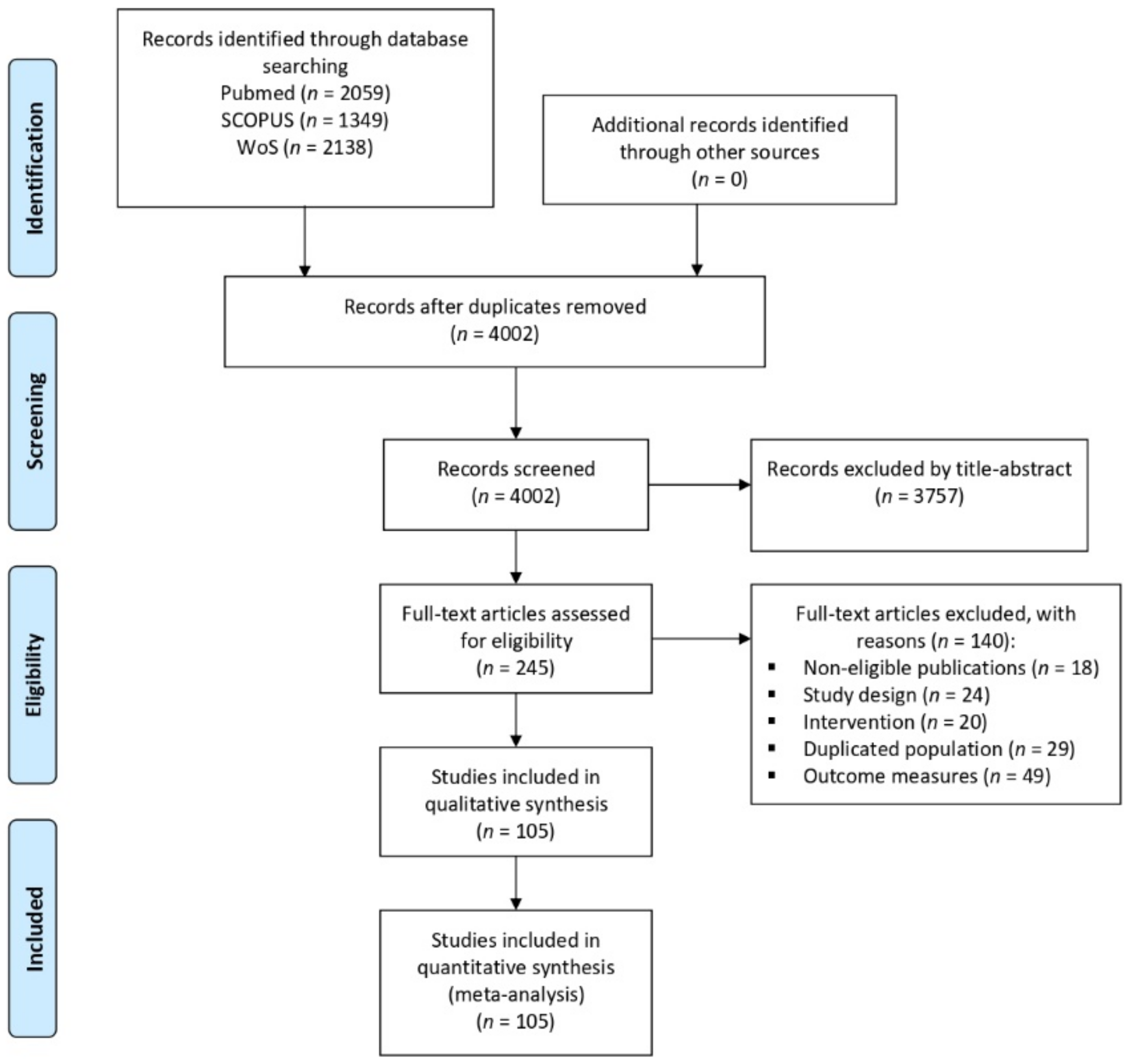
3.1. Results of the Literature Search
3.2. Study Characteristics
3.3. Assessment of Transitivity and Consistency
3.4. Risk of Bias
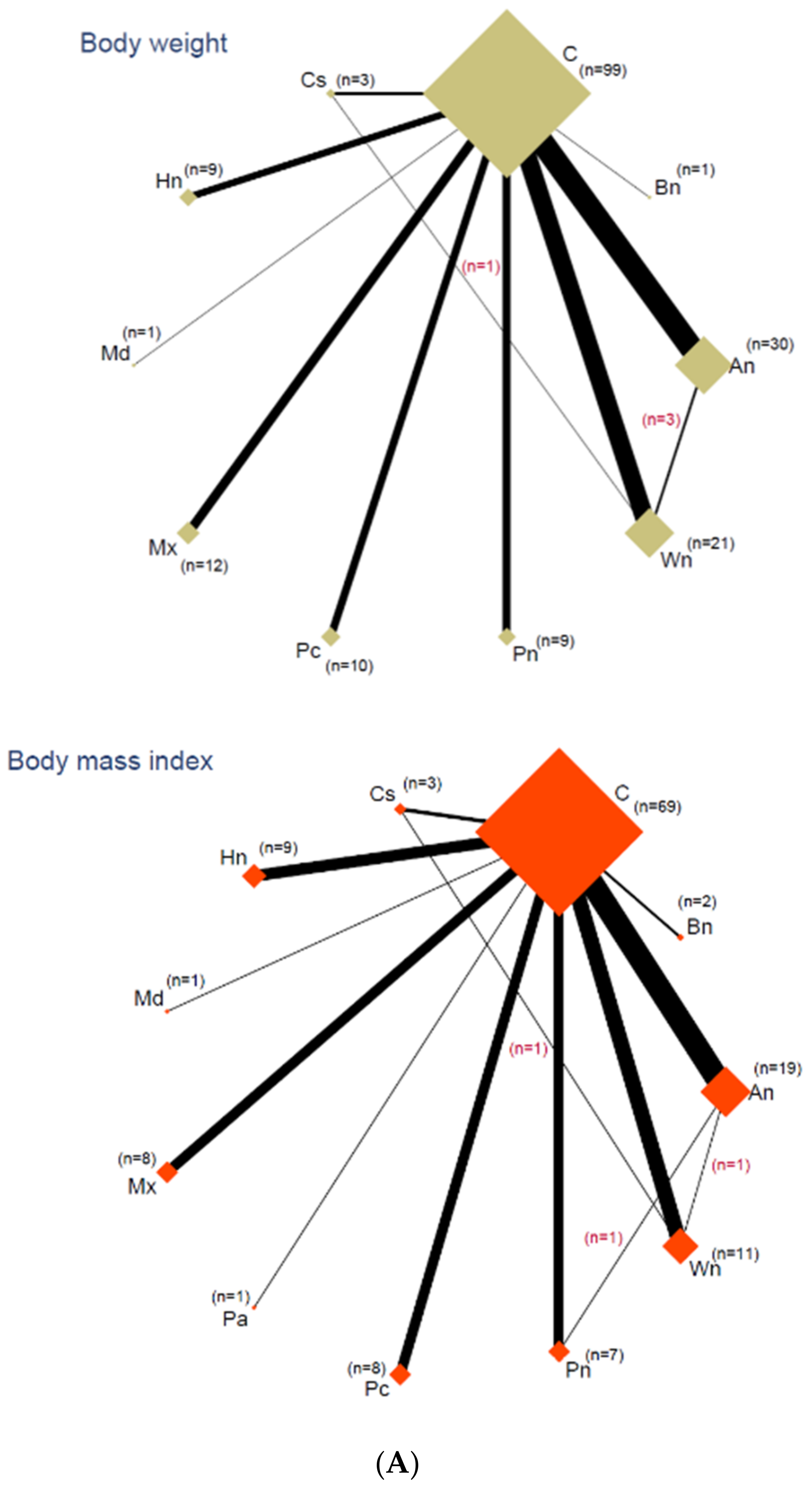
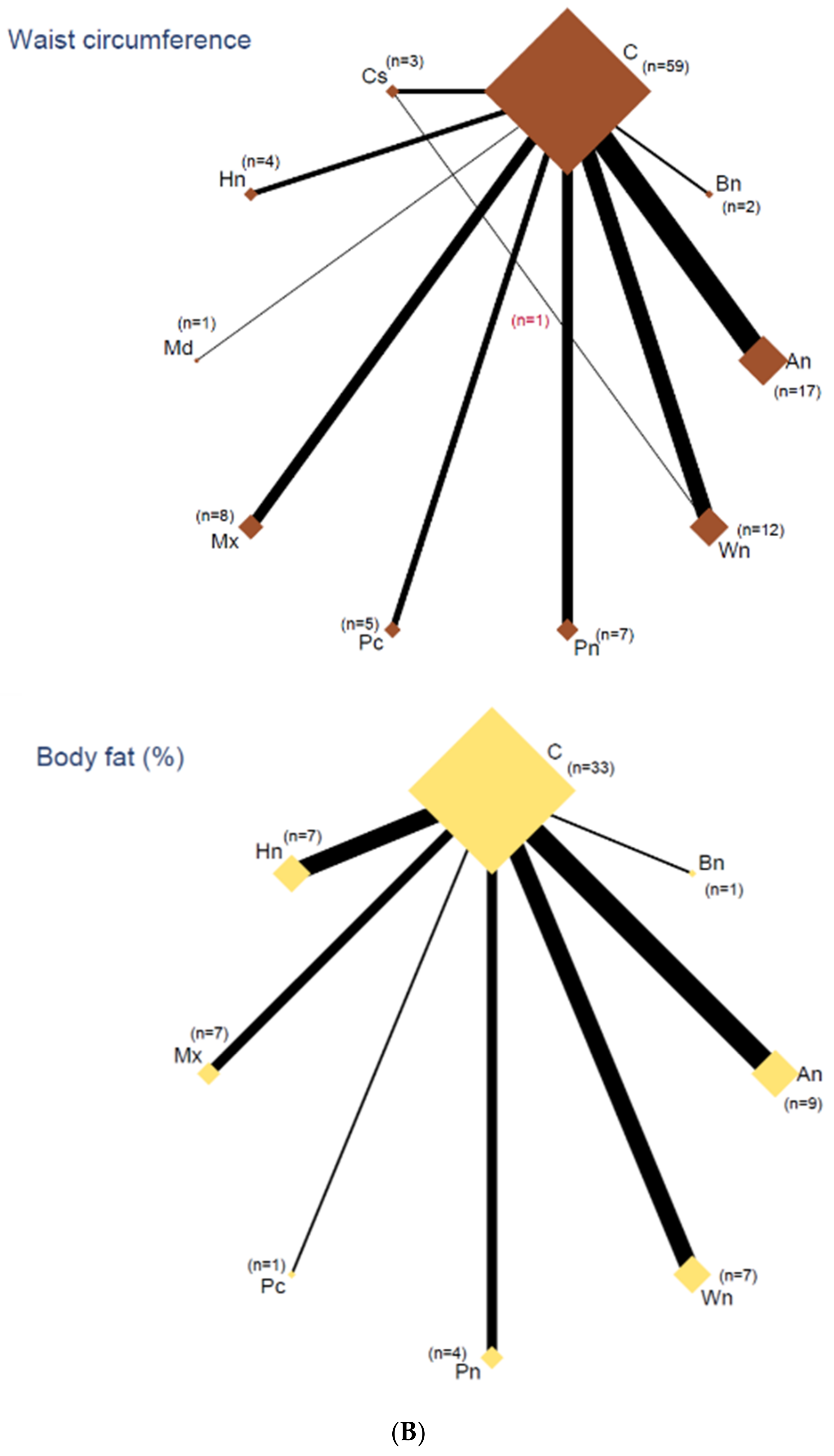
3.5. Network Analyses
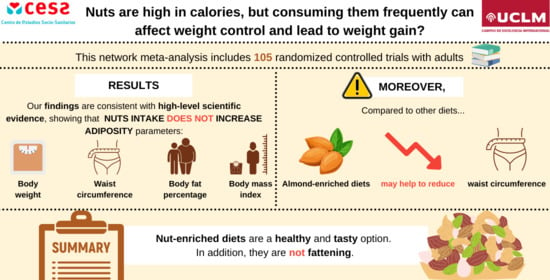
3.6. Outcomes
3.7. Clinical Percentage of Change
3.8. Quality of Evidence
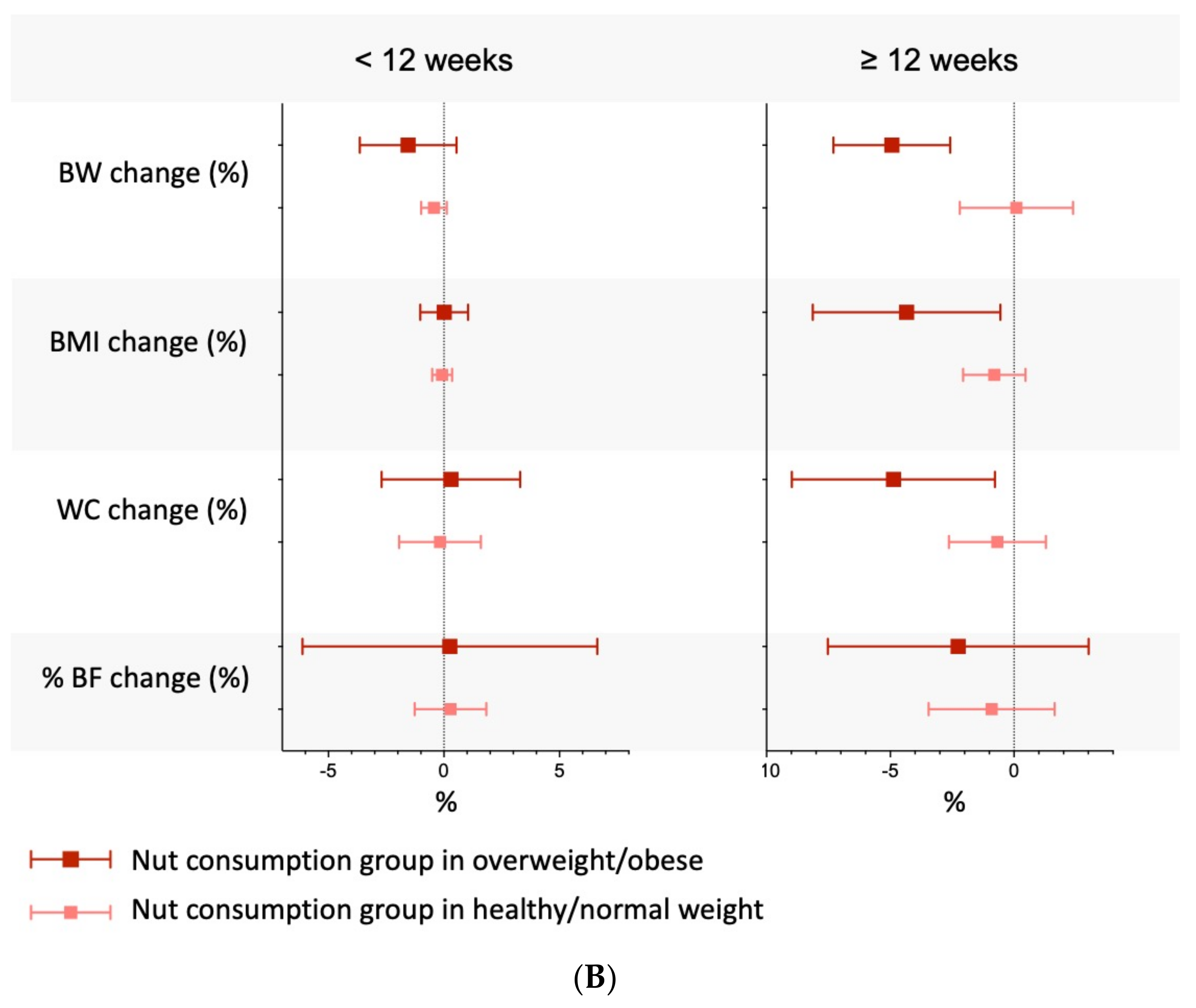
3.9. Subgroup Analyses, Meta-Regressions, Sensitivity Analyses, and Small-Study Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bentham, J.; Di Cesare, M.; Bilano, V. NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Wharton, S.; Lau, D.C.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boulé, N.; et al. Obesity in adults: A clinical practice guideline. Can. Med. Assoc. J. 2020, 192, E875–E891. [Google Scholar] [CrossRef] [PubMed]
- Canuto, R.; Garcez, A.; de Souza, R.V.; Kac, G.; Olinto, M.T.A. Nutritional intervention strategies for the management of overweight and obesity in primary health care: A systematic review with meta-analysis. Obes. Rev. 2021, 22, e13143. [Google Scholar] [CrossRef]
- Obesity National Task Force on the Prevention and Treatment of Obesity. Overweight, Obesity, and Health Risk. Arch. Intern. Med. 2000, 160, 898–904. [Google Scholar] [CrossRef]
- Chopra, S.; Malhotra, A.; Ranjan, P.; Vikram, N.K.; Sarkar, S.; Siddhu, A.; Kumari, A.; Kaloiya, G.S.; Kumar, A. Predictors of successful weight loss outcomes amongst individuals with obesity undergoing lifestyle interventions: A systematic review. Obes. Rev. 2021, 22, e13148. [Google Scholar] [CrossRef]
- Tobias, D.K.; Chen, M.; Manson, J.A.E.; Ludwig, D.S.; Willett, W.; Hu, F.B. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 968–979. [Google Scholar] [CrossRef] [Green Version]
- Dikariyanto, V.; Smith, L.; Francis, L.; Robertson, M.; Kusaslan, E.; O’Callaghan-Latham, M.; Palanche, C.; D’Annibale, M.; Christodoulou, D.; Basty, N.; et al. Snacking on whole almonds for 6 weeks improves endothelial function and lowers LDL cholesterol but does not affect liver fat and other cardiometabolic risk factors in healthy adults: The ATTIS study, a randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 1178–1189. [Google Scholar] [CrossRef]
- Carughi, A.; Bellisle, F.; Dougkas, A.; Giboreau, A.; Feeney, M.J.; Higgs, J. A randomized controlled pilot study to assess effects of a daily pistachio (pistacia vera) afternoon snack on next-meal energy intake, satiety, and anthropometry in french women. Nutrients 2019, 11, 767. [Google Scholar] [CrossRef] [Green Version]
- Bowen, J.; Luscombe-Marsh, N.D.; Stonehouse, W.; Tran, C.; Rogers, G.B.; Johnson, N.; Thompson, C.H.; Brinkworth, G.D. Effects of almond consumption on metabolic function and liver fat in overweight and obese adults with elevated fasting blood glucose: A randomised controlled trial. Clin. Nutr. ESPEN 2019, 30, 10–18. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salas-Salvadó, J.; Ros, E.; Sabaté, J. An overview. In Health Benefits of Nuts and Dried Fruits, 1st ed.; Taylor Francis: Abingdon, UK, 2020; pp. 1–9. [Google Scholar]
- Tan, S.Y.; Dhillon, J.; Mattes, R.D. A review of the effects of nuts on appetite, food intake, metabolism, and body weight. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 412–422. [Google Scholar] [CrossRef] [Green Version]
- Bolling, B.W.; Chen, C.Y.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [Green Version]
- Razquin, C.; Sanchez-Tainta, A.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Fito, M.; Ros, E.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; et al. Dietary energy density and body weight changes after 3 years in the PREDIMED study. Int. J. Food Sci. Nutr. 2017, 68, 865–872. [Google Scholar] [CrossRef]
- Konieczna, J.; Romaguera, D.; Pereira, V.; Fiol, M.; Razquin, C.; Estruch, R.; Asensio, E.M.; Babio, N.; Fitó, M.; Gómez-Gracia, E.; et al. Longitudinal association of changes in diet with changes in body weight and waist circumference in subjects at high cardiovascular risk: The PREDIMED trial. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 139. [Google Scholar] [CrossRef]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food Groups and Risk of Overweight, Obesity, and Weight Gain: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2019, 10, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Abazarfard, Z.; Salehi, M.; Keshavarzi, S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: A randomized controlled clinical trial. J. Res. Med. Sci. 2014, 19, 457–464. [Google Scholar]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between mediterranean dietary polyphenol intake and obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef] [Green Version]
- Bo, S.; Fadda, M.; Fedele, D.; Pellegrini, M.; Ghigo, E.; Pellegrini, N. A critical review on the role of food and nutrition in the energy balance. Nutrients 2020, 12, 1161. [Google Scholar] [CrossRef]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [Green Version]
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; ter Horst, K.W.; Serlie, M.J. The pathogenesis of obesity. Metabolism 2019, 92, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Mohammadifard, N.; Haghighatdoost, F.; Mansourian, M.; Hassannejhad, R.; Sadeghi, M.; Roohafza, H.; Sajjadi, F.; Maghroun, M.; Alikhasi, H.; Sarrafzadegan, N. Long-term association of nut consumption and cardiometabolic risk factors. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 972–982. [Google Scholar] [CrossRef]
- Eslampour, E.; Moodi, V.; Asbaghi, O.; Ghaedi, E.; Shirinbakhshmasoleh, M.; Hadi, A.; Miraghajani, M. The effect of almond intake on anthropometric indices: A systematic review and meta-analysis. Food Funct. 2020, 11, 7340–7355. [Google Scholar] [CrossRef]
- Fang, Z.; Dang, M.; Zhang, W.; Wang, Y.; Kord-Varkaneh, H.; Nazary-Vannani, A.; Santos, H.O.; Tan, S.C.; Clark, C.C.; Zanghelini, F.; et al. Effects of walnut intake on anthropometric characteristics: A systematic review and dose-response meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 50, 102395. [Google Scholar] [CrossRef]
- Jamshidi, S.; Moradi, Y.; Nameni, G.; Mohsenpour, M.A.; Vafa, M. Effects of cashew nut consumption on body composition and glycemic indices: A meta-analysis and systematic review of randomized controlled trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 605–613. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Yuan, S.; Jin, Y.; Lu, J. Nut consumption and risk of metabolic syndrome and overweight/obesity: A meta-analysis of prospective cohort studies and randomized trials. Nutr. Metab. 2018, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sugizaki, C.S.A.; Naves, M.M.V. Potential prebiotic properties of nuts and edible seeds and their relationship to obesity. Nutrients 2018, 10, 1654. [Google Scholar] [CrossRef] [Green Version]
- Rouse, B.; Chaimani, A.; Li, T. Network Meta-Analysis: An Introdcution for Clinicians. Intern. Emerg. Med. 2017, 12, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). The Cochrane Collaboration. 2011. Available online: www.cochrane-handbook.org (accessed on 20 January 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PloS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, I4898. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; Debeer, H. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Chaimani, A.; Caldwell, D.M.; Li, T.; Higgins, J.P.T.; Salanti, G. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. Chapter 11: Undertaking Network Meta-Analyses; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2021. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Keith, R.; Abrams, J.P.M. Bayesian Approaches to Clinical Trials and Health-Care Evaluation; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Nikolakopoulou, A.; Higgins, J.P.T.; Papakonstantinou, T.; Chaimani, A.; Del Giovane, C.; Egger, M.; Salanti, G. Cinema: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020, 17, e1003082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G.; Marinho, V.; Higgins, J.P.T. A case study of multiple-treatments meta-analysis demonstrates that covariates should be considered. J. Clin. Epidemiol. 2009, 62, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Sterne, J.A.C.; Higgins, J.P.T.; Egger, M. Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: A review. Res. Synth. Methods 2020, 12, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Bashan, I.; Bakman, M. The Effect of Daily Walnut Consumption on Dyslipidemia. J. Food Qual. 2018, 2018, 4731826. [Google Scholar] [CrossRef]
- Barbour, J.A.; Howe, P.R.C.; Buckley, J.D.; Bryan, J.; Coates, A.M. Effect of 12 weeks high oleic peanut consumption on cardio-metabolic risk factors and body composition. Nutrients 2015, 7, 7381–7398. [Google Scholar] [CrossRef] [Green Version]
- Alves, R.D.M.; Moreira, A.P.B.; Macedo, V.S.; Alfenas, R.D.C.G.; Bressan, J.; Mattes, R.; Costa, N.M.B. Regular intake of high-oleic peanuts improves fat oxidation and body composition in overweight/obese men pursuing a energy-restricted diet. Obesity 2014, 22, 1422–1429. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Pérez, J.; Villegas, A.S.; Díaz-Benítez, E.M.; Ruano-Rodríguez, C.; Corella, D.; Martínez-González, Á.N.; Estruch, R.; Salas-Salvadó, J.; Serra-Majem, L.; for the PREDIMED Study Investigators. Influence of a Mediterranean Dietary Pattern on Body Fat Distribution: Results of the PREDIMED–Canarias Intervention Randomized Trial. J. Am. Coll. Nutr. 2016, 35, 568–580. [Google Scholar] [CrossRef]
- Abbaspour, N.; Roberts, T.; Hooshmand, S.; Kern, M.; Hong, M.Y. Mixed nut consumption may improve cardiovascular disease risk factors in overweight and obese adults. Nutrients 2019, 11, 1488. [Google Scholar] [CrossRef] [Green Version]
- Zambón, D.; Sabaté, J.; Muñoz, S.; Campero, B.; Casals, E.; Merlos, M.; Laguna, J.C.; Ros, E. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann. Intern. Med. 2000, 132, 538–546. [Google Scholar] [CrossRef]
- Wien, M.; Oda, K.; Sabaté, J. A randomized controlled trial to evaluate the effect of incorporating peanuts into an American Diabetes Association meal plan on the nutrient profile of the total diet and cardiometabolic parameters of adults with type 2 diabetes. Nutr. J. 2014, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Wien, M.; Bleich, D.; Raghuwanshi, M.; Gould-Forgerite, S.; Gomes, J.; Monahan-Couch, L.; Oda, K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J. Am. Coll. Nutr. 2010, 29, 189–197. [Google Scholar] [CrossRef]
- Wien, M.A.; Sabaté, J.M.; Iklé, D.N.; Cole, S.E.; Kandeel, F.R. Almonds vs complex carbohydrates in a weight reduction program. Int. J. Obes. 2003, 27, 1365–1372. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Sun, L.; Liu, X.; Niu, Z.; Chen, S.; Tang, L.; Zheng, H.; Chen, X.; Li, H.; Lu, L.; et al. Replacing white rice bars with peanuts as snacks in the habitual diet improves metabolic syndrome risk among Chinese adults: A randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 28–35. [Google Scholar] [CrossRef]
- Tindall, A.M.; Petersen, K.S.; Skulas-Ray, A.C.; Richter, C.K.; Proctor, D.N.; Kris-Etherton, P.M. Replacing Saturated Fat With Walnuts or Vegetable Oils Improves Central Blood Pressure and Serum Lipids in Adults at Risk for Cardiovascular Disease: A Randomized Controlled-Feeding Trial. J. Am. Heart Assoc. 2019, 8, 9–12. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Batterham, M.; Teuss, G.; Tan, S.-Y.; Dalton, S.; Quick, C.J.; Gillen, L.J.; Charlton, K. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur. J. Clin. Nutr. 2009, 63, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Tapsell, L.C.; Gillen, L.J.; Patch, C.S.; Batterham, M.; Owen, A.; Baré, M.; Kennedy, M. Including walnuts in a low-fat/modified-faf diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004, 27, 2777–2783. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.Y.; Mattes, R.D. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: A randomized, controlled trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef] [Green Version]
- Spiller, G.A.; Gates, J.E.; Cragen, L.N.; Bruce, B.; Jenkins, D.A.J.; Bosello, O. Nuts and plasma lipids: An almond-based diet lowers ldl-c while preserving hdl-C. J. Am. Coll. Nutr. 1998, 17, 285–290. [Google Scholar] [CrossRef]
- Spaccarotella, K.J.S.; Kris-Etherton, P.M.; Stone, W.L.; Bagshaw, D.M.; Fishell, V.K.; West, S.G.; Lawrence, F.R.; Hartman, T.J. The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr. J. 2008, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Somerset, S.M.; Graham, L.; Markwell, K. Isoenergetic replacement of dietary saturated with monounsaturated fat via macadamia nuts enhances endothelial function in overweight subjects. ESPEN J. 2013, 8, e113–e119. [Google Scholar] [CrossRef]
- Sheridan, M.J.; Cooper, J.N.; Erario, M.; Cheifetz, C.E. Pistachio nut consumption and serum lipid levels. J. Am. Coll. Nutr. 2007, 26, 141–148. [Google Scholar] [CrossRef]
- Schutte, A.E.; Van Rooyen, J.M.; Huisman, H.W.; Mukuddem-Petersen, J.; Oosthuizen, W.; Hanekom, S.M.; Jerling, J. Modulation of Baroreflex Sensitivity by Walnuts Versus Cashew Nuts in Subjects with Metabolic Syndrome. Am. J. Hypertens. 2006, 19, 629–636. [Google Scholar] [CrossRef]
- Sabaté, J.; Haddad, E.; Tanzman, J.S.; Jambazian, P.; Rajaram, S. Serum lipid response to the graduated enrichment of a Step I diet with almonds: A randomized feeding trial. Am. J. Clin. Nutr. 2003, 77, 1379–1384. [Google Scholar] [CrossRef]
- Ruisinger, J.F.; Gibson, C.A.; Backes, J.M.; Smith, B.K.; Sullivan, D.K.; Moriarty, P.M.; Kris-Etherton, P. Statins and almonds to lower lipoproteins (the STALL study). J. Clin. Lipidol. 2015, 9, 58–64. [Google Scholar] [CrossRef]
- Ros, E.; Núñez, I.; Pérez-Heras, A.; Serra, M.; Gilabert, R.; Casals, E.; Deulofeu, R. A Walnut Diet Improves Endothelial Function in Hypercholesterolemic Subjects: A Randomized Crossover Trial. Circulation 2004, 109, 1609–1614. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An almond-based low carbohydrate diet improves depression and glycometabolism in patients with type 2 diabetes through modulating gut microbiota and glp-1: A randomized controlled trial. Nutrients 2020, 12, 3036. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Zunshine, E.; Nguyen, H.T.; Perez, A.O.; Zoumas, C.; Pakiz, B.; White, M.M. Effects of Pistachio Consumption in a Behavioral Weight Loss Intervention on Weight Change, Cardiometabolic Factors, and Dietary Intake. Nutrients 2020, 12, 2155. [Google Scholar] [CrossRef] [PubMed]
- Razquin, C.; Martinez, J.A.; Martinez-Gonzalez, M.A.; Fernández-Crehuet, J.; Santos, J.M.; Marti, A. A mediterranean diet rich in virgin olive oil may reverse the effects of the-174g/c il6 gene variant on 3-year body weight change. Mol. Nutr. Food Res. 2010, 54 (Suppl. 1), 75–82. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.R.; Tey, S.L.; Gray, A.R.; Chisholm, A.; Brown, R.C. Energy compensation and nutrient displacement following regular consumption of hazelnuts and other energy-dense snack foods in non-obese individuals. Eur. J. Nutr. 2017, 56, 1255–1267. [Google Scholar] [CrossRef]
- Parham, M.; Heidari, S.; Khorramirad, A.; Hozoori, M.; Hosseinzadeh, F.; Bakhtyari, L.; Vafaeimanesh, J. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: A randomized crossover trial. Rev. Diabet. Stud. 2014, 11, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Orem, A.; Yucesan, F.B.; Örem, C.; Akcan, B.; Kural, B.V.; Alasalvar, C.; Shahidi, F. Hazelnut-enriched diet improves cardiovascular risk biomarkers beyond a lipid-lowering effect in hypercholesterolemic subjects. J. Clin. Lipidol. 2013, 7, 123–131. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Granado-Lorencio, F.; Herrero-Barbudo, C.; Blanco-Navarro, I.; Blázquez-García, S.; Pérez-Sacristán, B. Consumption of Restructured Meat Products with Added Walnuts Has a Cholesterol-Lowering Effect in Subjects at High Cardiovascular Risk: A Randomised, Crossover, Placebo-Controlled Study. J. Am. Coll. Nutr. 2008, 27, 342–348. [Google Scholar] [CrossRef]
- O’Byrne, D.J.; Knauft, D.A.; Shireman, R.B. Low fat-monounsaturated rich diets containing high-oleic peanuts improve serum lipoprotein profiles. Lipids 1997, 32, 687–695. [Google Scholar] [CrossRef]
- Njike, V.Y.; Kavak, Y.; Treu, J.A.; Doughty, K.; Katz, D.L. Snacking, Satiety, and Weight: A Randomized, Controlled Trial. Am. J. Health Promot. 2017, 31, 296–301. [Google Scholar] [CrossRef]
- Mukuddem-Petersen, J.; Stonehouse Oosthuizen, W.; Jerling, J.C.; Hanekom, S.M.; White, Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: A controlled feeding trial. Br. J. Nutr. 2007, 97, 1144–1153. [Google Scholar] [CrossRef]
- Mohan, V.; Gayathri, R.; Jaacks, L.; Lakshmipriya, N.; Anjana, R.M.; Spiegelman, D.; Jeevan, R.G.; Balasubramaniam, K.K.; Shobana, S.; Jayanthan, M.; et al. Cashew nut consumption increases HDL cholesterol and reduces systolic blood pressure in Asian Indians with type 2 diabetes: A 12-week randomized controlled trial. J. Nutr. 2018, 148, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Morgan, W.; Beverly, J.; Clayshulte, M. Pecans lower low-density lipoprotein cholesterol in people with normal lipid levels. J. Am. Diet Assoc. 2000, 100, 312–318. [Google Scholar] [CrossRef]
- Mercanlıgil, S.M.; Arslan, P.; Alasalvar, C.; Okut, E.; Akgül, E.; Pınar, A.; Geyik, P.Ö.; Tokgözoğlu, L.; Shahidi, F. Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. Eur. J. Clin. Nutr. 2007, 61, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Njike, V.Y.; Millet, J.; Dutta, S.; Doughty, K.; Treu, J.A.; Katz, D. Effects of walnut consumption onendothelial function in type 2 diabetic subjects: A randomized controlled crossover trial. Diabetes Care 2010, 33, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Berryman, C.E.; West, S.G.; Chen, C.O.; Blumberg, J.B.; Lapsley, K.G.; Preston, A.G.; Fleming, J.A.; Kris-Etherton, P.M. Effects of dark chocolate and almonds on cardiovascular risk factors in overweight and obese individuals: A randomized controlled-feeding trial. J. Am. Heart Assoc. 2017, 6, e005162. [Google Scholar] [CrossRef] [Green Version]
- Le, T.; Flatt, S.W.; Natarajan, L.; Pakiz, B.; Quintana, E.L.; Heath, D.D.; Rana, B.K.; Rock, C.L. Effects of diet composition and insulin resistance status on plasma lipid levels in a weight loss intervention in women. J. Am. Heart Assoc. 2016, 5, e002771. [Google Scholar] [CrossRef] [Green Version]
- Kaseb, F.; Rashidi, M.; Afkhami-Ardekani, M.; Fallahzadeh, H. Effect of olive, almond and walnut oil on cardiovascular risk factors in type 2 diabetic patients. Int. J. Diabetes Dev. Ctries. 2013, 33, 115–119. [Google Scholar] [CrossRef]
- Lamarche, B.; Desroches, S.; Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A.; Faulkner, D.; Vidgen, E.; Lapsley, K.G.; Trautwein, E.A.; Parker, T.L.; et al. Combined effects of a dietary portfolio of plant sterols, vegetable protein, viscous fibre and almonds on LDL particle size. Br. J. Nutr. 2004, 92, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Li, S.C.; Liu, Y.H.; Liu, J.F.; Chang, W.H.; Chen, C.M.; Chen, C.Y.O. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, R.; Nguyen, C.; Zerlin, A.; Karp, H.; Naowamondhol, K.; Thames, G.; Gao, K.; Li, L.; Tseng, C.-H.; et al. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J. Am. Coll. Nutr. 2010, 29, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Koylu, A.A.; Keles, H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Trier, C.M.; Fleming, K.R. The effect of peanut and grain bar preloads on postmeal satiety, glycemia, and weight loss in healthy individuals: An acute and a chronic randomized intervention trial. Nutr. J. 2013, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Banach, M.S.; Srichaikul, K.; Vidgen, E.; Mitchell, S.; Parker, T.; Nishi, S.; Bashyam, B.; De Souza, R.; et al. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 2011, 34, 1706–1711. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Parker, T.L.; Connelly, P.W.; Qian, W.; Haight, J.S.; Faulkner, D.; Vidgen, E.; Lapsley, K.G.; et al. Dose response of almonds on coronary heart disease risk factors: Blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: A randomized, controlled, crossover trial. Circulation 2002, 106, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Popovich, D.G.; Kendall, C.W.; Vidgen, E.; Tariq, N.; Ransom, T.P.; Wolever, T.M.; Vuksan, V.; Mehling, C.C.; Boctor, D.L.; et al. Effect of a diet high in vegetables, fruit, and nuts on serum lipids. Metabolism 1997, 46, 530–537. [Google Scholar] [CrossRef]
- Hwang, H.J.; Liu, Y.; Kim, H.S.; Lee, H.; Lim, Y.; Park, H. Daily walnut intake improves metabolic syndrome status and increases circulating adiponectin levels: Randomized controlled crossover trial. Nutr. Res. Pract. 2019, 13, 105–114. [Google Scholar] [CrossRef]
- Gebauer, S.K.; West, S.G.; Kay, C.D.; Alaupovic, P.; Bagshaw, D.; Kris-Etherton, P.M. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: A dose-response study. Am. J. Clin. Nutr. 2008, 88, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Foster, G.D.; Shantz, K.L.; Veur, S.S.V.; Oliver, T.L.; Lent, M.R.; Virus, A.; Szapary, P.O.; Rader, D.J.; Zemel, B.S.; Gilden-Tsai, A. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am. J. Clin. Nutr. 2012, 96, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, J.; Thorwald, M.; De La Cruz, N.; Vu, E.; Asghar, S.A.; Kuse, Q.; Rios, L.K.D.; Ortiz, R.M. Glucoregulatory and cardiometabolic profiles of almond vs. Cracker snacking for 8 weeks in young adults: A randomized controlled trial. Nutrients 2018, 10, 960. [Google Scholar] [CrossRef] [Green Version]
- Damavandi, R.D.; Eghtesadi, S.; Shidfar, F.; Heydari, I.; Foroushani, A.R. Effects of hazelnuts consumption on fasting blood sugar and lipoproteins in patients with type 2 diabetes. J. Res. Med. Sci. 2013, 18, 314–321. [Google Scholar]
- Silva, L.M.C.E.; Melo, M.L.; Reis, F.V.F.; Monteiro, M.C.; Santos, S.M.D.; Gomes, B.A.Q.; Da Silva, L.H.M. Comparison of the effects of Brazil nut oil and soybean oil on the cardiometabolic parameters of patients with metabolic syndrome: A randomized trial. Nutrients 2020, 12, 46. [Google Scholar] [CrossRef] [Green Version]
- Claesson, A.L.; Holm, G.; Ernersson, Å.; Lindström, T.; Nystrom, F.H. Two weeks of overfeeding with candy, but not peanuts, increases insulin levels and body weight. Scand. J. Clin. Lab. Investig. 2009, 69, 598–605. [Google Scholar] [CrossRef]
- Chisholm, A.; Mc Auley, K.; Mann, J.; Williams, S.; Skeaff, M. Cholesterol lowering effects of nuts compared with a Canola oil enriched cereal of similar fat composition. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 284–292. [Google Scholar] [CrossRef]
- Casas-Agustench, P.; López-Uriarte, P.; Bulló, M.; Ros, E.; Cabré-Vila, J.J.; Salas-Salvadó, J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 126–135. [Google Scholar] [CrossRef]
- Canales, A.; Benedí, J.; Nus, M.; Librelotto, J.; Sánchez-Montero, J.M.; Sánchez-Muniz, F.J. Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra chd-risk factor. J. Am. Coll. Nutr. 2007, 26, 225–232. [Google Scholar] [CrossRef]
- Chen, C.-Y.O.; Liu, J.-F.; Li, S.-C.; Huang, C.-L.; Hsirh, A.-T.; Weng, S.-F.; Chang, M.-L.; Li, H.-T.; Mohn, E. Almonds ameliorate glycemic control in Chinese patients with better controlled type 2 diabetes: A randomized, crossover, controlled feeding trial. Nutr. Metab. 2017, 14, 51. [Google Scholar] [CrossRef]
- Kalgaonkar, S.; Almario, R.U.; Gurusinghe, D.; Garamendi, E.M.; Buchan, W.; Kim, K.; Karakas, S.E. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur. J. Clin. Nutr. 2011, 65, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Chen, C.Y.O.; Blumberg, J.B.; Kwak, H.K. The effect of almonds on vitamin E status and cardiovascular risk factors in Korean adults: A randomized clinical trial. Eur. J. Nutr. 2018, 57, 2069–2079. [Google Scholar] [CrossRef] [Green Version]
- Bullã, M.; Amigã-Correig, P.; Mãrquez-Sandoval, F.; Babio, N.; Martã nez-Gonzãlez, M.A.; Estruch, R.; Basora, J.; Solà, R.; Salas-Salvadã, J.; Amigó-Correig, P.; et al. Mediterranean diet and high dietary acid load associated with mixed nuts: Effect on bone metabolism in elderly subjects. J. Am. Geriatr. Soc. 2009, 57, 1789–1798. [Google Scholar] [CrossRef]
- Hou, Y.-Y.; Ojo, O.; Wang, L.-L.; Wang, Q.; Jiang, Q.; Shao, X.-Y.; Wang, X.-H. A randomized controlled trial to compare the effect of peanuts and almonds on the cardio-metabolic and inflammatory parameters in patients with type 2 diabetes mellitus. Nutrients 2018, 10, 1565. [Google Scholar] [CrossRef] [Green Version]
- Hollis, J.; Mattes, R. Effect of chronic consumption of almonds on body weight in healthy humans. Br. J. Nutr. 2007, 98, 651–656. [Google Scholar] [CrossRef]
- Damasceno, N.R.; Sala-Vila, A.; Cofan, M.; Pérez-Heras, A.M.; Fitó, M.; Ruiz-Gutiérrez, V.; Martínez-González, Á.M.; Corella, D.; Arós, F.; Estruch, R.; et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis 2013, 230, 347–353. [Google Scholar] [CrossRef]
- Dhillon, J.; Tan, S.Y.; Mattes, R.D. Almond consumption during energy restriction lowers truncal fat and blood pressure in compliant overweight or obese adults. J. Nutr. 2016, 146, 2513–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damasceno, N.; Pérez-Heras, A.; Serra, M.; Cofan, M.; Sala-Vila, A.; Salas-Salvadó, J.; Ros, E. Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. 1), 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.P.N.; Cominetti, C.; Simões Filho, A.; Naves, M.M.V. Baru almond improves lipid profile in mildly hypercholesterolemic subjects: A randomized, controlled, crossover study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T.; Bergeron, N.; Chiu, S.; Krauss, R.M. A randomized, controlled trial on the effects of almonds on lipoprotein response to a higher carbohydrate, lower fat diet in men and women with abdominal adiposity. Lipids Health Dis. 2019, 18, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Pan, A.; Yu, Z.; Qi, Q.; Lu, L.; Zhang, G.; Yu, D.; Zong, G.; Zhou, Y.; Chen, X.; et al. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J. Nutr. 2010, 140, 1937–1942. [Google Scholar] [CrossRef] [Green Version]
- Nezhad, M.J.Z.; Aghasadeghi, K.; Hakimi, H.; Yarmohammadi, H.; Nikaein, F. The effect of walnut oil consumption on blood sugar in patients with diabetes mellitus type 2. Int. J. Endocrinol. Metab. 2016, 14, e34889. [Google Scholar] [CrossRef] [Green Version]
- Caldas, A.P.S.; Alves, R.D.M.; Hermsdorff, H.H.M.; De Oliveira, L.L.; Bressan, J. Effects of high-oleic peanuts within a hypoenergetic diet on inflammatory and oxidative status of overweight men: A randomised controlled trial. Br. J. Nutr. 2020, 123, 673–680. [Google Scholar] [CrossRef]
- Coates, A.M.; Morgillo, S.; Yandell, C.; Scholey, A.; Buckley, J.D.; Dyer, K.A.; Hill, A. Effect of a 12-Week Almond-Enriched Diet on Biomarkers of Cognitive Performance, Mood, and Cardiometabolic Health in Older Overweight Adults. Nutrients 2020, 12, 1180. [Google Scholar] [CrossRef] [Green Version]
- Chiang, Y.L.; Haddad, E.; Rajaram, S.; Shavlik, D.; Sabaté, J. The effect of dietary walnuts compared to fatty fish on eicosanoids, cytokines, soluble endothelial adhesion molecules and lymphocyte subsets: A randomized, controlled crossover trial. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 111–117. [Google Scholar] [CrossRef]
- Abdrabalnabi, A.A.; Rajaram, S.; Bitok, E.; Oda, K.; Beeson, W.L.; Kaur, A.; Cofán, M.; Serra-Mir, M.; Roth, I.; Ros, E.; et al. Effects of supplementing the usual diet with a daily dose of walnuts for two years on metabolic syndrome and its components in an elderly cohort. Nutrients 2020, 12, 451. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, H.Ö.; Özyildirim, B. Evaluation of The Effects of Raisins and Hazelnuts Added To the Diet on Lipid Profiles and Anthropometric Measurements in Women with Hyperlipidemia. Bezmialem Sci. 2019, 7, 294–306. [Google Scholar] [CrossRef]
- Tey, S.L.; Gray, A.A.; Chisholm, A.A.; Delahunty, C.C.; Brown, R.R. The dose of hazelnuts influences acceptance and diet quality but not inflammatory markers and body composition in overweight and obese individuals. J. Nutr. 2013, 143, 1254–1262. [Google Scholar] [CrossRef] [Green Version]
- Berryman, C.E.; West, S.G.; Fleming, J.A.; Bordi, P.L.; Kris-Etherton, P.M. Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL-cholesterol: A randomized controlled trial. J. Am. Heart Assoc. 2015, 4, e000993. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Nam, G.E.; Seo, J.A.; Yoon, T.; Seo, I.; Lee, J.H.; Im, D.; Bahn, K.-N.; Jeong, S.A.; Kang, T.S.; et al. Nut consumption has favorable effects on lipid profiles of Korean women with metabolic syndrome. Nutr. Res. 2014, 34, 814–820. [Google Scholar] [CrossRef]
- Kasliwal, R.R.; Bansal, M.; Mehrotra, R.; Yeptho, K.P.; Trehan, N. Effect of pistachio nut consumption on endothelial function and arterial stiffness. Nutrition 2015, 31, 678–685. [Google Scholar] [CrossRef]
- Sauder, K.A.; McCrea, C.E.; Ulbrecht, J.S.; Kris-Etherton, P.M.; West, S.G. Effects of pistachios on the lipid/lipoprotein profile, glycemic control, inflammation, and endothelial function in type 2 diabetes: A randomized trial. Metabolism 2015, 64, 1521–1529. [Google Scholar] [CrossRef] [Green Version]
- Njike, V.Y.; Ayettey, R.; Petraro, P.; Treu, J.A.; Katz, D.L. Walnut ingestion in adults at risk for diabetes: Effects on body composition, diet quality, and cardiac risk measures. BMJ Open Diabetes Res. Care 2015, 3, e000115. [Google Scholar] [CrossRef] [Green Version]
- Maranhão, P.A.; Kraemer-Aguiar, L.G.; De Oliveira, C.L.; Kuschnir, M.C.; Vieira, Y.R.; Souza, M.G.; Koury, J.C.; Bouskela, E. Brazil nuts intake improves lipid profile, oxidative stress and microvascular function in obese adolescents: A randomized controlled trial. Nutr. Metab. 2011, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Katz, D.L.; Davidhi, A.; Ma, Y.; Kavak, Y.; Bifulco, L.; Njike, V.Y. Effects of walnuts on endothelial function in overweight adults with visceral obesity: A randomized, controlled, crossover trial. J. Am. Coll. Nutr. 2012, 31, 415–423. [Google Scholar] [CrossRef]
- Nouran, M.G.; Kimiagar, M.; Abadi, A.; Mirzazadeh, M.; Harrison, G. Peanut consumption and cardiovascular risk. Public Health Nutr. 2010, 13, 1581–1586. [Google Scholar] [CrossRef] [Green Version]
- Sabaté, J.; Cordero-MacIntyre, Z.; Siapco, G.; Torabian, S.; Haddad, E. Does regular walnut consumption lead to weight gain? Br. J. Nutr. 2005, 94, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Rock, C.L.; Flatt, S.W.; Barkai, H.S.; Pakiz, B.; Heath, D.D. Walnut consumption in a weight reduction intervention: Effects on body weight, biological measures, blood pressure and satiety. Nutr. J. 2017, 16, 76. [Google Scholar] [CrossRef] [Green Version]
- De Souza, R.G.M.; Gomes, A.C.; de Castro, I.A.; Mota, J.F. A baru almond–enriched diet reduces abdominal adiposity and improves high-density lipoprotein concentrations: A randomized, placebo-controlled trial. Nutrition 2018, 55, 154–160. [Google Scholar] [CrossRef]
- Fatahi, S.; Haghighatdoost, F.; Larijani, B.; Azadbakht, L. Effect of Weight Reduction Diets Containing Fish, Walnut or Fish plus Walnut on Cardiovascular Risk Factors in Overweight and Obese Women. Arch. Iran. Med. 2019, 22, 574–583. [Google Scholar]
- Domènech, M.; Serra-Mir, M.; Roth, I.; Freitas-Simoes, T.; Valls-Pedret, C.; Cofán, M.; López, A.; Sala-Vila, A.; Calvo, C.; Rajaram, S.; et al. Effect of a walnut diet on office and 24-h ambulatory blood pressure in elderly individuals: Findings from the WAHA randomized trial. Hypertension 2019, 73, 1049–1057. [Google Scholar] [CrossRef]
- Gozde, O.; Mercanligil, S.M.; Kabaran, S.; Ogmen, S. Effects of walnut-enriched diet on blood lipids and glucose profiles in hyperlipidemic subjects: A randomized controlled trial. Prog. Nutr. 2019, 21, 825–835. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; Lapetra, J.; et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2016, 4, 666–676. [Google Scholar] [CrossRef]
- Fantino, M.; Bichard, C.; Mistretta, F.; Bellisle, F. Daily consumption of pistachios over 12 weeks improves dietary profile without increasing body weight in healthy women: A randomized controlled intervention. Appetite 2020, 144, 104483. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M.; Bhatt, S.P.; Saluja, S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: A 24-wk, randomized control trial. Nutrition 2014, 30, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Sultan, F.A.T.; Iqbal, R.; Gilani, A.H. Dietary Almonds Increase Serum HDL Cholesterol in Coronary Artery Disease Patients in a Randomized Controlled Trial1-3. J. Nutr. 2015, 145, 2287–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Baldrich-Mora, M.; Juanola-Falgarona, M.; Bulló, M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: A randomized clinical trial. Diabetes Care 2014, 37, 3098–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapsell, L.C.; Lonergan, M.; Batterham, M.J.; Neale, E.; Martin, A.; Thorne, R.; Deane, F.; Peoples, G. Effect of interdisciplinary care on weight loss: A randomised controlled trial. BMJ Open 2017, 7, e014533. [Google Scholar] [CrossRef]
- Damavandi, R.D.; Mousavi, S.N.; Shidfar, F.; Mohammadi, V.; Rajab, A.; Hosseini, S.; Heshmati, J. Effects of Daily Consumption of Cashews on Oxidative Stress and Atherogenic Indices in Patients with Type 2 Diabetes: A Randomized, Controlled-Feeding Trial. Int. J. Endocrinol. Metab. 2019, 17, e70744. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvadó, J.; Fernández-Ballart, J.; Rosa, L.-R.; Martínez-González, M.-A.; Fitó, M.; Estruch, R.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the PREDIMED randomized trial. Arch. Intern. Med. 2008, 168, 2449–2458. [Google Scholar] [CrossRef] [Green Version]
- Bitok, E.; Rajaram, S.; Jaceldo-Siegl, K.; Oda, K.; Sala-Vila, A.; Serra-Mir, M.; Ros, E.; Sabaté, J. Effects of long-term walnut supplementation on body weight in free-living elderly: Results of a randomized controlled trial. Nutrients 2018, 10, 1317. [Google Scholar] [CrossRef] [Green Version]
- Casas, R.; Sacanella, E.; Urpi, M.; Corella, D.; Castañer, O.; Lamuela-Raventos, R.-M.; Salas-Salvadó, J.; Martínez-González, M.-A.; Ros, E.; Estruch, R. Long-term immunomodulatory effects of a mediterranean diet in adults at high risk of cardiovascular disease in the prevención con dieta mediterŕanea (predimed) randomized controlled trial. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef]
- Arab, L.; Guo, R.; Elashoff, D. Lower Depression Scores among Walnut Consumers in NHANES. Nutrients 2019, 11, 275. [Google Scholar] [CrossRef] [Green Version]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, I.; et al. Almonds (Prunus Dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef] [Green Version]
- Trombetta, D.; Smeriglio, A.; Denaro, M.; Zagami, R.; Tomassetti, M.; Pilolli, R.; De Angelis, E.; Monaci, L.; Mandalari, G. Understanding the Fate of Almond (Prunus dulcis (Mill.) D.A. Webb) Oleosomes during Simulated Digestion. Nutrients 2020, 12, 3397. [Google Scholar] [CrossRef]
- Atzeni, A.; Galié, S.; Muralidharan, J.; Babio, N.; Tinahones, F.; Vioque, J.; Corella, D.; Castañer, O.; Vidal, J.; Moreno-Indias, I.; et al. Gut microbiota profile and changes in body weight in elderly subjects with overweight/obesity and metabolic syndrome. Microorganisms 2021, 9, 346. [Google Scholar] [CrossRef]
- Julibert, A.; Bibiloni, M.D.M.; Gallardo-Alfaro, L.; Abbate, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Martínez, J.A.; Alonso-Gómez, Á.M.; et al. Metabolic Syndrome Features and Excess Weight Were Inversely Associated with Nut Consumption after 1-Year Follow-Up in the PREDIMED-Plus Study. J. Nutr. 2020, 150, 3161–3170. [Google Scholar] [CrossRef]
- Neale, E.P.; Tran, G.; Brown, R.C. Barriers and facilitators to nut consumption: A narrative review. Int. J. Environ. Res. Public Health 2020, 17, 9127. [Google Scholar] [CrossRef]

| Control | Walnuts | Almonds | Mixed | Pistachios | Peanuts | Hazelnuts | Cashews | Brazil Nuts | Macadamia | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.03 (−0.05 to 0.11) | −0.07 (−0.16 to 0.02) | 0.02 (−0.03 to 0.07) | <0.01 (−0.15 to 0.16) | 0.15 (−0.03 to 0.33) | 0.07 (−0.12 to 0.25) | 0.03 (−0.18 to 0.24) | −0.07 (−1.01 to 0.58) | −0.12 (−0.47 to 0.23) | |
| Walnuts | 0.01 (−0.08 to 0.09) | <−0.01 (−0.42 to 0.41) | NA | NA | NA | NA | 0.03 (−0.58 to 0.64) | NA | NA | |
| Almonds | −0.06 (−0.15 to 0.02) | −0.07 (−0.19 to 0.05) | NA | NA | NA | NA | NA | NA | NA | |
| Mixed | 0.02 (−0.02 to 0.07) | 0.02 (−0.08 to 0.11) | 0.09 (−0.01 to 0.19) | NA | NA | NA | NA | NA | NA | |
| Pistachios | <0.01 (−0.16 to 0.17) | <−0.01 (−0.18 to 0.19) | 0.07 (−0.11 to 0.26) | −0.01 (−0.19 to 0.16) | NA | NA | NA | NA | NA | |
| Peanuts | 0.05 (−0.11 to 0.20) | 0.04 (−0.14 to 0.21) | 0.11 (−0.07 to 0.29) | 0.02 (−0.14 to 0.19) | 0.04 (−0.19 to 0.26) | NA | NA | NA | NA | |
| Hazelnuts | 0.10 (−0.08 to 0.28) | 0.09 (−0.11 to 0.29) | 0.16 (−0.04 to 0.36) | 0.07 (−0.11 to 0.26) | 0.09 (−0.15 to 0.33) | 0.05 (−0.18 to 0.29) | NA | NA | NA | |
| Cashews | 0.04 (−0.16 to 0.24) | 0.03 (−0.18 to 0.24) | 0.10 (−0.11 to 0.32) | 0.02 (−0.19 to 0.22) | 0.03 (−0.19 to 0.26) | −0.01 (−0.26 to 0.25) | −0.06 (−0.32 to 0.21) | NA | NA | |
| Brazil nuts | −0.05 (−1 to 0.90) | −0.06 (−1.01 to 0.90) | 0.01 (−0.95 to 0.96) | −0.07 (−1.03 to 0.88) | −0.06 (−1.02 to 0.91) | −0.10 (−1.06 to 0.87) | −0.15 (−1.12 to 0.82) | −0.09 (−1.06 to 0.88) | NA | |
| Macadamia | −0.11 (−0.46 to 0.24) | −0.12 (−0.48 to 0.24) | −0.05 (−0.40 to 0.31) | −0.13 (−0.48 to 0.22) | −0.12 (−0.50 to 0.26) | −0.16 (−0.54 to 0.22) | −0.21(−0.60 to 0.18) | −0.15 (−0.55 to 0.25) | −0.06 (−1.07 to 0.95) |
| Control | Walnuts | Almonds | Mixed | Pistachios | Peanuts | Hazelnuts | Cashews | Brazil nuts | Macadamia | Pecan | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.04 (−0.06 to 0.14) | −0.08 (−0.23 to 0.06) | −0.05 (−0.20 to 0.10) | <0.01 (−0.17 to 0.19) | 0.06 (−0.11 to 0.22) | 0.07 (−0.12 to 0.25) | <0.01 (−0.21 to 0.21) | −0.13 (−0.70 to 0.44) | −0.07 (−0.41 to 0.28) | 0 (−0.9 to 0.9) | |
| Walnuts | 0.02 (−0.08 to 0.12) | 0.27 (−0.44 to 0.98) | NA | NA | 0.13 (−0.66 to 0.92) | NA | −0.04 (−0.57 to 0.65) | NA | NA | NA | |
| Almonds | −0.04 (−0.15 to 0.07) | −0.06 (−0.20 to 0.08) | NA | NA | NA | NA | NA | NA | NA | NA | |
| Mixed | 0.05 (−0.10 to 0.20) | 0.03 (−0.15 to 0.20) | 0.09 (−0.10 to 0.27) | NA | NA | NA | NA | NA | NA | NA | |
| Pistachios | 0.03 (−0.15 to 0.21) | 0.01 (−0.19 to 0.21) | 0.07 (−0.14 to 0.28) | −0.02 (−0.25 to 0.22) | NA | NA | NA | NA | NA | NA | |
| Peanuts | <−0.01 (−0.17 to 0.17) | −0.02 (−0.21 to 0.18) | 0.04 (−0.16 to 0.24) | −0.04 (−0.27 to 0.18) | −0.03 (−0.28 to 0.22) | NA | NA | NA | NA | NA | |
| Hazelnuts | 0.09 (−0.10 to 0.28) | 0.07 (−0.14 to 0.28) | 0.13 (−0.09 to 0.35) | 0.04 (−0.19 to 0.28) | 0.06 (−0.20 to 0.32) | 0.09 (−0.16 to 0.34) | NA | NA | NA | NA | |
| Cashews | −0.10 (−0.30 to 0.10) | −0.12 (−0.34 to 0.10) | −0.06 (−0.29 to 0.17) | −0.14 (−0.39 to 0.10) | −0.13 (−0.40 to 0.14) | −0.10 (−0.36 to 0.16) | −0.19 (−0.46 to 0.08) | NA | NA | NA | |
| Brazil nuts | −0.16 (−0.72 to 0.41) | −0.18 (−0.75 to 0.40) | −0.12 (−0.70 to 0.46) | −0.20 (−0.79 to 0.39) | −0.19 (−0.78 to 0.41) | −0.16 (−0.75 to 0.43) | −0.25 (−0.85 to 0.35) | −0.06 (−0.66 to 0.54) | NA | NA | |
| Macadamia | −0.09 (−0.43 to 0.26) | −0.11 (−0.47 to 0.25) | −0.05 (−0.41 to 0.31) | −0.14 (−0.51 to 0.24) | −0.12 (−0.51 to 0.27) | −0.09 (−0.48 to 0.29) | −0.18 (−0.57 to 0.21) | <0.01 (−0.39 to 0.41) | 0.07 (−0.60 to 0.73) | NA | |
| Pecan | <0.01 (−0.9 to 0.9) | −0.02 (−0.92 to 0.88) | 0.04 (−0.87 to 0.94) | −0.05 (−0.96 to 0.86) | −0.03 (−0.95 to 0.89) | <−0.01 (−0.92 to 0.91) | −0.09 (−1.01 to 0.83) | 0.10 (−0.82 to 1.02) | 0.16 (−0.91 to 1.22) | 0.09 (−0.87 to 1.05) |
| Control | Walnuts | Almonds | Mixed | Pistachios | Peanuts | Hazelnuts | Cashews | Brazil nuts | Macadamia | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | <−0.01 (−0.12 to 0.11) | −0.15 (−0.29 to −0.02) | −0.15 (−0.35 to 0.05) | 0.14 (−0.06 to 0.35) | −0.05 (−0.22 to 0.11) | 0.30 (0.02 to 0.59) | <−0.01 (−0.22 to 0.20) | 0.07 (−0.49 to 0.64) | −0.07 (−0.42 to 0.28) | |
| Walnuts | <0.01 (−0.11 to 0.12) | NA | NA | NA | NA | NA | 0.06 (−0.55 to 0.67) | NA | NA | |
| Almonds | −0.15 (−0.27 to −0.02) | −0.15 (−0.32 to 0.01) | NA | NA | NA | NA | NA | NA | NA | |
| Mixed | −0.12 (−0.27 to 0.02) | −0.02 (−0.62 to 0.59) | 0.02 (−0.16 to 0.21) | NA | NA | NA | NA | NA | NA | |
| Pistachios | 0.16 (−0.07 to 0.38) | 0.15 (−0.10 to 0.40) | 0.30 (0.04 to 0.55) | 0.28 (0.01 to 0.54) | NA | NA | NA | NA | NA | |
| Peanuts | −0.01 (−0.20 to 0.19) | −0.01 (−0.24 to 0.21) | 0.14 (−0.09 to 0.37) | 0.11 (−0.13 to 0.36) | −0.16 (−0.46 to 0.14) | NA | NA | NA | NA | |
| Hazelnuts | 0.18 (−0.12 to 0.49) | 0.18 (−0.15 to 0.51) | 0.33 (−0.001 to 0.66) | 0.30 (−0.03 to 0.64) | 0.03 (−0.35 to 0.41) | 0.19 (−0.17 to 0.55) | NA | NA | NA | |
| Cashews | <0.01 (−0.24 to 0.24) | <−0.01 (−0.27 to 0.26) | 0.15 (−0.12 to 0.41) | 0.12 (−0.15 to 0.40) | −0.15 (−0.48 to 0.17) | <0.01 (−0.29 to 0.31) | −0.18 (−0.57 to 0.21) | NA | NA | |
| Brazil nuts | −0.01 (−0.60 to 0.58) | −0.02 (−0.62 to 0.59) | 0.14 (−0.46 to 0.74) | 0.11 (−0.49 to 0.72) | −0.17 (−0.80 to 0.46) | <−0.01 (−0.62 to 0.61) | −0.19 (−0.86 to 0.48) | −0.01 (−0.65 to 0.63) | NA | |
| Macadamia | −0.07 (−0.47 to 0.33) | −0.07 (−0.50 to 0.35) | 0.08 (−0.33 to 0.48) | 0.05 (−0.36 to 0.47) | −0.23 (−0.68 to 0.22) | −0.06 (−0.50 to 0.37) | −0.25 (−0.76 to 0.26) | −0.07 (−0.54 to 0.40) | −0.06 (−0.78 to 0.66) |
| Control | Walnuts | Almonds | Mixed | Pistachios | Peanuts | Hazelnuts | Brazil Nuts | |
|---|---|---|---|---|---|---|---|---|
| Control | −0.16 (−0.40 to 0.09) | 0.04 (−0.11 to 0.20) | −0.23 (−0.52 to 0.06) | −0.07 (−0.73 to 0.68) | −0.16 (−0.49 to 0.18) | 0.05 (−0.16 to 0.25) | −0.02 (−0.73 to 0.69) | |
| Walnuts | −0.19 (−0.41 to 0.03) | NA | NA | NA | NA | NA | NA | |
| Almonds | 0.03 (−0.18 to 0.24) | 0.22 (−0.09 to 0.52) | NA | NA | NA | NA | NA | |
| Mixed | −0.23 (−0.56 to 0.10) | −0.04 (−0.44 to 0.35) | −0.26 (−0.65 to 0.13) | NA | NA | NA | NA | |
| Pistachios | −0.06 (−0.71 to 0.59) | 0.13 (−0.56 to 0.82) | −0.09 (−0.77 to 0.60) | 0.17 (−0.56 to 0.90) | NA | NA | NA | |
| Peanuts | −0.19 (−0.59 to 0.22) | <0.01 (−0.45 to 0.46) | −0.21 (−0.67 to 0.24) | 0.05 (−0.47 to 0.56) | −0.13 (−0.89 to 0.64) | NA | NA | |
| Hazelnuts | 0.03 (−0.24 to 0.29) | 0.21 (−0.13 to 0.56) | <−0.01 (−0.34 to 0.34) | 0.26 (−0.16 to 0.68) | 0.09 (−0.62 to 0.79) | 0.21 (−0.27 to 0.69) | NA | |
| Brazil nuts | −0.03 (−0.85 to 0.79) | 0.16 (−0.69 to 1.00) | −0.06 (−0.90 to 0.79) | 0.20 (−0.68 to 1.08) | 0.03 (−1.02 to 1.08) | 0.16 (−0.76 to 1.07) | −0.06 (−0.92 to 0.80) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Rodríguez, R.; Mesas, A.E.; Garrido-Miguel, M.; Martínez-Ortega, I.A.; Jiménez-López, E.; Martínez-Vizcaíno, V. The Relationship of Tree Nuts and Peanuts with Adiposity Parameters: A Systematic Review and Network Meta-Analysis. Nutrients 2021, 13, 2251. https://doi.org/10.3390/nu13072251
Fernández-Rodríguez R, Mesas AE, Garrido-Miguel M, Martínez-Ortega IA, Jiménez-López E, Martínez-Vizcaíno V. The Relationship of Tree Nuts and Peanuts with Adiposity Parameters: A Systematic Review and Network Meta-Analysis. Nutrients. 2021; 13(7):2251. https://doi.org/10.3390/nu13072251
Chicago/Turabian StyleFernández-Rodríguez, Rubén, Arthur E. Mesas, Miriam Garrido-Miguel, Isabel A. Martínez-Ortega, Estela Jiménez-López, and Vicente Martínez-Vizcaíno. 2021. "The Relationship of Tree Nuts and Peanuts with Adiposity Parameters: A Systematic Review and Network Meta-Analysis" Nutrients 13, no. 7: 2251. https://doi.org/10.3390/nu13072251
APA StyleFernández-Rodríguez, R., Mesas, A. E., Garrido-Miguel, M., Martínez-Ortega, I. A., Jiménez-López, E., & Martínez-Vizcaíno, V. (2021). The Relationship of Tree Nuts and Peanuts with Adiposity Parameters: A Systematic Review and Network Meta-Analysis. Nutrients, 13(7), 2251. https://doi.org/10.3390/nu13072251