The Role of Micronutrients in Ageing Asia: What Can Be Implemented with the Existing Insights
Abstract
1. Introduction
- The significance of the health issue, namely, which NCDs create the greater individual and societal burden.
- The strength of evidence between the nutrient status/intake and the incidence/severity of NCDs. The strength of evidence is graded as:
- ○
- Associative, when evidence is based on associational data, but data from intervention studies are not yet available
- ○
- Probable, when the evidence is based on both associational data and a plausible mechanism of action for the association
- ○
- Convincing, when there is a high level of causality as demonstrated in intervention studies, supported by data regarding the mechanism of action.
1.1. Ageing Populations
1.2. Age and Health
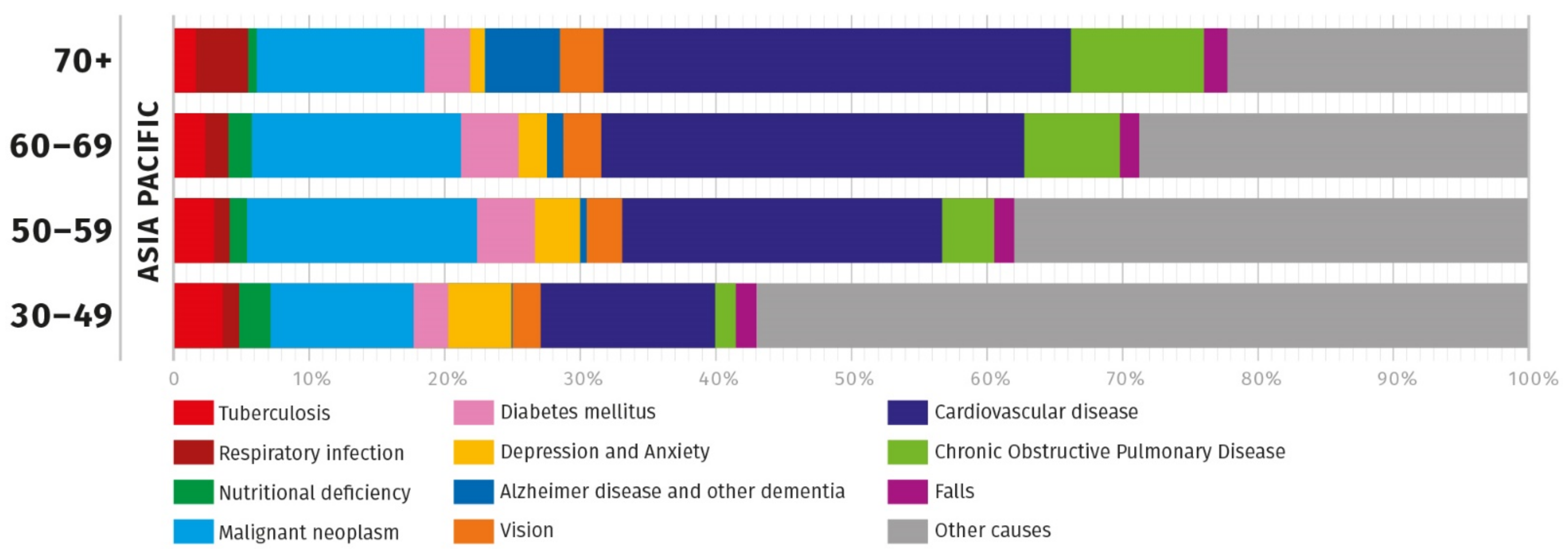
2. Obstacles to Healthy Ageing in Asia
3. When Does the Burden of Ageing Commence?
- programmed ageing of specific components (e.g., cells, organs);
- ageing caused by cumulative damage and a loss of resilience to that damage (e.g., cumulative damage to DNA or to other biological macromolecules); and
- general wearing out of body parts and functions due to extended use (loss of fitness and resilience).
4. Role of Nutrition in Healthy Ageing in Asia
4.1. Cognition
4.2. Mobility (Musculoskeletal Health)
4.3. Diabetes
4.4. Heart Function
4.5. Immunity
4.6. Eye Health
4.7. Nutrition in a Clinical Setting
5. Nutritional Adequacy and Nutritional Status in Asia
- Recommended Dietary Allowance (RDA): the average daily dietary intake level that is sufficient to meet the nutrient requirement of nearly all (97 to 98 percent) healthy individuals in a group.
- Adequate Intake (AI): a value based on observed or experimentally determined approximations of nutrient intake by a group (or groups) of healthy people—used when an RDA cannot be determined.
- Tolerable Upper Intake Level (UL): the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population. As intake increases above the UL, the risk of adverse effects increases.
- Estimated Average Requirement (EAR): a nutrient intake value that is estimated to meet the requirement of half the healthy individuals in a group.
6. Factors Affecting Adequate Nutritional Status in the Elderly
7. Potential Approaches to Optimize Nutrient Intake
- Accurate data on nutritional status, including micronutrient status, in population cohorts of ages from 40 years old onwards. This will provide the baseline of nutrient intake data, and, should it be created, will be managed and held by policymakers. However, the information should be freely available both to public and to individuals so they can determine their own nutrient status. It should be used to define optimal and sub-optimal nutrition.
- The creation of systematic data on the correlation between nutritional status and NCDs development at different stages in life including early biomarkers of current and future optimum health, healthy resilience and nutritional status. The extent of nutritional insufficiency should be determined through interaction between policy makers and the medical profession. The latter will be a key source of information on actual incidences of potentially diet-related NCDs. While accumulating longitudinal data is important, it is vital to leverage the existing information to support evidence-led interventions on a population basis in some areas. These should be driven by the size of the heath problem, the impact of the potential benefit as well as the weight of scientific evidence. By beginning such interventions now, we will be able to advance the knowledge about the impact and effectiveness of nutritional optimization on healthy ageing and finetune the recommendations in providing benefits to individuals, populations and societies.
- Intervention carried out by industry and government at a population level focusing on the effect of specific nutrition interventions on the occurrence and progression of NCDs. These interventions will be based upon existing knowledge and also contribute to the development of a new knowledge base in parallel to the above. Based on the existing evidence, we can measure post-intervention improvements in nutritional status. Government, in partnership with the private sector and other stakeholders, can work on specific actions including food fortification and nutrition education in order to optimize the approach and its effectiveness.
8. Conclusions and Outlook
- Vitamin A (under supervision) for immune function and eye health. Deficiency remains to be an issue.
- Vitamin D for musculoskeletal health, heart health and immune function. A significant proportion of the population is at risk for inadequate and deficient status. Intake data is missing in national nutritional survey for multiple countries.
- Vitamin E for diabetes and associated cardiovascular complications, immune function and eye health. A significant proportion of the population is at risk for inadequate and deficient status.
- Vitamin C for diabetes, immune function and eye health. A significant proportion of the population is at risk for inadequate and deficient status.
- B vitamins for cognition and immune function. In particular, vitamin B-12 has a high prevalence of deficiency and impaired absorption with age.
- Zinc for immune function and eye health. Deficiency remains to be an issue. Intake data is missing in national nutritional survey for multiple countries.
- Omega-3 LC-PUFAs for cognition, eye health, heart health and immune function. A significant proportion of the population is at risk for inadequate and deficient status. Intake data is missing in national nutritional survey for multiple countries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Brazeau, N.; Verity, R.; Jenks, S.; Fu, H.; Whittaker, C.; Winskill, P.; Dorigatti, I.; Walker, P.; Riley, S.; Schnekenberg, R.; et al. Report 34: COVID-19 Infection Fatality Ratio: Estimates from Seroprevalence; Imperial College London: London, UK, 2021. [Google Scholar]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates 2019: Disease burden by Cause, Age, Sex, by Country and by Region, 2000–2019. Available online: https://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html (accessed on 18 April 2021).
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerlan, 2015. [Google Scholar]
- United Nations. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; Working Paper No. ESA/P/WP/248; United Nations: Geneva, Switzerland, 2017. [Google Scholar]
- United Nations. World Population Prospects. Available online: https://population.un.org/wpp/ (accessed on 18 April 2021).
- World Health Organization. Global Health Observatory Data Repository. Life Expectancy and Healthy Life Expectancy. Data by Countr. Available online: https://apps.who.int/gho/data/node.main.688?lang=en (accessed on 18 April 2021).
- World Health Organisation. Global Health Estimates: Life Expectancy and Healthy Life Expectancy. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy (accessed on 18 April 2021).
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analy-sis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, E.; Miller, M.; Yaxley, A.; Isenring, E. Malnutrition in the elderly: A narrative review. Maturitas 2013, 76, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.T.; de Oliveira Otto, M.C.; Lemaitre, R.N.; McKnight, B.; Song, X.; King, I.B.; Chaves, P.H.; Odden, M.C.; Newman, A.B.; Siscovick, D.S.; et al. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: Prospective cohort study. BMJ 2018, 363, k4067. [Google Scholar] [CrossRef] [PubMed]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional Considerations for Healthy Aging and Reduction in Age-Related Chronic Disease. Adv. Nutr. 2017, 8, 17–26. [Google Scholar] [CrossRef]
- Engelheart, S.; Brümmer, R. Assessment of nutritional status in the elderly: A proposed function-driven model. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H.; Anthony, J.C.; Carvajal, R.; Chae, L.; Khoo, C.S.H.; Latulippe, M.E.; Matusheski, N.; McClung, H.L.; Rozga, M.; Schmid, C.H.; et al. Perspective: Guiding Principles for the Implementation of Personalized Nutrition Approaches That Benefit Health and Function. Adv. Nutr. 2019, 11, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Copenhagen Consensus Centre. The Second Copenhagen Consensus 2008. Available online: https://www.copenhagenconsensus.com/copenhagen-consensus-ii/outcomes (accessed on 18 April 2021).
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.R.; Singh, L.; Sharma, P.; Yadav, P.; Sharma, S.; Kalaskar, S.; Singh, H.; Adinarayanan, S.; Joshua, V.; Kulothungan, V.; et al. National Burden Estimates of healthy life lost in India, 2017: An analysis using direct mortality data and indirect disability data. Lancet Glob. Health 2019, 7, e1675–e1684. [Google Scholar] [CrossRef]
- Tee, E.-S.; Yap, R.W.K. Type 2 diabetes mellitus in Malaysia: Current trends and risk factors. Eur. J. Clin. Nutr. 2017, 71, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. National Health and Morbidity Survey 2019. Technical Report Vol. I: Non-Communicable Diseases: Risk Factors & Other Health Problems; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2020.
- Cabinet Office of Japan. Annual Report on the Ageing Society; Cabinet Office of Japan: Tokyo, Japan, 2019.
- Alzheimer’s Disease International. Available online: https://www.alzint.org/resource/world-alzheimer-report-2018/ (accessed on 25 June 2021).
- World Health Organisation. Looming dementia epidemic in Asia. Bull. W.H.O. 2011, 89, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Afshar, S.; Roderick, P.J.; Kowal, P.; Dimitrov, B.D.; Hill, A.G. Multimorbidity and the inequalities of global ageing: A cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Uttamacharya, U.; Jain, K.; Biritwum, R.B.; Yawson, A.E.; Wu, F.; Guo, Y.; Maximova, T.; Espinoza, B.M.; Rodríguez, A.S.; et al. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: What does the study on global ageing and adult health (SAGE) reveal? BMC Med. 2015, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Hamid, F.; Pati, S.; Atun, R.; Millett, C. Impact of Noncommunicable Disease Multimorbidity on Healthcare Utilisation and Out-Of-Pocket Expenditures in Middle-Income Countries: Cross Sectional Analysis. PLoS ONE 2015, 10, e0127199. [Google Scholar] [CrossRef]
- Chen, C.; Si, T.-M.; Xiang, Y.-T.; Ungvari, G.S.; Wang, C.-Y.; Pichet, U.; Kua, E.H.; Fujii, S.; Sim, K.; Trivedi, J.K.; et al. Prevalence and Prescription of Antidepressants in Depression with Somatic Comorbidity in Asia. Chin. Med J. 2015, 128, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Soveral, I. The immune system and aging: A review. Gynecol. Endocrinol. 2013, 30, 16–22. [Google Scholar] [CrossRef]
- Kempen, G.I.J.M.; Ballemans, J.; Ranchor, A.V.; Van Rens, G.H.M.B.; Zijlstra, G.A.R. The impact of low vision on activities of daily living, symptoms of depression, feelings of anxiety and social support in community-living older adults seeking vision rehabilitation services. Qual. Life Res. 2011, 21, 1405–1411. [Google Scholar] [CrossRef]
- Wittenborn, J.S.; Zhang, X.; Feagan, C.W.; Crouse, W.L.; Shrestha, S.; Kemper, A.R.; Hoerger, T.J.; Saaddine, J.B. The Economic Burden of Vision Loss and Eye Disorders among the United States Population Younger than 40 Years. Ophthalmology 2013, 120, 1728–1735. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- World Health Organisation. World Report on Vision; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Baugreet, S.; Hamill, R.M.; Kerry, J.P.; McCarthy, S.N. Mitigating Nutrition and Health Deficiencies in Older Adults: A Role for Food Innovation? J. Food Sci. 2017, 82, 848–855. [Google Scholar] [CrossRef]
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-Gavilán, J.; Bulló, M.; Salas-Salvadó, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. 2019, 11, 576–601. [Google Scholar] [CrossRef] [PubMed]
- Harte, A.L.; Da Silva, N.F.; Miller, M.A.; Cappuccio, F.P.; Kelly, A.; O’Hare, J.P.; Barnett, A.H.; Al-Daghri, N.M.; Al-Attas, O.; Alokail, M.; et al. Telomere Length Attrition, a Marker of Biological Senescence, Is Inversely Correlated with Triglycerides and Cholesterol in South Asian Males with Type 2 Diabetes Mellitus. Exp. Diabetes Res. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Campisi, J.; Vijg, J. Does Damage to DNA and Other Macromolecules Play a Role in Aging? If So, How? J. Gerontol. Ser. A: Boil. Sci. Med Sci. 2009, 64, 175–178. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and Oxidative Stress: Friend or Foe? Oxidative Med. Cell. Longev. 2018, 2018, 1–24. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Belsky, D.W.; Caspi, A.; Sugden, K.; Williams, B.; Yashin, A.I.; Poulton, R.; Moffitt, T.E.; Houts, R.; Cohen, H.J.; Corcoran, D.L.; et al. Quantification of biological aging in young adults. Proc. Natl. Acad. Sci. USA 2015, 112, E4104–E4110. [Google Scholar] [CrossRef] [PubMed]
- Péter, S.; Saris, W.H.M.; Mathers, J.C.; Feskens, E.; Schols, A.; Navis, G.; Kuipers, F.; Weber, P.; Eggersdorfer, M. Nutrient Status Assessment in Individuals and Populations for Healthy Aging—Statement from an Expert Workshop. Nutrients 2015, 7, 10491–10500. [Google Scholar] [CrossRef]
- European Food Safety Authority. “General Function” Health Claims under Article 13. Available online: https://www.efsa.europa.eu/en/topics/topic/general-function-health-claims-under-article-13 (accessed on 18 April 2021).
- Jernerén, F.; Elshorbagy, A.; Oulhaj, A.; Smith, S.M.; Refsum, H.; Smith, A.D. Brain atrophy in cognitively impaired elderly: The importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 215–221. [Google Scholar] [CrossRef]
- Van Der Zwaluw, N.L.; Brouwer-Brolsma, E.M.; Kessels, R.P.C.; Smeets, P.A.M.; Kok, F.J.; Dhonukshe-Rutten, R.A.M.; De Groot, L.C.P.G.M.; Van De Rest, O.; Van Wijngaarden, J.P.; Veld, P.H.I.T.; et al. Folate and Vitamin B12-Related Biomarkers in Relation to Brain Volumes. Nutrients 2016, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement1. J. Alzheimer’s Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef]
- Oulhaj, A.; Jernerén, F.; Refsum, H.; Smith, A.D.; De Jager, C.A. Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2016, 50, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.-T.; Jiang, Y.-W.; Pan, X.-F.; Feng, L.; Yuan, J.-M.; Pan, A.; Koh, W.-P. Association Between Dietary Intakes of B Vitamins in Midlife and Cognitive Impairment in Late-Life: The Singapore Chinese Health Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2019, 75, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic Acid and Adult Memory: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W. Does Consumption of LC Omega-3 PUFA Enhance Cognitive Performance in Healthy School-Aged Children and throughout Adulthood? Evidence from Clinical Trials. Nutrients 2014, 6, 2730–2758. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between Serum and Brain Carotenoids,α-Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Adair, L.S.; Plassman, B.L.; Batis, C.; Edwards, L.; Popkin, B.M.; Mendez, M.A. Dietary Patterns and Cognitive Decline Among Chinese Older Adults. Epidemiology 2015, 26, 758–768. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Facts and Statistics | International Osteoporosis Foundation. Available online: https://www.iofbonehealth.org/facts-statistics (accessed on 18 April 2021).
- Rizzoli, R.; Biver, E.; Bonjour, J.-P.; Coxam, V.; Goltzman, D.; Kanis, J.A.; Lappe, J.; Rejnmark, L.; Sahni, S.; Weaver, C.; et al. Benefits and safety of dietary protein for bone health—An expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos. Int. 2018, 29, 1933–1948. [Google Scholar] [CrossRef]
- Wallace, T.C. Optimizing Dietary Protein for Lifelong Bone Health. Nutr. Today 2019, 54, 107–115. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- International Osteoporosis Foundation. Vitamin D. Available online: https://www.iofbonehealth.org/osteoporosis-musculoskeletal-disorders/osteoporosis/prevention/vitamin-d (accessed on 18 April 2021).
- Frost and Sullivan. Healthcare Cost Savings of Calcium and Vitamin D Food Supplements in the European Union; Frost & Sullivan: Mountain View, CA, USA, 2017. [Google Scholar]
- Man, P.W.; Van Der Meer, I.M.; Lips, P.; Middelkoop, B.J.C. Vitamin D status and bone mineral density in the Chinese population: A review. Arch. Osteoporos. 2016, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Population Nutrient Intake Goals for Preventing Diet-Related Chronic Disease; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Rodacki, C.L.N.; Rodacki, A.L.F.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef]
- Da Boit, M.; Hunter, A.; Gray, S.R. Fit with good fat? The role of n-3 polyunsaturated fatty acids on exercise performance. Metabolism 2017, 66, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Muir, S.W.; Montero-Odasso, M. Effect of Vitamin D Supplementation on Muscle Strength, Gait and Balance in Older Adults: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2011, 59, 2291–2300. [Google Scholar] [CrossRef]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.-Y.; Bruyere, O. The Effects of Vitamin D on Skeletal Muscle Strength, Muscle Mass, and Muscle Power: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.; Kim, M.K.; Yun, B.H.; Cho, S.; Choi, Y.S.; Lee, B.S.; Seo, S.K. Relationships between 25(OH)D concentration, sarcopenia and HOMA-IR in postmenopausal Korean women. Climacteric 2017, 21, 40–46. [Google Scholar] [CrossRef]
- World Health Organization. Diet, nutrition and the prevention of chronic diseases. In World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- Fernandez-Mejia, C. Pharmacological effects of biotin. J. Nutr. Biochem. 2005, 16, 424–427. [Google Scholar] [CrossRef]
- Valdes-Ramos, R.; Laura, G.-L.A.; Elina, M.-C.B.; Donají, B.-A.A. Vitamins and Type 2 Diabetes Mellitus. Endocrine, Metab. Immune Disord. Drug Targets 2015, 15, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.-H.; Wareham, N.J.; Bingham, S.A.; Khaw, K.; Luben, R.; Welch, A.; Forouhi, N.G. Plasma Vitamin C Level, Fruit and Vegetable Consumption, and the Risk of New-Onset Type 2 Diabetes MellitusThe European Prospective Investigation of Cancer–Norfolk Prospective Study. Arch. Intern. Med. 2008, 168, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Rasmussen, B.; van Loon, L.J.; Salmon, J.; Wadley, G.D. Ascorbic acid supplementation improves postprandial glycaemic control and blood pressure in individuals with type 2 diabetes: Findings of a randomized cross-over trial. Diabetes Obes. Metab. 2019, 21, 674–682. [Google Scholar] [CrossRef]
- Rajalakshmy, P. Role of Micronutrients on Type Ii Diabetes Mellitus. Acta Sci. Nutr. Health 2019, 3, 44–47. [Google Scholar] [CrossRef]
- De Paula, T.P.; Kramer, C.K.; Viana, L.V.; Azevedo, M.J. Effects of individual micronutrients on blood pressure in patients with type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2017, 7, 40751. [Google Scholar] [CrossRef] [PubMed]
- Milman, U.; Blum, S.; Shapira, C.; Aronson, D.; Miller-Lotan, R.; Anbinder, Y.; Alshiek, J.; Bennett, L.; Kostenko, M.; Landau, M.; et al. Vitamin E Supplementation Reduces Cardiovascular Events in a Subgroup of Middle-Aged Individuals with Both Type 2 Diabetes Mellitus and the Haptoglobin 2-2 Genotype. Arter. Thromb. Vasc. Biol. 2008, 28, 341–347. [Google Scholar] [CrossRef]
- Dalan, R.; Liew, H.; Goh, L.L.; Gao, X.; Chew, D.E.; O Boehm, B.; Leow, M.K.S. The haptoglobin 2-2 genotype is associated with inflammation and carotid artery intima-media thickness. Diabetes Vasc. Dis. Res. 2016, 13, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Nakada, N.; Abe, K. Genetic Polymorphism of Haptoglobin Subtypes in a Japanese Population. Hum. Hered. 1987, 37, 376–380. [Google Scholar] [CrossRef]
- Goliasch, G.; Silbernagel, G.; Kleber, M.E.; Grammer, T.B.; Pilz, S.; Tomaschitz, A.; Bartko, P.E.; Maurer, G.; Koenig, W.; Niessner, A.; et al. Refining Long-Term Prediction of Cardiovascular Risk in Diabetes–The VILDIA Score. Sci. Rep. 2017, 7, 4700. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- World Health Organization. The Atlas of Heart Disease and Stroke; Mackay, J., Mensah, G., Eds.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Effects of Icosapent Ethyl on Total Ischemic Events. J. Am. Coll. Cardiol. 2019, 73, 2791–2802. [Google Scholar] [CrossRef]
- The Economist Intelligence Unit. The Cost of Silence: Cardiovascular Disease in Asia a Report; The Economist: London, UK, 2018. [Google Scholar]
- Alexander, D.D.; Miller, P.E.; Van Elswyk, M.E.; Kuratko, C.N.; Bylsma, L.C. A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin. Proc. 2017, 92, 15–29. [Google Scholar] [CrossRef]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-Chain Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid and Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R. Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 95, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Childs, C.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; Goodall, E.C.; et al. Vitamin D supplementation to prevent acute respiratory infections: Individual participant data meta-analysis. Health Technol. Assess. 2019, 23, 1–44. [Google Scholar] [CrossRef] [PubMed]
- A Jolliffe, D.; A Camargo, C.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.J.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef]
- MacNeil, A.; Glaziou, P.; Sismanidis, C.; Maloney, S.; Floyd, K. Global Epidemiology of Tuberculosis and Progress Toward Achieving Global Targets—2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 263–266. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and immune function: An overview. In Proceedings of the Nutrition Society; Cambridge University Press: Cambridge, UK, 2012; Volume 71, pp. 50–61. [Google Scholar]
- Jolliffe, D.A.; Ganmaa, D.; Wejse, C.; Raqib, R.; Haq, M.A.; Salahuddin, N.; Daley, P.K.; Ralph, A.P.; Ziegler, T.R.; Martineau, A.R. Adjunctive vitamin D in tuberculosis treatment: Meta-analysis of individual participant data. Eur. Respir. J. 2019, 53, 1802003. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Age-Related Macular Degeneration. Available online: https://www.who.int/blindness/causes/priority/en/index7.html (accessed on 18 April 2021).
- Sideri, O.; Tsaousis, K.T.; Li, H.J.; Viskadouraki, M.; Tsinopoulos, I. The potential role of nutrition on lens pathology: A systematic review and meta-analysis. Surv. Ophthalmol. 2019, 64, 668–678. [Google Scholar] [CrossRef]
- Jiang, H.; Yin, Y.; Wu, C.-R.; Liu, Y.; Guo, F.; Li, M.; Ma, L. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am. J. Clin. Nutr. 2019, 109, 43–54. [Google Scholar] [CrossRef] [PubMed]
- National Eye Institute. Age-Related Eye Disease Study (AREDS). Available online: https://www.nei.nih.gov/research/clinical-trials/age-related-eye-disease-study-areds (accessed on 18 April 2021).
- Chew, E.Y.; Lindblad, A.S.; Clemons, T. Age-Related Eye Disease Study Research Group Summary Results and Recommendations From the Age-Related Eye Disease Study. Arch. Ophthalmol. 2009, 127, 1678–1679. [Google Scholar] [CrossRef]
- Frost and Sullivan. The Economic Benefits of Using Lutein and Zeaxanthin Food Supplements in the European Union; Frost & Sullivan: Mountain View, CA USA, 2017. [Google Scholar]
- Gayton, J.L. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009, 3, 405–412. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Bernabei, F.; Roda, M.; Taroni, L.; Versura, P.; Campos, E.C. Efficacy of Omega-3 Fatty Acid Supplementation for Treatment of Dry Eye Disease: A Meta-Analysis of Randomized Clinical Trials. Cornea 2019, 38, 565–573. [Google Scholar] [CrossRef]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.I.S.D.S.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.; Deutz, N.; Erickson, N.; Laviano, A.; Lisanti, M.; Lobo, D.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Baracos, V.E. Skeletal muscle anabolism in patients with advanced cancer. Lancet Oncol. 2015, 16, 13–14. [Google Scholar] [CrossRef]
- Barrera, R. Nutritional Support in Cancer Patients. J. Parenter. Enter. Nutr. 2002, 26, S63–S71. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, G.; Almeida, A.; Ravasco, P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition 2015, 31, 549–555. [Google Scholar] [CrossRef]
- Pizato, N.; Bonatto, S.; Yamazaki, R.K.; Aikawa, J.; Nogata, C.; Mund, R.C.; Nunes, E.A.; Piconcelli, M.; Naliwaiko, K.; Curi, R.; et al. Ratio of n6 to n-3 Fatty Acids in the Diet Affects Tumor Growth and Cachexia in Walker 256 Tumor-Bearing Rats. Nutr. Cancer 2005, 53, 194–201. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Brooks, D.; White, J.; Goldstein, R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2012, 12, CD000998. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, L.; Man, Q.; Wang, J.; Zhao, W.; Zhang, J. Dietary Micronutrients Intake Status among Chinese Elderly People Living at Home: Data from CNNHS 2010–2012. Nutrients 2019, 11, 1787. [Google Scholar] [CrossRef]
- Nozue, M.; Ishikawa, M.; Takemi, Y.; Kusama, K.; Fukuda, Y.; Yokoyama, T.; Nakaya, T.; Nishi, N.; Yoshiba, K.; Murayama, N. Prevalence of Inadequate Nutrient Intake in Japanese Community-Dwelling Older Adults Who Live Alone. J. Nutr. Sci. Vitaminol. 2016, 62, 116–122. [Google Scholar] [CrossRef]
- Angeles-Agdeppa, I.; Sun, Y.; Denney, L.; Tanda, K.V.; Octavio, R.A.D.; Carriquiry, A.; Capanzana, M.V. Food sources, energy and nutrient intakes of adults: 2013 Philippines National Nutrition Survey. Nutr. J. 2019, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mark, H.E.; Houghton, L.A.; Gibson, R.S.; Monterrosa, E.; Kraemer, K. Estimating dietary micronutrient supply and the prevalence of inadequate intakes from national Food Balance Sheets in the South Asia region. Asia Pac. J. Clin. Nutr. 2016, 25, 368–376. [Google Scholar] [CrossRef]
- Ter Borg, S.; Verlaan, S.; Hemsworth, J.; Mijnarends, D.M.; Schols, J.M.G.A.; Luiking, Y.C.; De Groot, L.C.P.G.M. Micronutrient intakes and potential inadequacies of community-dwelling older adults: A systematic review. Br. J. Nutr. 2015, 113, 1195–1206. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Akobundu, U.; Jensen, G.; Johnson, M.A.; Mackay, D.; Marshall, K.; Meydani, S.N.; Tucker, K.L.; Bailey, R.L.; Shlisky, J.; et al. Hidden Hunger: Solutions for America’s Aging Populations. Nutrients 2018, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef]
- Malik, A.; Eggersdorfer, M.; Trilok-Kumar, G. Vitamin E status in healthy population in Asia: A review of current literature. Int. J. Vitam. Nutr. Res. 2021, 91, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef]
- Ferguson, E.L.; Watson, L.; Berger, J.; Chea, M.; Chittchang, U.; Fahmida, U.; Khov, K.; Kounnavong, S.; Le, B.M.; Rojroongwasinkul, N.; et al. Realistic Food-Based Approaches Alone May Not Ensure Dietary Adequacy for Women and Young Children in South-East Asia. Matern. Child Health J. 2018, 23, 55–66. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
- Victora, C.G.; Christian, P.; Vidaletti, L.P.; Gatica-Domínguez, G.; Menon, P.; E Black, R. Revisiting maternal and child undernutrition in low-income and middle-income countries: Variable progress towards an unfinished agenda. Lancet 2021, 397, 1388–1399. [Google Scholar] [CrossRef]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am. J. Clin. Nutr. 2010, 91, 1733–1744. [Google Scholar] [CrossRef]
- Sweeney, M.R.; Staines, A.; Daly, L.; Traynor, A.; Daly, S.; Bailey, S.W.; Alverson, P.B.; E Ayling, J.; Scott, J.M. Persistent circulating unmetabolised folic acid in a setting of liberal voluntary folic acid fortification. Implications for further mandatory fortification? BMC Public Health 2009, 9, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Dong, B.; Wang, Z. Serum folate concentrations and all-cause, cardiovascular disease and cancer mortality: A cohort study based on 1999–2010 National Health and Nutrition Examination Survey (NHANES). Int. J. Cardiol. 2016, 219, 136–142. [Google Scholar] [CrossRef]
- Hans, K.B.; Jana, T. Micronutrients in the life cycle: Requirements and sufficient supply. NFS J. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Deshpande, S.S.; Lubree, H.G.; Naik, S.S.; Bhat, D.; Uradey, B.S.; Deshpande, J.A.; Rege, S.S.; Refsum, H.; Yudkin, J.S. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J. Assoc. Physicians India 2006, 54, 775–782. [Google Scholar]
- Bailey, R.L.; Carmel, R.; Green, R.; Pfeiffer, C.M.; Cogswell, M.E.; Osterloh, J.D.; Sempos, C.T.; Yetley, E.A. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am. J. Clin. Nutr. 2011, 94, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Damji, A.; Uppaluri, A. Vitamin B12 deficiency. Prevalence among South Asians at a Toronto clinic. Can. Fam. Physician Med. Fam. Can. 2004, 50, 743–747. [Google Scholar]
- Onoue, H.; Koyama, T.; Zamami, Y.; Hagiya, H.; Tatebe, Y.; Mikami, N.; Shinomiya, K.; Kitamura, Y.; Hinotsu, S.; Sendo, T.; et al. Trends in Polypharmacy in Japan: A Nationwide Retrospective Study. J. Am. Geriatr. Soc. 2018, 66, 2267–2273. [Google Scholar] [CrossRef]
- Mohn, E.S.; Kern, H.J.; Saltzman, E.; Mitmesser, S.H.; McKay, D.L. Evidence of Drug–Nutrient Interactions with Chronic Use of Commonly Prescribed Medications: An Update. Pharmaceutics 2018, 10, 36. [Google Scholar] [CrossRef]
- König, M.; Spira, D.; Demuth, I.; Steinhagen-Thiessen, E.; Norman, K. Polypharmacy as a Risk Factor for Clinically Relevant Sarcopenia: Results From the Berlin Aging Study II. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2017, 73, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, T.; Hosomi, K.; Yokoyama, S.; Takada, M. Polypharmacy in elderly patients in Japan: Analysis of Japanese real-world databases. J. Clin. Pharm. Ther. 2020, 45, 991–996. [Google Scholar] [CrossRef]
- Kim, H.-A.; Shin, J.; Kim, M.-H.; Park, B.-J. Prevalence and Predictors of Polypharmacy among Korean Elderly. PLoS ONE 2014, 9, e98043. [Google Scholar] [CrossRef]
- Tan, Y.W.; Suppiah, S.; Bautista, M.A.C.; Malhotra, R. Polypharmacy among community-dwelling elderly in Singapore: Prevalence, risk factors and association with medication non-adherence. Proc. Singap. Healthcare 2019, 28, 224–231. [Google Scholar] [CrossRef]
- Davies, L.E.; Spiers, G.; Kingston, A.; Todd, A.; Adamson, J.; Hanratty, B. Adverse Outcomes of Polypharmacy in Older People: Systematic Review of Reviews. J. Am. Med Dir. Assoc. 2020, 21, 181–187. [Google Scholar] [CrossRef]
- Monographs in Oral Science. In The Impact of Nutrition and Diet on Oral Health; Zohoori, F., Duckworth, V., Ralph, M., Eds.; Karger: Basel, Switzerland, 2020; Volume 28. [Google Scholar]
- Gossweiler, A.G.; Martinez-Mier, E.A. Chapter 6: Vitamins and Oral Health. In Monographs in Oral Science; S. Karger AG: Basel, Switzerland, 2019; Volume 28, pp. 59–67. [Google Scholar]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Massironi, S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World J. Gastroenterol. 2017, 23, 563–572. [Google Scholar] [CrossRef]
- Thomas, D.M.; Mirowski, G.W. Nutrition and oral mucosal diseases. Clin. Dermatol. 2010, 28, 426–431. [Google Scholar] [CrossRef]
- Eke, P.I.; Thornton-Evans, G.; Dye, B.; Genco, R. Advances in Surveillance of Periodontitis: The Centers for Disease Control and Prevention Periodontal Disease Surveillance Project. J. Periodontol. 2012, 83, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Corbet, E.F.; Zee, K.-Y.; Lo, E.C.M. Periodontal diseases in Asia and Oceania. Periodontol. 2000 2002, 29, 122–152. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Seeberger, G.K.; Hennedige, O. Burden of oral diseases and noncommunicable diseases: An asia-pacific perspective. Indian J. Dent. Res. 2018, 29, 820. [Google Scholar] [CrossRef]
- Caroleo, M.; Carbone, E.A.; Primerano, A.; Foti, D.; Brunetti, A.; Segura-Garcia, C. The role of hormonal, metabolic and inflammatory biomarkers on sleep and appetite in drug free patients with major depression: A systematic review. J. Affect. Disord. 2019, 250, 249–259. [Google Scholar] [CrossRef]
- Wei, J.; Fan, L.; Zhang, Y.; Li, S.; Partridge, J.; Claytor, L.; Sulo, S. Association Between Malnutrition and Depression Among Community-Dwelling Older Chinese Adults. Asia Pac. J. Public Health 2018, 30, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.T.; Harmsen, I.; Assies, J.; Koeter, M.W.J.; Ruhé, H.G.; Schene, A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liang, H.; Tong, Y.; Li, Y. Association of dietary n-3 polyunsaturated fatty acids intake with depressive symptoms in midlife women. J. Affect. Disord. 2020, 261, 164–171. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Álvarez-Pérez, J.; Toledo, E.; Salas-Salvadó, J.; Ortega-Azorín, C.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; Romaguera, D.; López, J.P.; et al. Seafood Consumption, Omega-3 Fatty Acids Intake, and Life-Time Prevalence of Depression in the PREDIMED-Plus Trial. Nutrients 2018, 10, 2000. [Google Scholar] [CrossRef]
- Ministry of Health Malaysia. Healthy Eating for Elderly; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2017.
- National Institute of Nutrition. Dietary Guidelines for Indians; Indian Council of Medical Research: Hyderabad, India, 2011.
- Lichtenstein, A.H.; Rasmussen, H.; Yu, W.W.; Epstein, S.R.; Russell, R.M. Modified MyPyramid for Older Adults. J. Nutr. 2008, 138, 5–11. [Google Scholar] [CrossRef]
- Drewnowski, A.; Fulgoni, I.V. Nutrient profiling of foods: Creating a nutrient-rich food index. Nutr. Rev. 2008, 66, 23–39. [Google Scholar] [CrossRef]
- National Institutes of Health. 2020–2030 Strategic Plan for NIH Nutrition Research. A Report of the NIH Nutrition Research Task Force; National Institutes of Health: Bethesda, MD, USA, 2020.
- Klemera, P.; Doubal, S. A new approach to the concept and computation of biological age. Mech. Ageing Dev. 2006, 127, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, T.; Itkonen, S.T.; Lundqvist, A.; Erkkola, M.; Koskela, T.; Lakkala, K.; Dowling, K.G.; Hull, G.; Kröger, H.; Karppinen, J.; et al. The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: Evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data. Am. J. Clin. Nutr. 2017, 105, ajcn151415. [Google Scholar] [CrossRef] [PubMed]
- Pietinen, P.; Männistö, S.; Valsta, L.M.; Sarlio-Lähteenkorva, S. Nutrition policy in Finland. Public Health Nutr. 2010, 13, 901–906. [Google Scholar] [CrossRef]
- Haque, N. Active Ageing Level of Older Persons: Regional Comparison in Thailand. J. Aging Res. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- UNECE/European Commission. 2018 Active Ageing Index: Analytical Report; ECE/WG.1/33; United Nations: Geneva, Switzerland, 2019. [Google Scholar]
- Help Age International. Policy Mapping on Ageing in Asia and the Pacific Analytical Report. Available online: http://ageingasia.org/mapping-of-ageing-policies/ (accessed on 18 April 2021).
- Asia Health and Wellbeing Initiative. Longitudinal Survey of Aging and Health in ASEAN Countries. Available online: https://www.ahwin.org/research-projects/longitudinal-survey-of-the-elderly-in-southeast-asia (accessed on 18 April 2021).
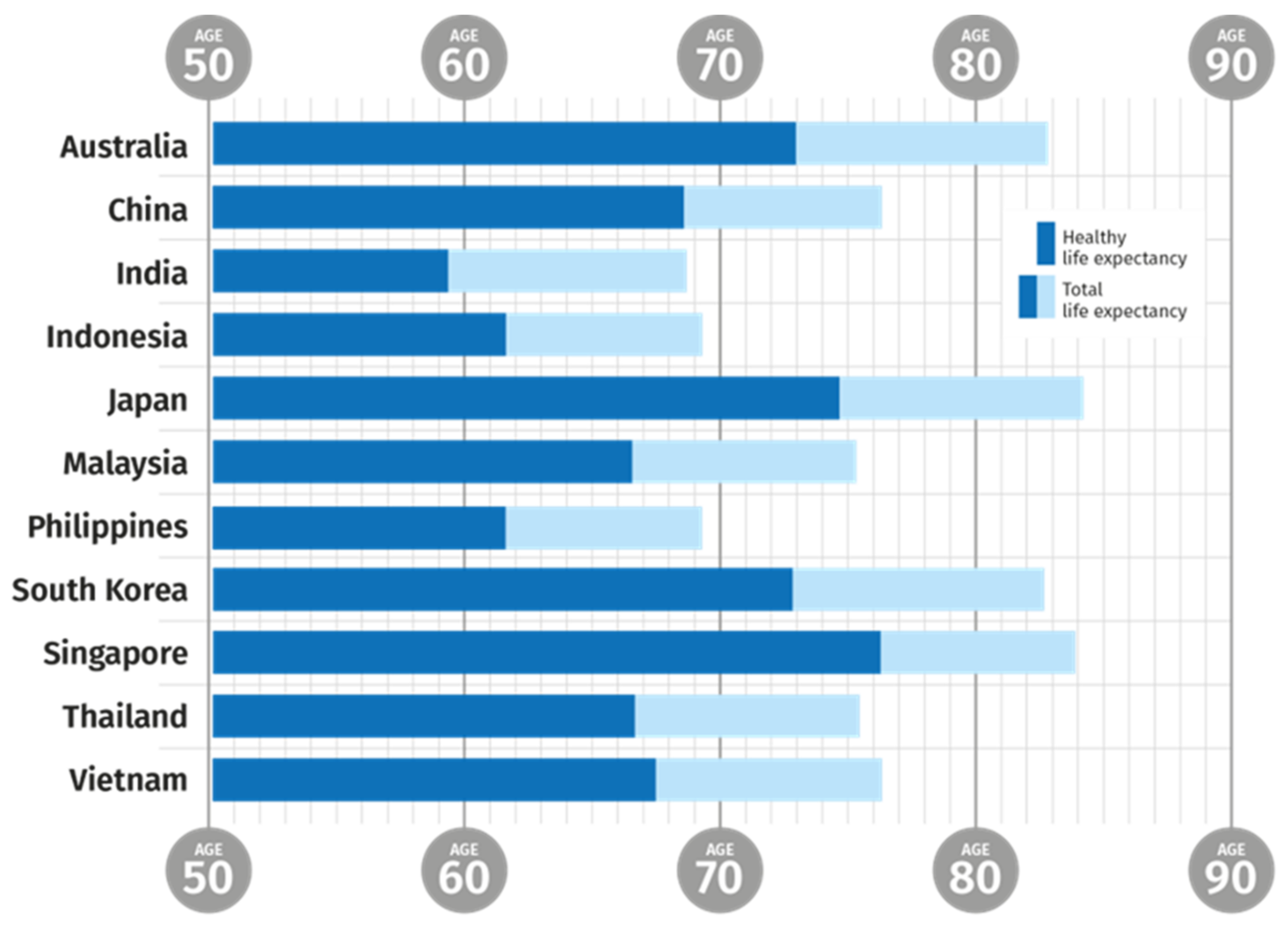
| Country | Life Expectancy at Birth (2018) | Birth Rate (Births Per Woman, 2018—World Bank) |
|---|---|---|
| China | 77 | 1.6 |
| Japan | 84 | 1.4 |
| Singapore | 83 | 1.1 |
| Thailand | 77 | 1.5 |
| Australia | 83 | 1.7 |
| United States | 79 | 1.7 |
| France | 83 | 1.9 |
| Deficiency | Disease | Occurrence |
|---|---|---|
| Vitamin A | Night blindness and xerophthalmia | Up to 30% in children in Asia |
| Vitamin B1 | Beri beri. Neurodevelopmental disorders | Up to 50% in parts of East Asia due to diet |
| Vitamin B3 | Pellagra | Comparatively rare but difficult to diagnose in isolation from B complex vitamin deficiency |
| Vitamin B12 | Anaemia | Up to 70% reported in adults in India |
| Vitamin C | Scurvy | 74% of adults in North India and 46% of adults in South India had low vitamin C intake. |
| Vitamin D | Ricketts | More common in Northern Europe in those of Asian descent. |
| Calcium | Ricketts and osteoporosis | Low intake in some parts of Asia, e.g., Japan |
| Iodine | Goitre | Prevalence of 2% in adults and up to 30% among children in some parts of Asia |
| Iron | Anaemia | Up to 50% among women of reproductive age in parts of Asia |
| Zinc | Stunting, impaired immunity | Over 25% of inadequate zinc intake in Southeast and South Asian countries |
| Vitamin A | Vitamin C | Vitamin D | Vitamin E | Vitamin B1 | Vitamin B2 | Niacin | Vitamin B6 | Folate | Vitamin B12 | Calcium | Iron | Zinc | Omega-3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia | ||||||||||||||
| India | * | * | * | * | * | * | * | * | * | |||||
| Indonesia | * | * | * | * | * | * | * | * | * | * | ||||
| Japan | ||||||||||||||
| South Korea | ||||||||||||||
| Malaysia | ||||||||||||||
| New Zealand | * | * | * | * | * | * | * | * | * | * | * | |||
| Philippines | ||||||||||||||
| Thailand | ** | ** | ** | ** | ** | ** | ** | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inui, T.; Hanley, B.; Tee, E.S.; Nishihira, J.; Tontisirin, K.; Van Dael, P.; Eggersdorfer, M. The Role of Micronutrients in Ageing Asia: What Can Be Implemented with the Existing Insights. Nutrients 2021, 13, 2222. https://doi.org/10.3390/nu13072222
Inui T, Hanley B, Tee ES, Nishihira J, Tontisirin K, Van Dael P, Eggersdorfer M. The Role of Micronutrients in Ageing Asia: What Can Be Implemented with the Existing Insights. Nutrients. 2021; 13(7):2222. https://doi.org/10.3390/nu13072222
Chicago/Turabian StyleInui, Taichi, Bryan Hanley, E Siong Tee, Jun Nishihira, Kraisid Tontisirin, Peter Van Dael, and Manfred Eggersdorfer. 2021. "The Role of Micronutrients in Ageing Asia: What Can Be Implemented with the Existing Insights" Nutrients 13, no. 7: 2222. https://doi.org/10.3390/nu13072222
APA StyleInui, T., Hanley, B., Tee, E. S., Nishihira, J., Tontisirin, K., Van Dael, P., & Eggersdorfer, M. (2021). The Role of Micronutrients in Ageing Asia: What Can Be Implemented with the Existing Insights. Nutrients, 13(7), 2222. https://doi.org/10.3390/nu13072222






