Vitamin D Status Is Associated with Modifiable Lifestyle Factors in Pre-Adolescent Children Living in Urban Kuala Lumpur, Malaysia
Abstract
:1. Introduction
2. Methodology
2.1. Participants
2.2. General Health Screening
2.3. Bone Parameters and Body Composition
2.4. Serum 25-OH Vitamin D and PTH
2.5. Calcium and Vitamin D Intake
2.6. Sun Index (SI)
2.7. Physical Activity
2.8. Anthropometry
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almoudi, M.M.; Hussein, A.S.; Abu Hassan, M.I.; Schroth, R.J. Dental caries and vitamin D status in children in Asia. Pediatr. Int. 2019, 61, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011, 14, 938–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. Horm. Res. Paediatr. 2016, 85, 83–106. [Google Scholar] [CrossRef] [Green Version]
- Khor, G.L.; Chee, W.S.S.; Shariff, Z.M.; Poh, B.K.; Arumugam, M.; Ab Rahman, J.; Theobald, H.E. High prevalence of vitamin D insufficiency and its association with BMI-for-age among primary school children in Kuala Lumpur, Malaysia. BMC Public Health 2011, 11, 95. [Google Scholar] [CrossRef] [Green Version]
- Poh, B.K.; Rojroongwasinkul, N.; Le Nguyen, B.K.; Sandjaja; Ruzita, A.T.; Yamborisut, U.; Hong, T.N.; Ernawati, F.; Deurenberg, P.; Parikh, P. 25-hydroxy-vitamin D demography and the risk of vitamin D insufficiency in the South East Asian Nutrition Surveys (SEANUTS). Asia Pac. J. Clin. Nutr. 2016, 25, 538. [Google Scholar]
- Taylor, S.N. Vitamin D in Preterm and Full-Term Infants. Ann. Nutr. Metab. 2020, 76, 30–41. [Google Scholar] [CrossRef]
- Heaney, R.P.; Dowell, M.S.; Hale, C.A.; Bendich, A. Calcium Absorption Varies within the Reference Range for Serum 25-Hydroxyvitamin D. J. Am. Coll. Nutr. 2003, 22, 142–146. [Google Scholar] [CrossRef]
- Steingrimsdottir, L.; Gunnarsson, O.; Indridason, O.S.; Franzson, L.; Sigurdsson, G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005, 294, 2336–2341. [Google Scholar] [CrossRef] [Green Version]
- Heaney, R.P.; Abrams, S.; Dawson-Hughes, B.; Looker, A.; Marcus, R.; Matkovic, V.; Weaver, C. Peak Bone Mass. Osteoporos. Int. 2001, 11, 985–1009. [Google Scholar] [CrossRef]
- Suriawati, A.A.; Majid, H.A.; Al-Sadat, N.; Mohamed, M.N.A.; Jalaludin, M.Y. Vitamin D and Calcium Intakes, Physical Activity, and Calcaneus BMC among School-Going 13-Year Old Malaysian Adolescents. Nutrients 2016, 8, 666. [Google Scholar] [CrossRef] [Green Version]
- Poh, B.K.; Ng, B.K.; Haslinda, M.D.S.; Shanita, S.N.; Wong, J.E.; Budin, S.B.; Ruzita, A.T.; Ng, L.O.; Khouw, I.; Norimah, A.K. Nutritional status and dietary intakes of children aged 6 months to 12 years: Findings of the Nutrition Survey of Malaysian Children (SEANUTS Malaysia). Br. J. Nutr. 2013, 110, S21–S35. [Google Scholar] [CrossRef] [Green Version]
- Duke, P.M.; Litt, I.F.; Gross, R.T. Adolescents’ self-assessment of sexual maturation. Pediatrics 1980, 66, 918–920. [Google Scholar] [PubMed]
- Tee, E.; Ismail, M.; Nasir, M.; Idris, K. Nutrient Composition of Malaysian Foods, 4th ed.; Institute for Medical Research: Kuala Lumpur, Malaysia, 1987.
- Health Promotion Board. Energy & Nutrient Composition of Food. Available online: https://focos.hpb.gov.sg/eservices/ENCF/ (accessed on 2 January 2021).
- Barger-Lux, M.J.; Heaney, R.P. Effects of Above Average Summer Sun Exposure on Serum 25-Hydroxyvitamin D and Calcium Absorption. J. Clin. Endocrinol. Metab. 2002, 87, 4952–4956. [Google Scholar] [CrossRef]
- Nurbazlin, M.; Chee, W.S.S.; Rokiah, P.; Tan, A.T.B.; Chew, Y.Y.; Nusaibah, A.R.S.; Chan, S.P. Effects of sun exposure on 25(OH) vitamin D concentration in urban and rural women in Malaysia. Asia Pac. J. Clin. Nutr. 2013, 22, 391. [Google Scholar]
- Nor Aini, J.; Poh, B.K.; Chee, W.S.S. Validity of a children’s physical activity questionnaire (cPAQ) for the study of bone health. Pediatr. Int. 2013, 55, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of Physical Activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemper, H.; Bakker, I.; Twisk, J.; van Mechelen, W. Validation of a physical activity questionnaire to measure the effect of mechanical strain on bone mass. Bone 2002, 30, 799–804. [Google Scholar] [CrossRef]
- WHO. Physical Status: The Use and Interpretation of Anthropometry, Report of a WHO Expert Committee; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- National Coordinating Committee on Food and Nutrition, Ministry of Health Malaysia. Recommended Nutrient Intakes for Malaysia. A Report of the Technical Working Group on Nutritional Guidelines; Ministry of Health Malaysia: Putrajaya, Malaysia, 2017.
- Department of Statistics Malaysia. Household Income and Basic Amenities Survey (HIS) Report 2019; Department of Statistics Malaysia: Putrajaya, Malaysia, 2019.
- Rosen, C.J.; Abrams, S.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S.; et al. IOM Committee Members Respond to Endocrine Society Vitamin D Guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1146–1152. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, F.M. Vitamin D status and its associated factors of free living Malay adults in a tropical country, Malaysia. J. Photochem. Photobiol. B Biol. 2011, 104, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Quah, S.W.; Majid, H.A.; Al-Sadat, N.; Yahya, A.; Su, T.T.; Jalaludin, M.Y. Risk factors of vitamin D deficiency among 15-year-old adolescents participating in the Malaysian Health and Adolescents Longitudinal Research Team Study (MyHeARTs). PLoS ONE 2018, 13, e0200736. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Wong, J.E.; Shanita, S.N.; Ismail, M.N.; Deurenberg, P.; Poh, B.K. Daily Physical Activity and Screen Time, but Not Other Sedentary Activities, Are Associated with Measures of Obesity during Childhood. Int. J. Environ. Res. Public Health 2014, 12, 146–161. [Google Scholar] [CrossRef]
- Barja-Fernández, S.; Aguilera, C.M.; Martínez-Silva, I.; Vazquez, R.; Gil-Campos, M.; Olza, J.; Bedoya, J.; Cadarso-Suárez, C.; Gil, Á.; Seoane, L.M.; et al. 25-Hydroxyvitamin D levels of children are inversely related to adiposity assessed by body mass index. J. Physiol. Biochem. 2017, 74, 111–118. [Google Scholar] [CrossRef]
- Asghari, G.; Yuzbashian, E.; Wagner, C.L.; Mahdavi, M.; Shamsi, R.; Hosseinpanah, F.; Mirmiran, P. The relation between circulating levels of vitamin D and parathyroid hormone in children and adolescents with overweight or obesity: Quest for a threshold. PLoS ONE 2019, 14, e0225717. [Google Scholar] [CrossRef]
- Plesner, J.L.; Dahl, M.; Fonvig, C.E.; Nielsen, T.R.H.; Kloppenborg, J.T.; Pedersen, O.; Hansen, T.; Holm, J.-C. Obesity is associated with vitamin D deficiency in Danish children and adolescents. J. Pediatr. Endocrinol. Metab. 2018, 31, 53–61. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Bouillon, R.; Bikle, D. Vitamin D Metabolism Revised: Fall of Dogmas. J. Bone Miner. Res. 2019, 34, 1985–1992. [Google Scholar] [CrossRef]
- Yang, W.; Burrows, T.; MacDonald-Wicks, L.; Williams, L.; Collins, C.; Chee, W. The Family Diet Study: A cross-sectional study into the associations between diet, food habits and body weight status in M alay families. J. Hum. Nutr. Diet. 2016, 29, 441–448. [Google Scholar] [CrossRef]
- Uday, S.; Hoegler, W. Nutritional Rickets and Osteomalacia in the Twenty-first Century. Curr. Osteoporos. Rep. 2017, 15, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Sharawat, I.K.; Dawman, L. Bone mineral density and its correlation with vitamin D status in healthy school-going children of Western India. Arch. Osteoporos. 2019, 14, 13. [Google Scholar] [CrossRef]
- Esterle, L.; Nguyen, M.; Walrantâ Debray, O.; Sabatier, J.; Garabedian, M. Adverse interaction of low calcium diet and low 25 (OH) D levels on lumbar spine mineralization in latepubertal girls. J. Bone Miner. Res. 2010, 25, 2392–2398. [Google Scholar] [CrossRef]
- Pekkinen, M.; Viljakainen, H.; Saarnio, E.; Lamberg-Allardt, C.; Mäkitie, O. Vitamin D Is a Major Determinant of Bone Mineral Density at School Age. PLoS ONE 2012, 7, e40090. [Google Scholar] [CrossRef] [PubMed]
- Hatun, S.; Islam, O.; Cizmecioglu, F.; Kara, B.; Babaoglu, K.; Berk, F.; Gökalp, A.S. Subclinical Vitamin D Deficiency Is Increased in Adolescent Girls Who Wear Concealing Clothing. J. Nutr. 2005, 135, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, Z.; White, S.; Dalvie, T.; Kruger, M.C.; Van Zyl, A.; Becker, P. Bone Health, Body Composition, and Vitamin D Status of Black Preadolescent Children in South Africa. Nutrients 2019, 11, 1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wu, F.; Winzenberg, T.; Jones, G. The Association of Vitamin D in Youth and Early Adulthood with Bone Mineral Density and Microarchitecture in Early Adulthood. Calcif. Tissue Int. 2019, 104, 605–612. [Google Scholar] [CrossRef]
- Baptista, F.; Barrigas, C.; Vieira, F.; Santa-Clara, H.; Homens, P.M.; Fragoso, I.; Teixeira, P.J.; Sardinha, L.B.; Vieira, M.F. The role of lean body mass and physical activity in bone health in children. J. Bone Miner. Metab. 2011, 30, 100–108. [Google Scholar] [CrossRef]
- Black, L.J.; Anderson, D.; Clarke, M.W.; Ponsonby, A.-L.; Lucas, R.M. Analytical Bias in the Measurement of Serum 25-Hydroxyvitamin D Concentrations Impairs Assessment of Vitamin D Status in Clinical and Research Settings. PLoS ONE 2015, 10, e0135478. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; März, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front. Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 243) | Boys (n = 127) | Girls (n = 116) | p-Value ¥ | |
|---|---|---|---|---|
| Age | 10.1 ± 1.0 | 10.2 ± 0.9 | 10.0 ± 1.0 | 0.122 |
| Ethnicity (n,%) | ||||
| Malay | 220 (90.5) | 110 (86.6) | 110 (94.8) | |
| Indian | 23 (9.5) | 17 (13.4) | 6 (5.2) | |
| Tanner stage (n,%) | ||||
| Stage 1 | 230 (94.7) | 125 (98.4) | 105 (90.5) | |
| Stage 2 | 13 (5.3) | 2 (1.6) | 11 (9.5) | |
| Monthly household income (n,%) # | 0.275 | |||
| Low < RM4849 | 183 (75.3) | 100 (78.7) | 83 (71.6) | |
| Middle income RM4850-10959 | 37 (15.2) | 17 (13.4) | 20 (17.2) | |
| High income ≥ RM10960 | 14 (5.8) | 6 (4.7) | 8 (6.9) | |
| No response | 9 (3.7) | 4 (3.2) | 5 (4.3) | |
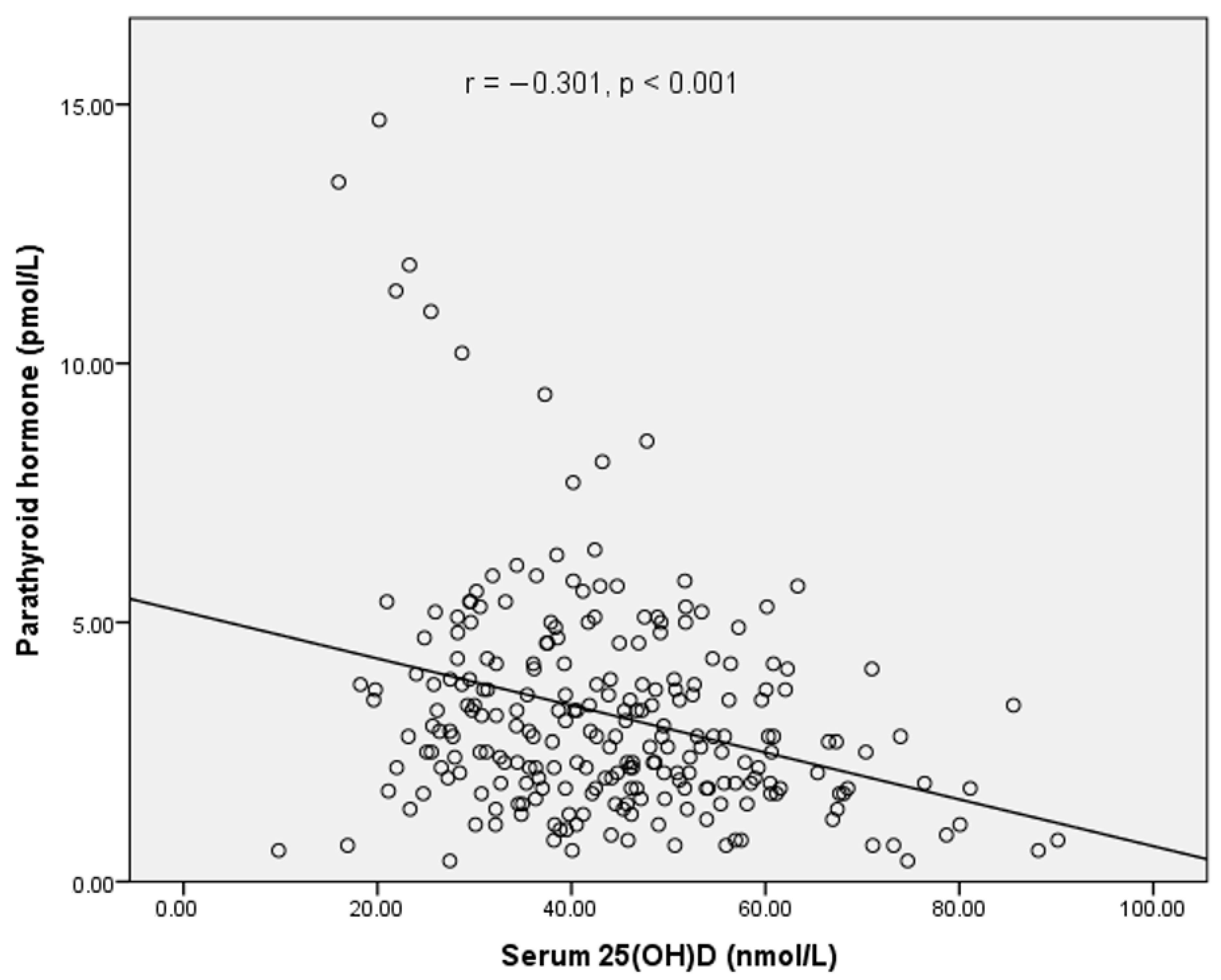
| Serum 25(OH)D (nmol/L) | 43.9 ± 14.5 | 50.3 ± 13.7 | 36.8 ± 11.9 | <0.001 ** |
| PTH (pmol/L) | 3.22 ± 2.14 | 2.81 ± 1.81 | 3.68 ± 2.37 | 0.001 * |
| BMI (kg/m2) | 18.0 ± 4.5 | 18.3 ± 4.8 | 17.7 ± 4.2 | 0.332 |
| BMI-for-age Z-score | 0.187 ± 1.693 | 0.321 ± 1.742 | 0.040 ± 1.632 | 0.198 |
| BMI Z-score classification, n (%) | ||||
| Thinness | 21 (8.7) | 9 (7.1) | 12 (10.3) | |
| Normal | 142 (58.4) | 74 (58.3) | 67 (57.8) | |
| Overweight | 37 (15.2) | 18 (14.2) | 21 (18.1) | |
| Obese | 43 (17.7) | 26 (20.5) | 16 (13.8) | |
| LSBMC (g) | 21.7 ± 5.2 | 21.6 ± 4.7 | 21.9 ± 5.7 | 0.725 |
| LSBMD (g/cm2) | 0.725 ± 0.091 | 0.715 ± 0.081 | 0.736 ± 0.100 | 0.064 |
| TBBMC (g) | 1129.5 ± 231.6 | 1160.4 ± 237.9 | 1095.6 ± 220.6 | 0.029 * |
| TBBMD (g/cm2) | 0.768 ± 0.075 | 0.780 ± 0.075 | 0.754 ± 0.072 | 0.006 ** |
| TBBMD z-score | 0.789 ± 0.960 | 0.890 ± 0.921 | 0.678 ± 0.994 | 0.087 |
| Lean mass (kg) | 21.79 ± 5.29 | 22.50 ± 5.40 | 21.00 ± 5.07 | 0.026 * |
| Fat mass (kg) | 10.93 ± 7.08 | 11.00 ± 7.98 | 10.85 ± 5.98 | 0.876 |
| Body fat percentage (%) | 29.87 ± 8.44 | 28.82 ± 9.17 | 31.06 ± 7.39 | 0.035 * |
| Energy (Kcal/day) | 1457 ± 450 | 1543 ± 463 | 1363 ± 417 | 0.002 * |
| Protein intake (g/day) | 61.4 ± 21.6 | 65.7 ± 23.3 | 56.6 ± 18.7 | 0.001 * |
| Calcium intake (mg/day) | 329 ± 202 | 355 ± 215 | 300 ± 185 | 0.035 * |
| Vitamin D intake (µg/day) | 1.6 ± 1.6 | 1.8 ± 1.8 | 1.3 ± 1.2 | 0.014 * |
| PA level (MET scores) | 822 ± 447 | 961 ± 502 | 670 ± 317 | <0.001 ** |
| Hours of sun exposure (h/week) | 6.7 ± 3.4 | 7.4 ± 3.7 | 5.9 ± 2.8 | <0.001 * |
| Body surface area exposed to sun | 0.14 ± 0.08 | 0.20 ± 0.06 | 0.09 ± 0.05 | <0.001 * |
| Sun Index | 1.08 ± 0.77 | 1.49 ± 0.78 | 0.62 ± 0.44 | <0.001 * |
| 25(OH)D Levels * | Total (n = 243) | Boys (n = 127) | Girls (n = 116) |
|---|---|---|---|
| <50 nmol/L | 169 (69.4) | 67 (52.8) | 102 (87.9) |
| <40 nmol/L | 103 (42.4) | 30 (23.6) | 73(62.9) |
| Inadequacy (30–40 nmol/L) | 57 (23.5) | 25 (19.7) | 32 (27.6) |
| Deficiency (<30 nmol/L) | 46 (18.9) | 5 (3.9) | 41 (35.3) |
| Predictors | Unstandardized | Standardized | t | p-Value | VIF | |
|---|---|---|---|---|---|---|
| B | SE | Beta | ||||
| (Constant) | 19.482 | 6.347 | 3.069 | 0.002 | ||
| Height, m | −0.029 | 0.050 | −0.045 | −0.577 | 0.565 | 1.815 |
| Fat mass, kg | 0.000 | 0.000 | −0.109 | −1.444 | 0.150 | 1.700 |
| MET score | 0.001 | 0.001 | 0.084 | 0.882 | 0.379 | 2.708 |
| Vit D, µg/day | 0.367 | 0.228 | 0.100 | 1.607 | 0.109 | 1.145 |
| Sun exposure (h/week) | −0.234 | 0.182 | −0.135 | −1.284 | 0.200 | 3.295 |
| BSA | −3.743 | 9.084 | −0.049 | −0.412 | 0.681 | 4.244 |
| Sun Index | 1.868 | 0.945 | 0.248 | 1.977 | 0.049 | 4.687 |
| BSA*gender | 15.490 | 6.299 | 0.282 | 2.459 | 0.015 | 3.904 |
| Predictors | Unstandardized | Standardized | t | p-Value | VIF | |
|---|---|---|---|---|---|---|
| B | SE | Beta | ||||
| (Constant) | 15.732 | 0.763 | 20.625 | 0.000 | ||
| Fat mass, kg | −0.0001 | 0.00005 | −0.149 | −2.558 | 0.011 | 1.005 |
| Sun Index | 1.497 | 0.537 | 0.199 | 2.787 | 0.006 | 1.511 |
| BSA*gender | 15.370 | 3.925 | 0.279 | 3.916 | 0.000 | 1.513 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chee, W.S.S.; Chang, C.Y.; Arasu, K.; Wong, S.Y.; Ong, S.H.; Yang, W.Y.; Chong, M.H.Z.; Mavinkurve, M.; Khoo, E.J.; Chinna, K.; et al. Vitamin D Status Is Associated with Modifiable Lifestyle Factors in Pre-Adolescent Children Living in Urban Kuala Lumpur, Malaysia. Nutrients 2021, 13, 2175. https://doi.org/10.3390/nu13072175
Chee WSS, Chang CY, Arasu K, Wong SY, Ong SH, Yang WY, Chong MHZ, Mavinkurve M, Khoo EJ, Chinna K, et al. Vitamin D Status Is Associated with Modifiable Lifestyle Factors in Pre-Adolescent Children Living in Urban Kuala Lumpur, Malaysia. Nutrients. 2021; 13(7):2175. https://doi.org/10.3390/nu13072175
Chicago/Turabian StyleChee, Winnie Siew Swee, Chung Yuan Chang, Kanimolli Arasu, Soon Yee Wong, Shu Hwa Ong, Wai Yew Yang, Megan Hueh Zan Chong, Meenal Mavinkurve, Erwin Jiayuan Khoo, Karuthan Chinna, and et al. 2021. "Vitamin D Status Is Associated with Modifiable Lifestyle Factors in Pre-Adolescent Children Living in Urban Kuala Lumpur, Malaysia" Nutrients 13, no. 7: 2175. https://doi.org/10.3390/nu13072175
APA StyleChee, W. S. S., Chang, C. Y., Arasu, K., Wong, S. Y., Ong, S. H., Yang, W. Y., Chong, M. H. Z., Mavinkurve, M., Khoo, E. J., Chinna, K., & Weaver, C. M. (2021). Vitamin D Status Is Associated with Modifiable Lifestyle Factors in Pre-Adolescent Children Living in Urban Kuala Lumpur, Malaysia. Nutrients, 13(7), 2175. https://doi.org/10.3390/nu13072175








