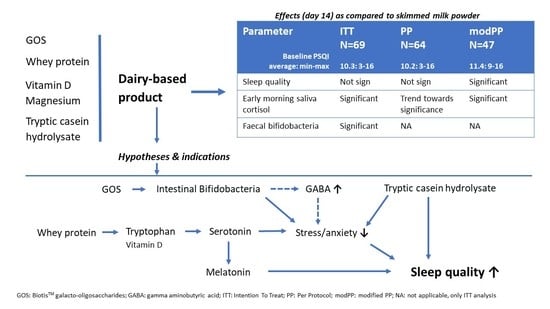
The Effect of A Whey-Protein and Galacto-Oligosaccharides Based Product on Parameters of Sleep Quality, Stress, and Gut Microbiota in Apparently Healthy Adults with Moderate Sleep Disturbances: A Randomized Controlled Cross-Over Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Products
2.4. Methods
2.5. Statistics
3. Results
3.1. Subjects
3.2. Sleep Quality
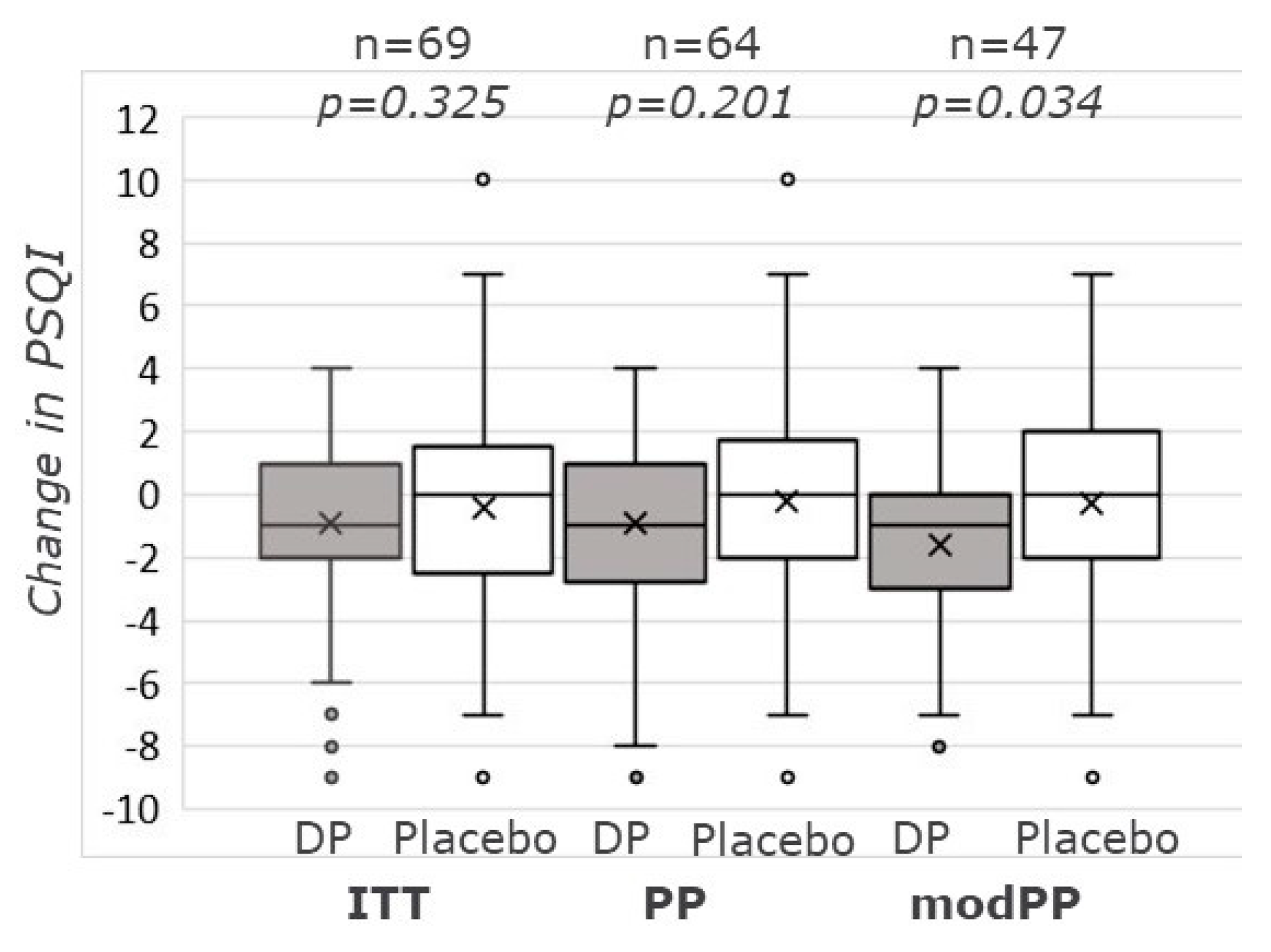
3.2.1. PSQI-Score
3.2.2. SmartSleep
3.3. Daily Lifestyle Questionnaire
3.4. Depression–Anxiety–Stress Outcomes
3.4.1. DASS-42 Questionnaire
3.4.2. Salivary Cortisol
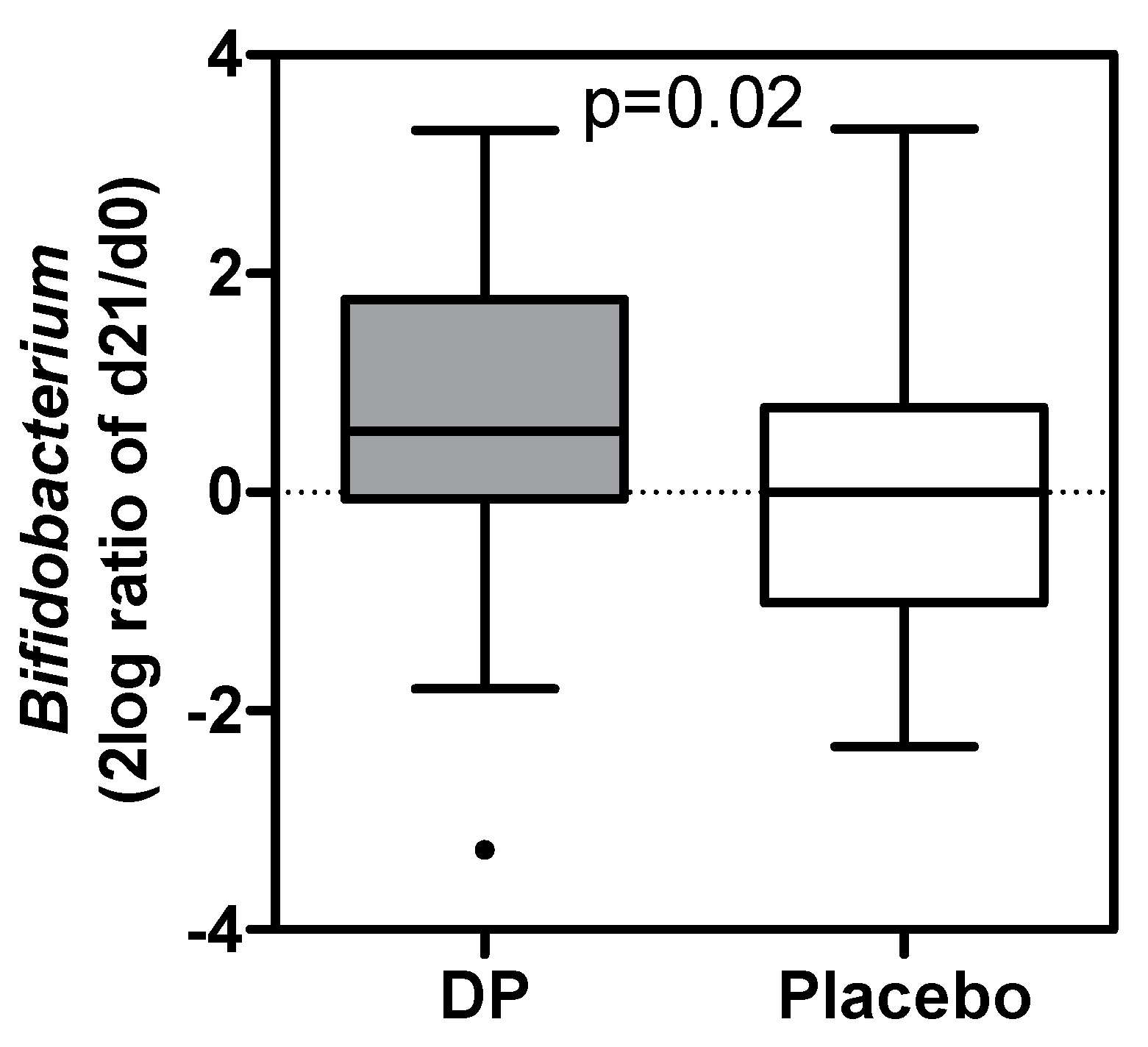
3.5. Gut Microbiota
3.6. Product Tolerability and Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chattu, V.; Manzar, M.; Kumary, S.; Burman, D.; Spence, D.; Pandi-Perumal, S. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef]
- Gulia, K.K.; Kumar, V.M. Sleep disorders in the elderly: A growing challenge. Psychogeriatrics 2018, 18, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Winer, J.R.; Jagust, W.J.; Walker, M.P. Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimers’s disease? Trends Neurosci. 2016, 39, 552–566. [Google Scholar] [CrossRef]
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock—Blue light sets the human rhythm. J. Biophotonics 2019, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.J.; Coates, A.M.; Kohler, M.; Banks, S. Caffeine consumption and sleep quality in Australian adults. Nutrients 2016, 8, E479. [Google Scholar] [CrossRef]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Rechtschaffen, A.; Siegel, J. Sleep and dreaming. In Principles of Neuroscience; Kandel, E.R., Schwartz, J.H., Jessell, T.M., Eds.; McGraw-Hill: New York, NY, USA, 2000; pp. 936–947. [Google Scholar]
- Saper, C.B.; Cano, G.; Scammell, T.E. Homeostatic, circadian, and emotional regulation of sleep. J. Comp. Neurol. 2005, 493, 92–98. [Google Scholar] [CrossRef]
- Ohno, K.; Sakurai, T. Orexin neuronal circuitry: Role in the regulation of sleep and wakefulness. Front. Neuroendocrinol. 2008, 29, 70–87. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for a-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- De Saint-Hilaire, Z.; Messaoudi, M.; Desor, D.; Kobayashi, T. Effects of a bovine alpha S1-casein tryptic hydrolysate (CTH) on sleep disorder in Japanese general population. Open Sleep J. 2009, 2, 26–32. [Google Scholar] [CrossRef]
- Guesdon, B.; Messaoudi, M.; Lefranc-Millot, C.; Fromentin, G.; Tomé, D.; Even, P.C. A tryptic hydrolysate from bovine milk αS1-casein improves sleep in rats subjected to chronic mild stress. Peptides 2006, 27, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, G.; Milgram, B.; Mougeot, I.; Kelly, S.; de Rivera, C. Therapeutic effects of an alpha-casozepine and L-tryptophan supplemented diet on fear and anxiety in the cat. J. Feline Med. Surg. 2017, 19, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, N.; Milesi, C.; Burn, O.; van den Bogert, B.; Nauta, A.; Hart, K.; Sowden, P.; Burnet, P.W.J.; Cohen Kadosh, K. Anxiolytic effects of a galacto-oligosaccharides prebiotic in healthy females (18–25 years) with corresponding changes in gut bacterial composition. Sci. Rep. 2021, 11, 8302. [Google Scholar] [CrossRef]
- Dela Peña, I.J.I.; Kim, H.J.; de la Peña, J.B.; Kim, M.; Botanas, C.J.; You, K.Y.; Woo, T.; Lee, Y.S.; Jung, J.C.; Kim, K.M.; et al. A tryptic hydrolysate from bovine milk αs1-casein enhances pentobarbital-induced sleep in mice via the GABAA receptor. Behav. Brain Res. 2016, 313, 184–190. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.; Lee, S.; Kim, B.; Kwon, E.; Lee, J.E.; Chun, M.Y.; Lee, C.Y.; Boulier, A.; Oh, S.; et al. A double-blind, randomized, placebo-controlled crossover clinical study of the effects of alpha-s1 casein hydrolysate on sleep disturbance. Nutrients 2019, 11, 1466. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, E. Meet the ‘psychobiome’: The gut bacteria that may alter how you think, feel, and act. Science 2020, 200. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Thompson, R.S.; Vargas, F.; Dorrestein, P.C.; Chichlowski, M.; Berg, B.M.; Fleshner, M. Dietary prebiotics alter novel microbial dependent fecal metabolites that improve sleep. Sci. Rep. 2020, 10, 3848. [Google Scholar] [CrossRef]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Drennan, M.D.; Kripke, D.F.; Klemfuss, H.A.; Moore, J.D. Potassium affects actigraph-identified sleep. Sleep 1991, 14, 357–360. [Google Scholar] [PubMed]
- Tarleton, E.K.; Littenberg, B. Magnesium Intake and Depression in Adults. J. Am. Board Fam. Med. 2015, 28, 249–256. [Google Scholar] [CrossRef]
- Tarleton, E.; Littenberg, B.; MacLean, C.D.; Kennedy, A.G.; Daley, C. Role of magnesium in the treatment of depression. PLoS ONE 2017, 32, S311. [Google Scholar] [CrossRef]
- Driessens, F.C.M.; Verbeeck, R.M.H.; Van Dijk, J.W.E. Is systemic treatment of osteoporosis and mandibular resorption possible? Acta Stomatol. Belg. 1990, 87, 107–124. [Google Scholar]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A.M.; Traversa, U. Mechanisms of inhibitory effects of zinc and cadmium ions on agonist binding to adenosine A1 receptors in rat brain. Biochem. Pharmacol. 1999, 58, 623–632. [Google Scholar] [CrossRef]
- Schetz, J.A.; Sibley, D.R. Zinc allosterically modulates antagonist binding to cloned D1 and D2 dopamine receptors. J. Neurochem. 1997, 68, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.H.; Peters, J.A.; Lambert, J.J. An electrophysiological investigation of the properties of a murine recombinant 5-HT3 receptor stably expressed in HEK 293 cells. Br. J. Pharmacol. 1995, 114, 1211–1221. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 18, 193–213. [Google Scholar] [CrossRef]
- De Beurs, E.; Van Dyck, R.; Marquenie, L.A.; Lange, A.; Blonk, R.W.B. De DASS: Een vragenlijst voor het meten van depressie, angst en stress. Gedragstherapie 2001, 34, 35–53. [Google Scholar]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.M.; Cercamondi, C.I.; Moretti, D.; Barth-Jaeggi, T.; Schwab, C.; Boekhorst, J.; Timmerman, H.M.; Lacroix, C.; et al. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in Kenyan infants. Gut 2017, 66, 1956–1967. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, 141–145. [Google Scholar] [CrossRef]
- Wellek, S.; Blettner, M. Vom richtigen Umgang mit dem Crossover-Design in klinischen Studien: Teil 18 der Serie zur Bewertung wissenschaftlicher Publikationen. Dtsch. Arztebl. Int. 2012, 109, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Twisk, J.W.R. Inleiding in de Toegepaste Statistiek, 3rd ed.; Twisk, J.W.R., Ed.; Bohn Staflei van Loghum: Houten, The Netherlands, 2016; ISBN 978-90-368-1533-8. [Google Scholar]
- Ter Braak, C.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Lovibond, P.F.; Lovibond, S.H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Axiety Inventories. Behav. Res. Ther. 1995, 33, 335–342. [Google Scholar] [CrossRef]
- Lovibond, S.H.; Lovibond, P.F. Psychology Foundation of Australia. In Manual for the Depression Anxiety Stress Scales; School of Psychology, University of New South Wales: Sydney, Australia, 1995. [Google Scholar]
- Scholey, A.; Benson, S.; Gibbs, A.; Perry, N.; Sarris, J.; Murray, G. Exploring the effect of lactiumTM and zizyphus complex on sleep quality: A double-blind, randomized placebo-controlled trial. Nutrients 2017, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Wait, A.; Tang, Y.-R.; Cheng, H.-M.; Tai, C.-J.; Chien, L.-Y. Acupressure effect on sleep quality: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 37, 24–34. [Google Scholar] [CrossRef]
- Hidese, S.; Ogawa, S.; Ota, M.; Ishida, I.; Yasukawa, Z.; Ozeki, M.; Kunugi, H. Effects of L-Theanine administration on stress-related symptoms and cognitive functions in healthy adults: A randomized controlled trial. Nutrients 2019, 11, 2362. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, P.A. The anxiolytic effect of Bifidobacterium longum NCC3001. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Kiers, J.L.; Bakker, G.C.M.; Bakker-zierikzee, A.M.; Smits, M.G.; Schaafsma, A. Sleep improving effects of enriched milk, a randomised double-blind trial in adult women with insomnia. Nutrafoods 2007, 6, 19–27. [Google Scholar]
- Merens, W.; Booij, L.; Markus, R.; Zitman, F.G.; Onkenhout, W.; Van der Does, A.J.W. The effects of a diet enriched with α-lactalbumin on mood and cortisol response in unmedicated recovered depressed subjects and controls. Br. J. Nutr. 2005, 94, 415–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Savignac, H.M.; Tramullas, M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015, 287, 59–72. [Google Scholar] [CrossRef]
- Sijbers, A.M.; Schoemaker, R.J.W.; Nauta, A.; Alkema, W. Revealing new leads for the impact of galacto-oligosaccharides on gut commensals and gut health benefits through text mining. Benef. Microbes 2020, 11, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Holscher, H.D. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr. Neurosci. 2020, 23, 237–250. [Google Scholar] [CrossRef]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Gastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome Study population. Alim. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, H.K.J.; Mazuy, C.; Rutters, F.; Martens, E.A.P.; Adam, T.C.; Westerterp-Plantenga, M.S. Sleep architecture when sleeping at an unusual circadian time and associations with insulin sensitivity. PLoS ONE 2013, 8, e72877. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kunz, D.; Mahlberg, R.; Müller, C.; Tilmann, A.; Bes, F. Melatonin in patients with reduced REM sleep duration: Two randomized controlled trials. J. Clin. Endocrinol. Metab. 2004, 89, 128–134. [Google Scholar] [CrossRef] [PubMed]
| Nutrient | Unit | DP 4 | Placebo |
|---|---|---|---|
| Amount per sachet 1 | g | 19 | 12 |
| Protein | g | 10 | 3.62 |
| whey protein | g | 9.5 | 0.72 |
| casein | g | 2.90 | |
| tryptophan | mg | 500 | 75 |
| other LNAA 1 | mg | 2930 | 573 |
| tryptic casein hydrolysate 2 | mg | 200 | |
| Lactose | g | 1.9 | 5.4 |
| GOS 3 | g | 5.2 | |
| Magnesium | mg | 200 | 13.1 |
| Zinc | mg | 5 | 0.12 |
| Vitamin B6 | mg | 1 | 0.03 |
| Niacin | mg | 10 | 0.10 |
| Vitamin D3 | µ | 10 | <0.15 |
| ITT | PP | modPP | |
|---|---|---|---|
| n | 69 | 64 | 47 |
| Gender: female/male (n) [ratio] | 54/15 [3.6] | 51/13 [3.9] | 37/10 [3.7] |
| Age (y) | 39.5 ± 6.3 | 39.1 ± 6.2 | 38.5 ± 6.4 |
| Height (m) | 1.71 ± 0.10 | 1.71 ± 0.09 | 1.71 ± 0.08 |
| Weight (kg) | 68.2 ± 11.2 | 67.3 ±11.0 | 68.2 ± 10.5 |
| BMI (kg/m2) | 23.1 ± 2.0 | 23.1 ± 2.0 | 23.3 ± 1.9 |
| PSQI (at baseline) | 10.3 ±2.8 | 10.2 ±2.7 | 11.5 ± 2.0 a |
| Normal time to bed (h:min) | 23:37 ± 01:11 | 23:39 ± 01:13 | 23:40 ± 01:14 |
| Normal time to rise (h:min) | 07:38 ± 00:55 | 07:39 ± 00:56 | 07:42 ± 00:59 |
| Daily caffeine containing coffee (cups, (min-max)) | 1.7 ± 1.5 (0-6.3) | 1.7 ± 1.5 (0-6.3) | 1.8 ± 1.5 (0-6.3) |
| Daily alcohol containing drink (servings (min-max)) | 0.7 ± 0.7 (0-3.1) | 0.6 ± 0.7 (0-3.0) | 0.6 ± 0.6 (0-7) |
| Watching TV 30 min before bedtime (n,%) | 25 (36.2) | 24 (37.5) | 17 (36.2) |
| Watching computer 30 min before bedtime (n,%) | 4 (5.7) | 4 (6.3) | 3 (6.4) |
| Using the telephone 30 min before bedtime (n,%) | 37 (53.6) | 36 (56.3) | 26 (55.3) |
| Reading a book 30 min before bedtime (n,%) | 5 (7.2) | 5 (7.8) | 2 (4.3) |
| Self-reported mood quality at rise (5-1: good-bad) | 2.7 ± 1.0 | 2.7 ± 1.0 | 3.0 ± 1.0 |
| Self-reported fitness quality at rise (5-1: good-bad) | 2.9 ± 1.0 | 2.9 ± 1.0 | 3.1 ± 1.0 |
| Products | p-Value 1 | ||
|---|---|---|---|
| DP | Placebo | ||
| ITT | |||
| Change d0–d7 | −0.72 ± 3.25 | −0.62 ± 2.64 | 0.841 |
| Change d0–d14 | −0.93 ± 2.84 | −0.41 ± 3.35 | 0.325 |
| Change d0–d21 | −0.46 ± 3.05 | −0.36 ± 3.14 | 0.848 |
| Time effect 2, p-value | 0.379 | 0.723 | |
| Post-hoc analysis | >0.370 | 1.0 | |
| PSQI d0 | 9.83 ± 2.90 | 9.91 ± 3.04 | 0.817 |
| PSQI d7 | 9.24 ± 2.90 | 9.29 ± 3.43 | 0.863 |
| PSQI d14 | 8.90 ± 2.61 | 9.51 ± 3.11 | 0.216 |
| PSQI d21 | 9.36 ± 3.08 | 9.55 ± 3.02 | 0.717 |
| Time effect 2, p-value | 0.072 | 0.389 | |
| Post-hoc analysis | >0.068 | >0.321 | |
| PP | |||
| Change d0–d7 | −0.63 ± 3.32 | −0.55 ± 2.49 | 0.841 |
| Change d0–d14 | −0.92 ± 2.93 | −0.22 ± 3.24 | 0.325 |
| Change d0–d21 | −0.50 ± 3.01 | −020 ± 2.99 | 0.848 |
| Time effect 2, p-value | 0.429 | 0.574 | |
| Post-hoc analysis | >0.487 | >0.992 | |
| PSQI d0 | 9.84 ± 3.00 | 9.89 ± 2.92 | 0.864 |
| PSQI d7 | 9.37 ± 2.89 | 9.34 ± 3.38 | 0.920 |
| PSQI d14 | 8.92 ± 2.67 | 9.67 ± 3.13 | 0.216 |
| PSQI d21 | 9.34 ± 3.01 | 9.69 ± 3.06 | 0.717 |
| Time effect 2, p-value | 0.101 | 0.511 | |
| Post-hoc analysis | >0.117 | >0.501 | |
| ModPP | |||
| Change d0–d7 | −0.91 ± 3.10 | −066 ± 2.60 | 0.673 |
| Change d0–d14 | −1.60 ± 2.53 | −0.30 ±3.28 | 0.034 |
| Change d0–d21 | −1.17 ± 2.76 | 0.43 ± 3.17 | 0.227 |
| Time effect 2, p-value | 0.228 | 0.682 | |
| Post-hoc analysis | >0.448 | 1.0 | |
| PSQI d0 | 10.85 ± 2.55 | 10.57 ± 2.68 | 0.609 |
| PSQI d7 | 9.87 ± 2.97 | 9.91 ± 3.28 | 0.944 |
| PSQI d14 | 9.26 ± 2.83 | 10.28 ± 2.85 | 0.085 |
| PSQI d21 | 9.68 ± 3.07 | 10.15 ± 3.25 | 0.474 |
| Time effect 2, p-value | 0.001 | 0.487 | |
| Post-hoc analysis | 0–14 d: 0.001 0–21 d: 0.055 | >0.531 | |
| Products | p-Value 1 | ||
|---|---|---|---|
| DP | Placebo | ||
| ITT | |||
| DASS total score d0 | 16.5 ± 15.4; 13.0 (13.5) | 14.2 ± 10.7; 12.0 (13.5) | 0.510 |
| DASS total score d21 | 14.9 ± 13.1; 11.0 (16.5) | 13.0 ± 11.5; 11.0 (14.0) | 0.427 |
| Within group2; p | 0.150 | 0.038 | |
| DASS stress d0 | 7.4 ± 5.9; 6.0 (8.0) | 6.7 ± 4.5; 6.0 (7.0) | 0.611 |
| DASS stress d21 | 6.7 ± 4.8; 6.0 (7.0) | 5.9 ± 4.8; 4.0 (7.0) | 0.255 |
| Within group; p | 0.318 | 0.041 | |
| DASS anxiety d0 | 3.1 ± 4.2; 2.0 (2.5) | 2.4 ± 2.9; 1.0 (2.0) | 0.291 |
| DASS anxiety d21 | 2.7 ± 3.4; 2.0 (3.0) | 2.1 ± 2.9; 1.0 (2.0) | 0.178 |
| Within group; p | 0.374 | 0.116 | |
| DASS depression d0 | 6.0 ± 7.3; 3.0 (6.0) | 5.1 ± 5.1; 3.0 (6.0) | 0.679 |
| DASS depression d21 | 5.5 ± 6.7; 3.0 (6.5) | 5.0 ± 5.3; 3.0 (7.0) | 0.918 |
| Within group; p | 0.260 | 0.649 | |
| PP | |||
| DASS total score d0 | 17.0 ± 15.9; 14.0 (14.8) | 14.3 ± 10.8; 12.0 (12.5) | 0.426 |
| DASS total score d21 | 14.7 ± 13.0; 11.0 (15.0) | 13.1 ± 11.8; 10.5 (13.5) | 0.492 |
| Within group; p | 0.055 | 0.055 | |
| DASS stress d0 | 7.7 ± 6.0; 6.0 (6.0) | 6.8 ± 4.5; 6.0 (6.8) | 0.441 |
| DASS stress d21 | 6.7 ± 4.7; 6.0 (6.8) | 5.9 ± 4.8; 4.5 (7.0) | 0.316 |
| Within group; p | 0.125 | 0.044 | |
| DASS anxiety d0 | 3.2 ± 4.3; 2.0 (3.0) | 2.5 ± 2.9; 1.0 (2.0) | 0.304 |
| DASS anxiety d21 | 2.7 ± 3.3; 1.5(2.8) | 2.2 ± 3.0; 1.0 (2.0) | 0.224 |
| Within group; p | 0.325 | 0.187 | |
| DASS depression d0 | 6.1 ± 7.5; 3.0 (7.5) | 5.0 ± 5.2; 3.0 (6.0) | 0.694 |
| DASS depression d21 | 5.3 ± 6.7; 3.0 (5.0) | 5.0 ± 5.4; 2.5 (6.8) | 0.927 |
| Within group; p | 0.102 | 0.810 | |
| modPP | |||
| DASS total score d0 | 19.1 ± 17.2; 16.0 (16.0) | 15.8 ± 11.3; 13.0 (13.0) | 0.382 |
| DASS total score d21 | 16.4 ± 13.9; 11.0 (19.0) | 14.4 ± 12.1; 12.0 (17.0) | 0.633 |
| Within group; p | 0.065 | 0.098 | |
| DASS stress d0 | 8.5 ± 6.3; 8.0 (6.0) | 7.5 ± 4.2; 7.0 (6.0) | 0.521 |
| DASS stress d21 | 7.3 ± 4.5; 7.0 (6.0) | 6.4 ± 5.0; 5.0 (6.0) | 0.275 |
| Within group; p | 0.131 | 0.048 | |
| DASS anxiety d0 | 3.5 ± 4.7; 2.0 (3.0) | 2.6 ± 3.2; 2.0 (2.0) | 0.319 |
| DASS anxiety d21 | 3.0 ± 3.7; 2.0 (5.0) | 2.2 ± 3.0; 1.0 (2.0) | 0.206 |
| Within group; p | 0.522 | 0.094 | |
| DASS depression d0 | 7.1 ± 8.2; 4.0 (8.0) | 5.6 ± 5.5; 3.0 (7.0) | 0.487 |
| DASS depression d21 | 6.1 ± 7.4; 4.0 (10.0) | 5.8 ± 5.6; 4.0 (9.0) | 0.706 |
| Within group; p | 0.067 | 0.674 | |
| Period 1 + 2 | p-Value 1 | ||
|---|---|---|---|
| DP | Placebo | ||
| ITT | |||
| Cortisol d0, 0 min | 3.36 ± 1.54; 3.20 (2.20) | 3.38 ± 1.44; 3.05 (2.18) | 0.973 |
| Cortisol d21, 0 min | 3.19 ± 1.45; 3.00 (2.20) | 3.78 ± 1.78; 3.50 (1.85) | 0.045 |
| Within group; p | 0.316 | 0.161 | |
| PP | |||
| Cortisol d0, 0 min | 3.38 ± 1.56; 3.20 (2.10) | 3.34 ± 1.44; 3.00 (2.05) | 0.784 |
| Cortisol d21, 0 min | 3.21 ± 1.45; 3.20 (2.20) | 3.83 ± 1.82; 3.50 (1.95) | 0.059 |
| Within group; p | 0.414 | 0.098 | |
| modPP | |||
| Cortisol d0, 0 min | 3.38 ± 1.64; 3.10 (1.90) | 3.38 ± 1.46; 3.10 (2.15) | 0.788 |
| Cortisol d21, 0 min | 3.03 ± 1.36; 2.90 (2.10) | 3.90 ± 1.91; 3.60 (2.05) | 0.033 |
| Within group; p | 0.248 | 0.170 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaafsma, A.; Mallee, L.; van den Belt, M.; Floris, E.; Kortman, G.; Veldman, J.; van den Ende, D.; Kardinaal, A. The Effect of A Whey-Protein and Galacto-Oligosaccharides Based Product on Parameters of Sleep Quality, Stress, and Gut Microbiota in Apparently Healthy Adults with Moderate Sleep Disturbances: A Randomized Controlled Cross-Over Study. Nutrients 2021, 13, 2204. https://doi.org/10.3390/nu13072204
Schaafsma A, Mallee L, van den Belt M, Floris E, Kortman G, Veldman J, van den Ende D, Kardinaal A. The Effect of A Whey-Protein and Galacto-Oligosaccharides Based Product on Parameters of Sleep Quality, Stress, and Gut Microbiota in Apparently Healthy Adults with Moderate Sleep Disturbances: A Randomized Controlled Cross-Over Study. Nutrients. 2021; 13(7):2204. https://doi.org/10.3390/nu13072204
Chicago/Turabian StyleSchaafsma, Anne, Leonard Mallee, Maartje van den Belt, Esther Floris, Guus Kortman, Jouke Veldman, Daan van den Ende, and Alwine Kardinaal. 2021. "The Effect of A Whey-Protein and Galacto-Oligosaccharides Based Product on Parameters of Sleep Quality, Stress, and Gut Microbiota in Apparently Healthy Adults with Moderate Sleep Disturbances: A Randomized Controlled Cross-Over Study" Nutrients 13, no. 7: 2204. https://doi.org/10.3390/nu13072204
APA StyleSchaafsma, A., Mallee, L., van den Belt, M., Floris, E., Kortman, G., Veldman, J., van den Ende, D., & Kardinaal, A. (2021). The Effect of A Whey-Protein and Galacto-Oligosaccharides Based Product on Parameters of Sleep Quality, Stress, and Gut Microbiota in Apparently Healthy Adults with Moderate Sleep Disturbances: A Randomized Controlled Cross-Over Study. Nutrients, 13(7), 2204. https://doi.org/10.3390/nu13072204