Graph Theory-Based Electroencephalographic Connectivity and Its Association with Ketogenic Diet Effectiveness in Epileptic Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol
2.3. EEG Recording
2.4. EEG Preprocessing
2.5. Functional Connectivity
2.6. Graph Theoretical Analysis
2.7. Statistical Analysis
3. Results
3.1. Patient Enrollment
3.2. Patient Demographics and Response to KDT
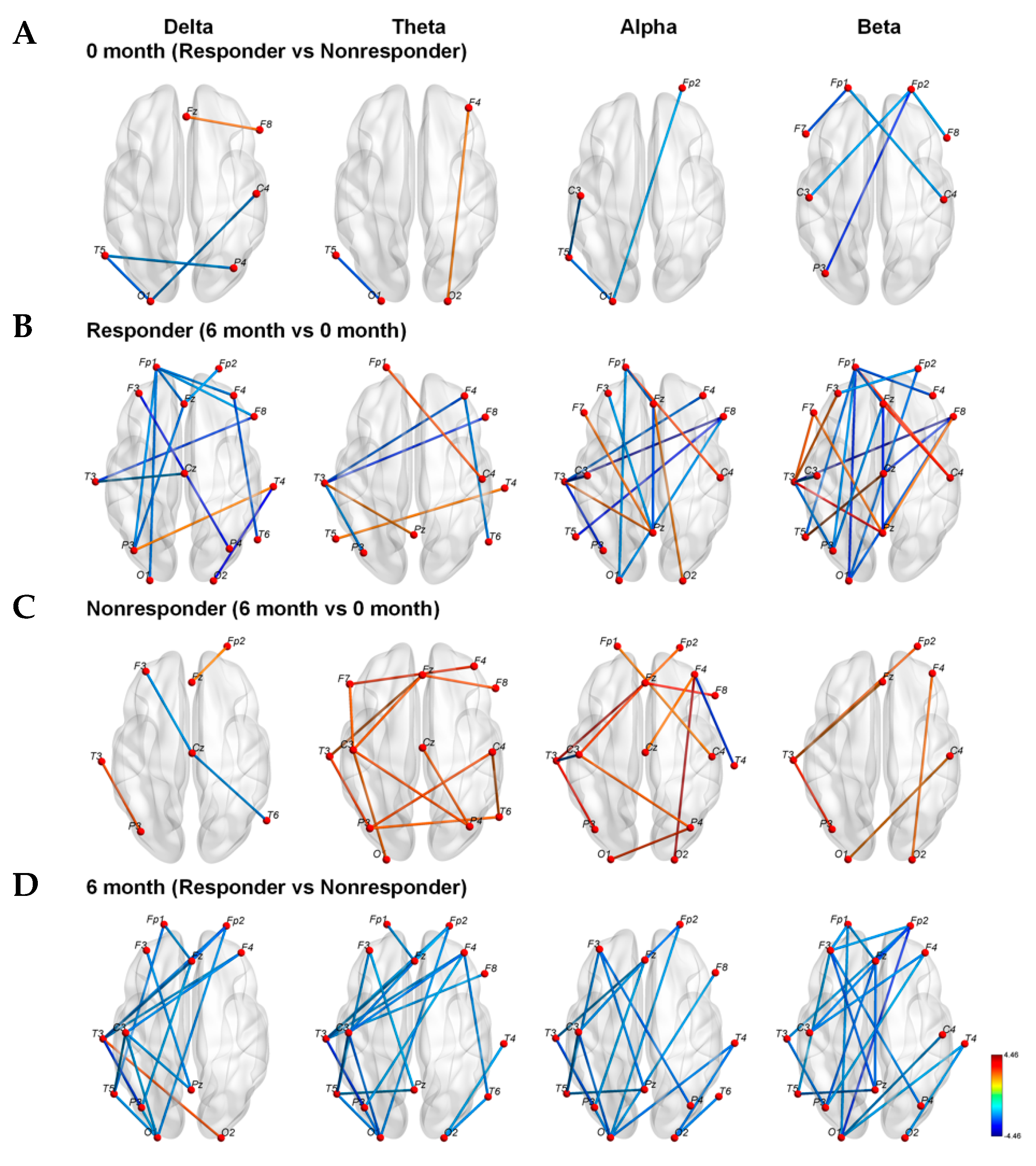
3.3. Strengths of Connections between Nodes
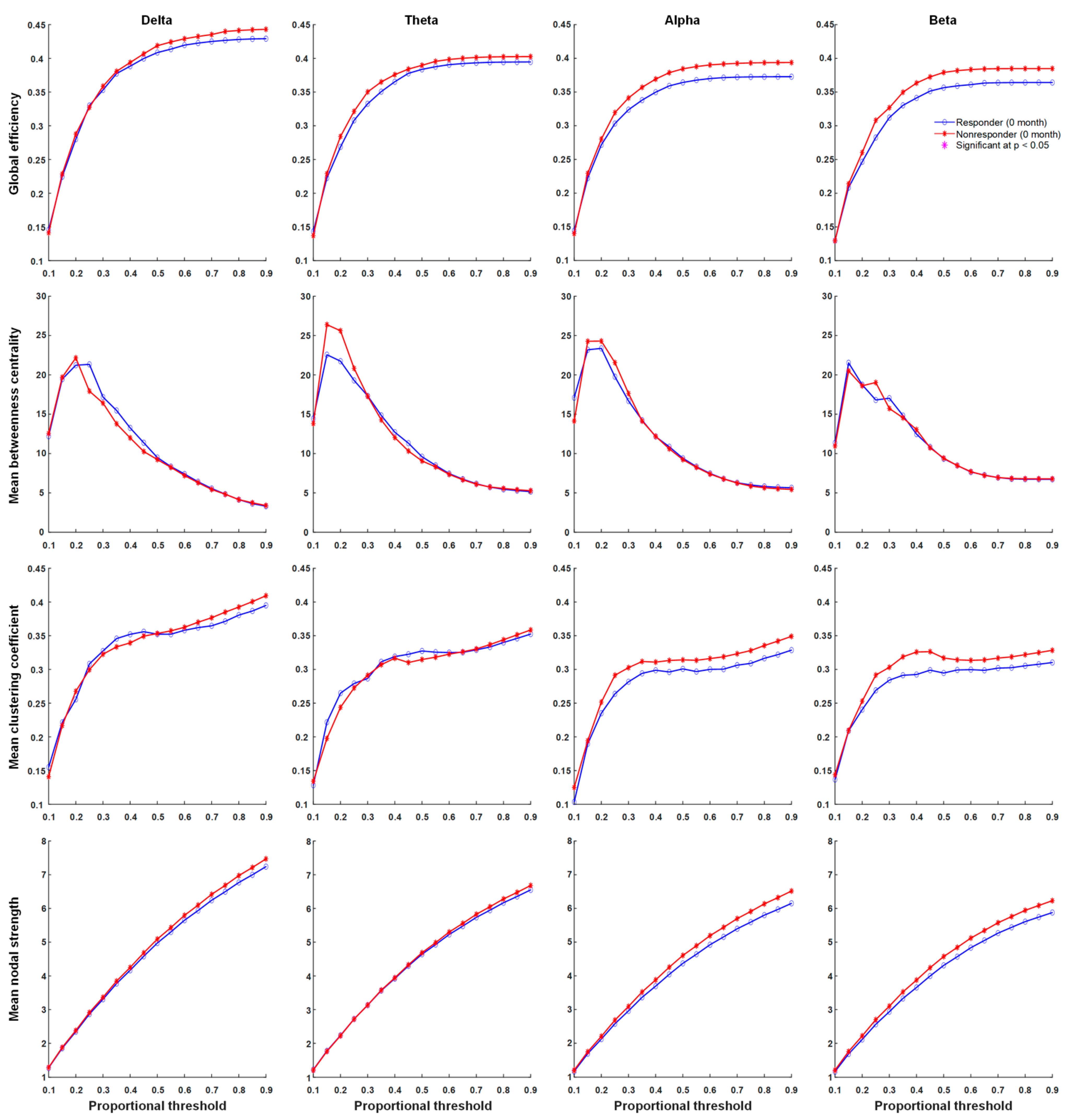
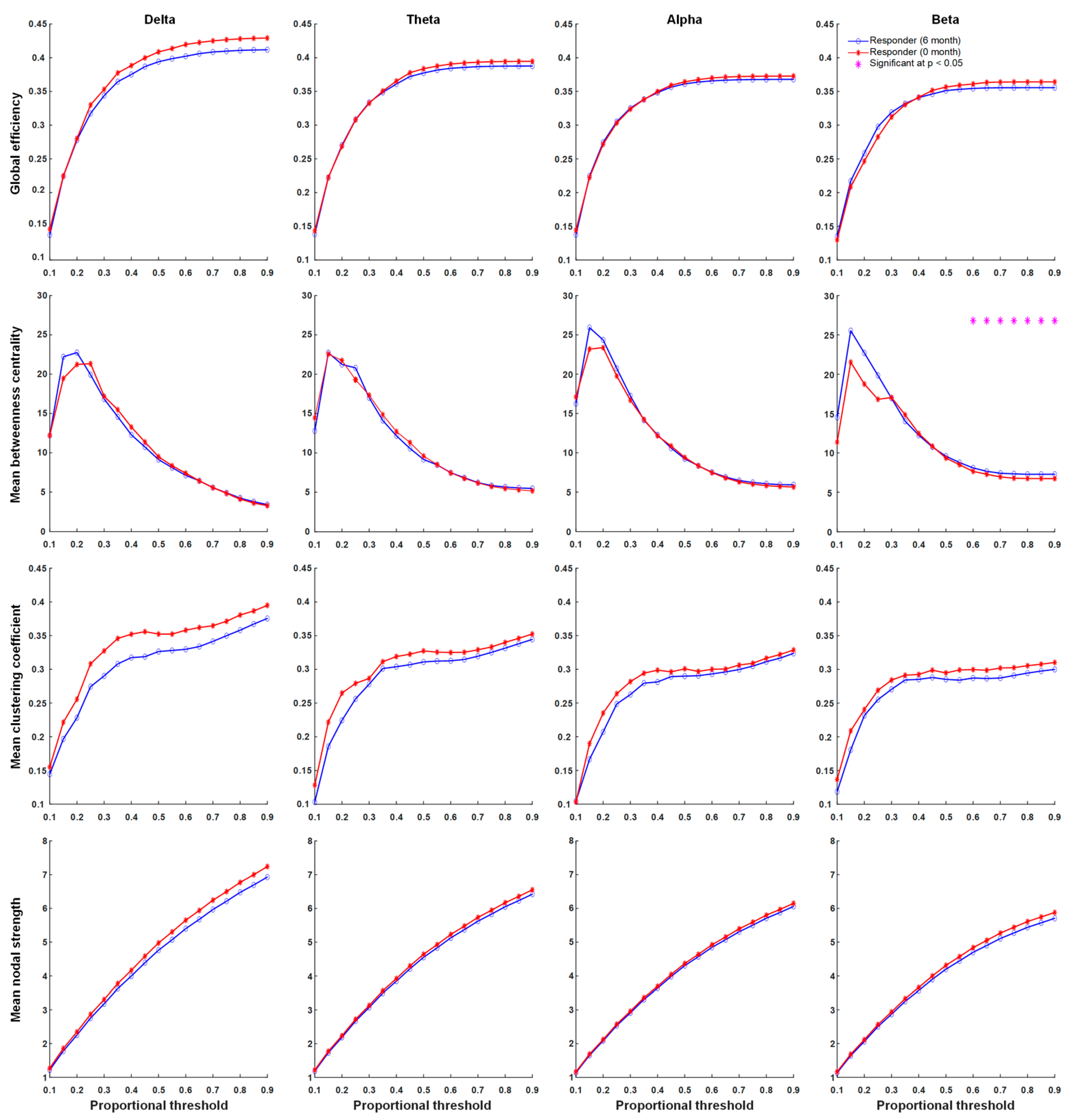
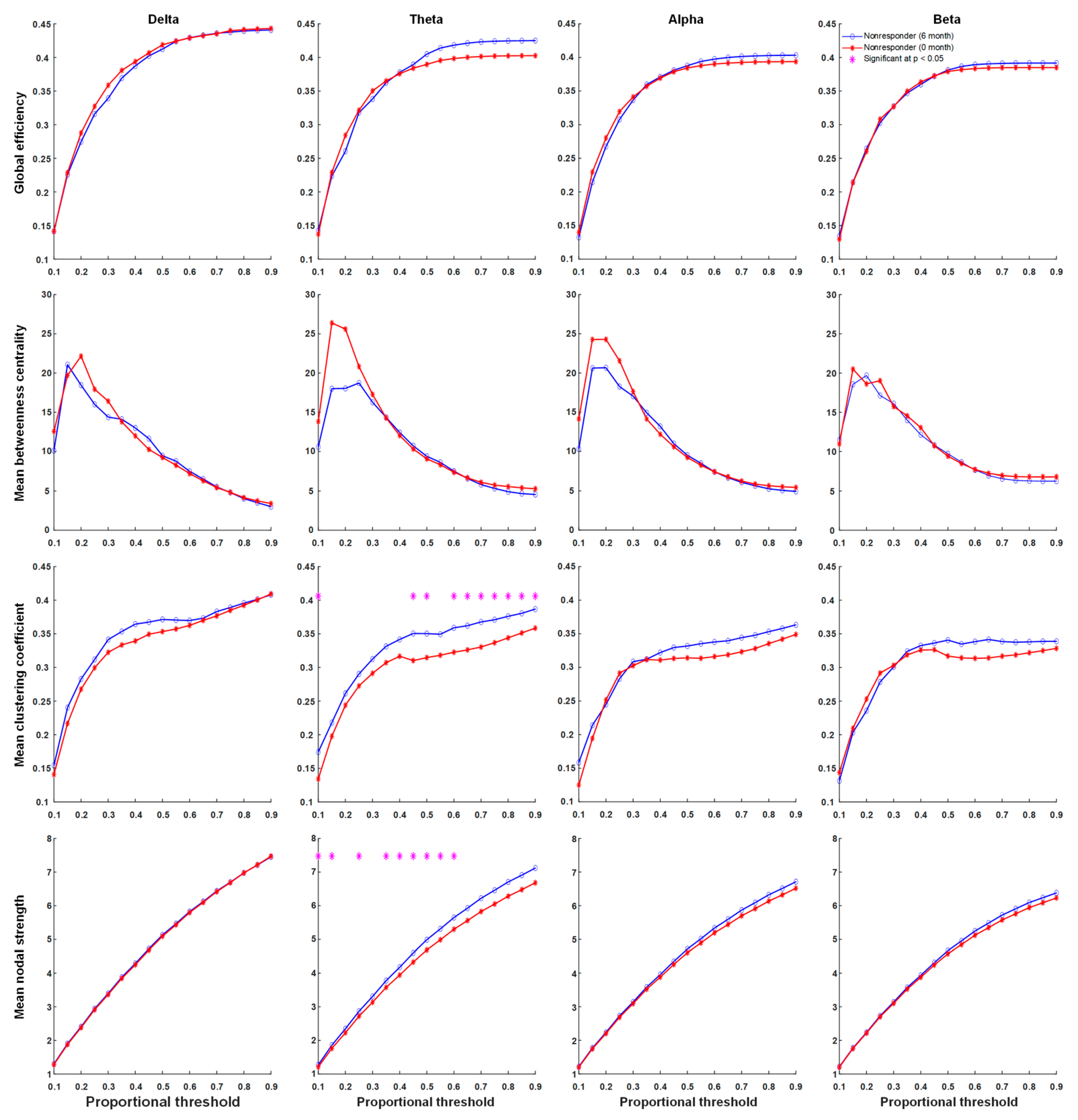
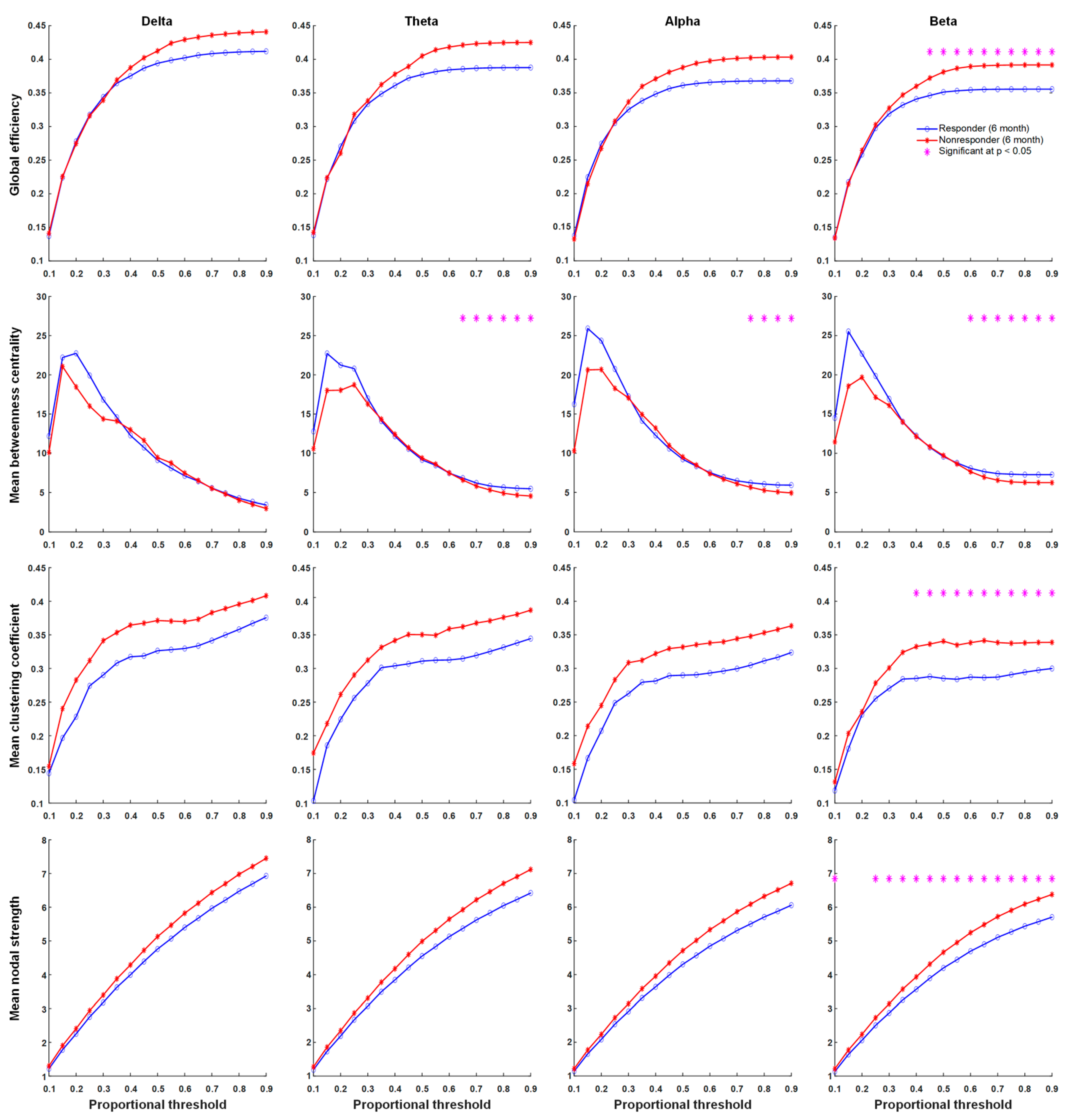
3.4. Functional Connectivity
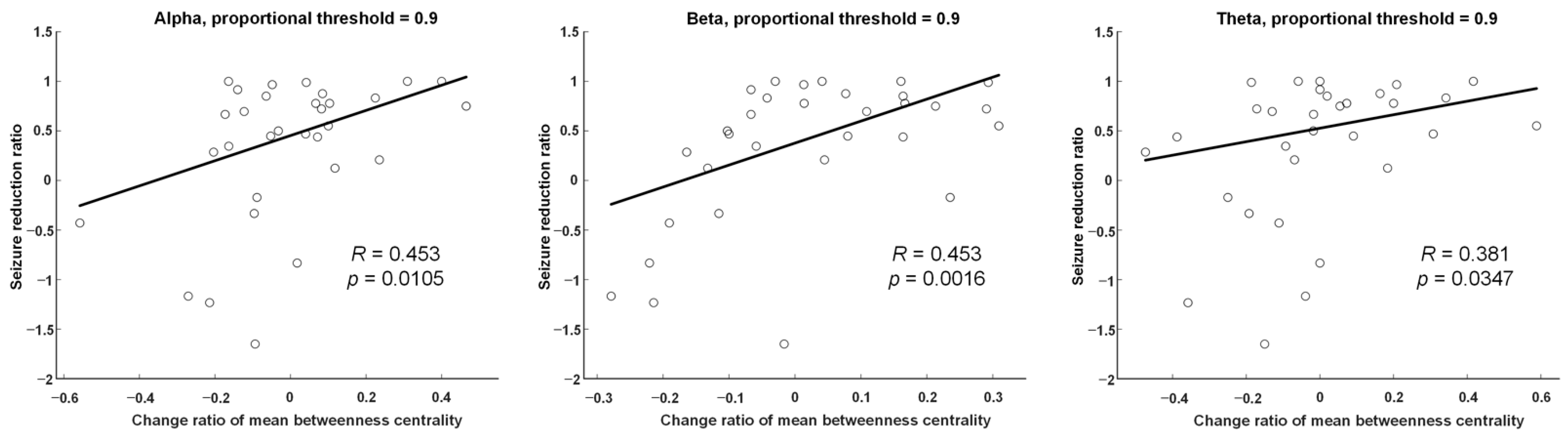
3.5. Correlations with Clinical Variates
4. Discussion
4.1. Neuronal Synchrony Was Persistently High in Non-Responders after Six Months of KDTs
4.2. Global Connectivity Was Weaker in Responders Than in Non-Responders after Six Months of KDTs
4.3. Betweenness Centrality Intensified in Responders after Six Months of KDTs
4.4. Betweenness Centrality Changes were Positively Correlated with Seizure Reduction Rates
4.5. Future Prospective
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keene, D.L. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediatr. Neurol. 2006, 35, 1–5. [Google Scholar] [CrossRef]
- Van der Louw, E.; van den Hurk, D.; Neal, E.; Leiendecker, B.; Fitzsimmon, G.; Dority, L.; Thompson, L.; Marchió, M.; Dudzińska, M.; Dressler, A.; et al. Ketogenic diet guidelines for infants with refractory epilepsy. Eur. J. Paediatr. Neurol. 2016, 20, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Mackay, M.T.; Bicknell-Royle, J.; Nation, J.; Humphrey, M.; Harvey, A.S. The ketogenic diet in refractory childhood epilepsy. J. Paediatr. Child Health 2005, 41, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, N.E.; Cross, J.H.; Sander, J.W.; Sisodiya, S.M. Can we predict a favourable response to Ketogenic Diet Therapies for drug-resistant epilepsy? Epilepsy Res. 2013, 106, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vehmeijer, F.O.L.; van der Louw, E.J.T.M.; Arts, W.F.M.; Catsman-Berrevoets, C.E.; Neuteboom, R.F. Can we predict efficacy of the ketogenic diet in children with refractory epilepsy? Eur. J. Paediatr. Neurol. 2015, 19, 701–705. [Google Scholar] [CrossRef]
- Schoeler, N.E.; Leu, C.; White, J.; Plagnol, V.; Ellard, S.; Matarin, M.; Yellen, G.; Thiele, E.A.; Mackay, M.; McMahon, J.M.; et al. Variants in KCNJ11 and BAD do not predict response to ketogenic dietary therapies for epilepsy. Epilepsy Res. 2015, 118, 22–28. [Google Scholar] [CrossRef]
- Bergqvist, A.G.; Schall, J.I.; Richard, E.L.; Gallagher, P.R.; Stallings, V.A. Predictive power of first morning glucose and the ketogenic diet. Neuropediatrics 2007, 38, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Maydell, B.V.; Wyllie, E.; Akhtar, N.; Kotagal, P.; Powaski, K.; Cook, K.; Weinstock, A.; Rothner, A.D. Efficacy of the ketogenic diet in focal versus generalized seizures. Pediatr. Neurol. 2001, 25, 208–212. [Google Scholar] [CrossRef]
- Dressler, A.; Stöcklin, B.; Reithofer, E.; Benninger, F.; Freilinger, M.; Hauser, E.; Reiter-Fink, E.; Seidl, R.; Trimmel-Schwahofer, P.; Feucht, M. Long-term outcome and tolerability of the ketogenic diet in drug-resistant childhood epilepsy—The Austrian experience. Seizure 2010, 19, 404–408. [Google Scholar] [CrossRef]
- Than, K.D.; Kossoff, E.H.; Rubenstein, J.E.; Pyzik, P.L.; McGrogan, J.R.; Vining, E.P. Can you predict an immediate, complete, and sustained response to the ketogenic diet? Epilepsia 2005, 46, 580–582. [Google Scholar] [CrossRef]
- Hallböök, T.; Köhler, S.; Rosén, I.; Lundgren, J. Effects of ketogenic diet on epileptiform activity in children with therapy resistant epilepsy. Epilepsy Res. 2007, 77, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.; Jose Miranda, M.; Alving, J.; Heber Povlsen, J.; Wolf, P. Effectiveness of the ketogenic diet in a broad range of seizure types and EEG features for severe childhood epilepsies. Acta Neurol. Scand. 2010, 121, 58–62. [Google Scholar] [CrossRef]
- Kessler, S.K.; Gallagher, P.R.; Shellhaas, R.A.; Clancy, R.R.; Bergqvist, A.G.C. Early EEG improvement after ketogenic diet initiation. Epilepsy Res. 2011, 94, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Ebus, S.C.M.; Lambrechts, D.A.J.E.; Herraets, I.J.T.; Majoie, M.J.M.; de Louw, A.J.; Boon, P.J.; Aldenkamp, A.P.; Arends, J.B. Can an early 24-h EEG predict the response to the ketogenic diet? A prospective study in 34 children and adults with refractory epilepsy treated with the ketogenic diet. Seizure 2014, 23, 468–474. [Google Scholar] [CrossRef][Green Version]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Makeig, S.; Jung, T.-P.; Bell, A.J.; Ghahremani, D.; Sejnowski, T.J. Blind separation of auditory event-related brain responses into independent components. Proc. Natl. Acad. Sci. USA 1997, 94, 10979–10984. [Google Scholar] [CrossRef]
- Stam, C.J.; Nolte, G.; Daffertshofer, A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007, 28, 1178–1193. [Google Scholar] [CrossRef]
- Genovese, C.R.; Lazar, N.A.; Nichols, T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002, 15, 870–878. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Staba, R.J.; Worrell, G.A. What is the importance of abnormal “background” activity in seizure generation? Adv. Exp. Med. Biol. 2014, 813, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Towle, V.L.; Carder, R.K.; Khorasani, L.; Lindberg, D. Electrocorticographic Coherence Patterns. Clin. Neurophysiol. 1999, 16, 528. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, H.P.; Pincus, S.M.; Goncharova, I.I.; Duckrow, R.B.; Spencer, D.D.; Spencer, S.S. Localization-related epilepsy exhibits significant connectivity away from the seizure-onset area. Neuroreport 2009, 20, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Schevon, C.A.; Cappell, J.; Emerson, R.; Isler, J.; Grieve, P.; Goodman, R.; McKhann, G., Jr.; Weiner, H.; Doyle, W.; Kuzniecky, R.; et al. Cortical abnormalities in epilepsy revealed by local EEG synchrony. Neuroimage 2007, 35, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Turner, G.M.; Gray, B.C. Anticonvulsant effects of the experimental induction of hippocampal theta activity. Epilepsy Res. 1994, 18, 195–204. [Google Scholar] [CrossRef]
- Douw, L.; van Dellen, E.; de Groot, M.; Heimans, J.J.; Klein, M.; Stam, C.J.; Reijneveld, J.C. Epilepsy is related to theta band brain connectivity and network topology in brain tumor patients. BMC Neurosci. 2010, 11, 103. [Google Scholar] [CrossRef]
- Song, J.; Nair, V.A.; Gaggl, W.; Prabhakaran, V. Disrupted Brain Functional Organization in Epilepsy Revealed by Graph Theory Analysis. Brain Connect. 2015, 5, 276–283. [Google Scholar] [CrossRef]
- Englot, D.J.; Konrad, P.E.; Morgan, V.L. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia 2016, 57, 1546–1557. [Google Scholar] [CrossRef]
- Laiou, P.; Avramidis, E.; Lopes, M.A.; Abela, E.; Müller, M.; Akman, O.E.; Richardson, M.P.; Rummel, C.; Schindler, K.; Goodfellow, M. Quantification and Selection of Ictogenic Zones in Epilepsy Surgery. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Costa, A.-M.; Lucchi, C.; Malkoç, A.; Rustichelli, C.; Biagini, G. Relationship between Delta Rhythm, Seizure Occurrence and Allopregnanolone Hippocampal Levels in Epileptic Rats Exposed to the Rebound Effect. Pharmaceuticals 2021, 14, 127. [Google Scholar] [CrossRef]
- Marchiò, M.; Roli, L.; Lucchi, C.; Costa, A.M.; Borghi, M.; Iughetti, L.; Trenti, T.; Guerra, A.; Biagini, G. Ghrelin Plasma Levels After 1 Year of Ketogenic Diet in Children With Refractory Epilepsy. Front. Nutr. 2019, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Carboni, M.; Rubega, M.; Iannotti, G.R.; De Stefano, P.; Toscano, G.; Tourbier, S.; Pittau, F.; Hagmann, P.; Momjian, S.; Schaller, K.; et al. The network integration of epileptic activity in relation to surgical outcome. Clin. Neurophysiol. 2019, 130, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xu, C.; Zhang, X.; Qiao, L.; Wang, X.; Zhang, X.; Yan, X.; Ni, D.; Yu, T.; Zhang, G.; et al. The changes in the topological properties of brain structural network based on diffusion tensor imaging in pediatric epilepsy patients with vagus nerve stimulators: A graph theoretical analysis. Brain Dev. 2021, 43, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Pasca, L.; Guglielmetti, M.; Marazzi, C.; Trentani, C.; Varesio, C.; Tagliabue, A.; De Giorgis, V. Comment on: Ketogenic diet therapy provision in the COVID-19 pandemic: Dual-center experience and recommendations. Epilepsy Behav. 2020, 112, 107399. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.-M.; Marchiò, M.; Bruni, G.; Bernabei, S.M.; Cavalieri, S.; Bondi, M.; Biagini, G. Evaluation of E-Health Applications for Paediatric Patients with Refractory Epilepsy and Maintained on Ketogenic Diet. Nutrients 2021, 13, 1240. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Guglielmetti, M.; Tamagni, E.; Trentani, C.; De Giorgis, V.; Pasca, L.; Varesio, C.; Ferraro, O.E.; Tagliabue, A. Use of Remote Monitoring by E-mail for Long-Term Management of the Classic Ketogenic Diet. Nutrients 2020, 12, 1833. [Google Scholar] [CrossRef]
| Responders | Non-Responders | p-Value | |
|---|---|---|---|
| Age (years, Mean ± SD) | 6.69 ± 5.58 | 7.33 ± 6.09 | 0.409 |
| Gender (n, %) | 0.870 | ||
| Male | 8 (47.1) | 7 (50.0) | |
| Female | 9 (52.9) | 7 (50.0) | |
| Main Seizure type (n, %) | 0.816 | ||
| Focal onset | 8 (47.1) | 5 (35.7) | |
| Generalized onset | 8 (47.1) | 8 (57.1) | |
| Both | 1 (5.9) | 1 (7.1) | |
| Etiology | 0.395 | ||
| Structural | 1 (5.9) | 2 (14.3) | |
| Genetic | 7 (41.2) | 7 (50.0) | |
| Infectious | 0 (0.0) | 1 (7.1) | |
| Metabolic | 0 (0.0) | 0 (0.0) | |
| Immune | 0 (0.0) | 1 (7.1) | |
| HIE | 4 (23.5) | 1 (7.1) | |
| Unknown | 5 (29.4) | 2 (14.3) | |
| No. of AEDs (Mean ± SD) | 2.76 ± 1.03 | 2.86 ± 1.23 | 0.917 |
| Dosage of AEDs (mg/kg/day, Mean ± SD) | |||
| LEV | 30.21 ± 14.48 | 30.32 ± 19.19 | 0.934 |
| VPA | 26.34 ± 12.95 | 23.90 ± 11.92 | 0.462 |
| VGB | 65.54 ± 40.88 | 86.79 ± 24.71 | 0.602 |
| CZP | 0.05 ± 0.03 | 0.04 ± 0.02 | 1.000 |
| TPM | 4.55 ± 2.50 | 2.96 ± 1.25 | 0.180 |
| LTG | 2.38 | 1.19 ± 0.84 | 0.157 |
| PB | 5.40 ± 0.89 | 4.14 ± 1.73 | 0.480 |
| OXC | 23.62 ± 8.52 | 16.51 ± 6.35 | 0.355 |
| PER | 0.02 | 0.53 | 0.317 |
| Baseline seizure frequency (per month, Mean ± SD) | 103.59 ± 137.69 | 63.64 ± 77.20 | 0.905 |
| 6M seizure frequency (per month, Mean ± SD) | 20.82 ± 39.23 | 59.36 ± 70.87 | 0.011 * |
| 6M Fasting blood sugar (mg/dl) | 74.63 ± 11.31 | 77.46 ± 12.64 | 0.455 |
| 6M βHB (mmol/L) | 2.54 ± 1.82 | 2.78 ± 1.86 | 0.836 |
| EEG lateralization (n, %) | 0.748 | ||
| Right | 2 (11.8) | 3 (21.4) | |
| Left | 1 (5.9) | 1 (7.1) | |
| Bilateral | 14 (82.4) | 10 (71.4) | |
| Dominant hand (n, %) | 0.493 | ||
| Right | 13 (76.5) | 8 (57.1) | |
| Left | 1 (5.9) | 1 (7.1) | |
| N/A (<2 years) | 3 (17.6) | 5 (35.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, T.-Y.; Hung, P.-L.; Chen, C.; Lin, Y.-J.; Peng, S.-J. Graph Theory-Based Electroencephalographic Connectivity and Its Association with Ketogenic Diet Effectiveness in Epileptic Children. Nutrients 2021, 13, 2186. https://doi.org/10.3390/nu13072186
Su T-Y, Hung P-L, Chen C, Lin Y-J, Peng S-J. Graph Theory-Based Electroencephalographic Connectivity and Its Association with Ketogenic Diet Effectiveness in Epileptic Children. Nutrients. 2021; 13(7):2186. https://doi.org/10.3390/nu13072186
Chicago/Turabian StyleSu, Ting-Yu, Pi-Lien Hung, Chien Chen, Ying-Jui Lin, and Syu-Jyun Peng. 2021. "Graph Theory-Based Electroencephalographic Connectivity and Its Association with Ketogenic Diet Effectiveness in Epileptic Children" Nutrients 13, no. 7: 2186. https://doi.org/10.3390/nu13072186
APA StyleSu, T.-Y., Hung, P.-L., Chen, C., Lin, Y.-J., & Peng, S.-J. (2021). Graph Theory-Based Electroencephalographic Connectivity and Its Association with Ketogenic Diet Effectiveness in Epileptic Children. Nutrients, 13(7), 2186. https://doi.org/10.3390/nu13072186







