Tolerability and Safety of a Novel Ketogenic Ester, Bis-Hexanoyl (R)-1,3-Butanediol: A Randomized Controlled Trial in Healthy Adults
Abstract
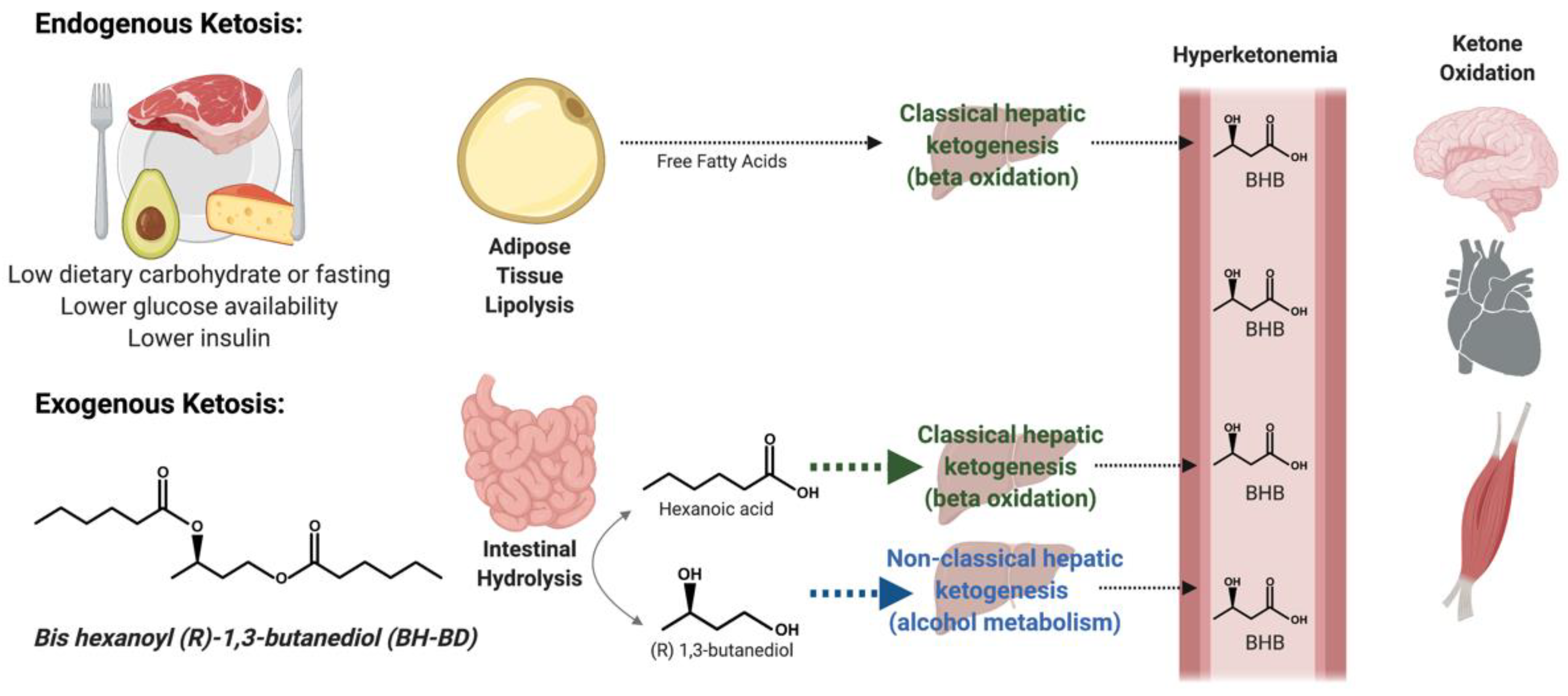
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Screening
2.3. Study Beverages
2.4. Standard Breakfast Meal
2.5. Study Questionnaires
2.5.1. Study Beverage Log
2.5.2. Beverage Tolerability Questionnaire (BTQ)
2.5.3. Brief Biphasic Alcohol Effect Scale (B-BAES)
2.6. In-Clinic Procedures
2.7. At-Home Procedures
2.8. Biological Sample Analysis
2.9. Statistical Methods
3. Results
3.1. Compliance and Adverse Events
3.2. Tolerability
3.3. Stimulation and Sedation
3.4. Safety Laboratory Tests
3.5. Acute Glucose and Ketone Measurement
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, A.M.; Williamson, D.H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 1980, 60, 143–187. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, L.M.; Parris, E.E.; Cahill, G.F. Starvation in Man. N. Engl. J. Med. 1970, 282, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.L.; Pyzik, P.L.; Freeman, J.M. The ketogenic diet: Seizure control correlates better with serum beta-hydroxybutyrate than with urine ketones. J. Child Neurol. 2000, 15, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Koeslag, J.H.; Noakes, T.D.; Sloan, A.W. Post-Exercise Ketosis. J. Physiol. Lond. 1980, 301, 79–90. [Google Scholar] [CrossRef]
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F. Brain Metabolism during Fasting*. J. Clin. Investig. 1967, 46, 1589–1595. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef]
- Krebs, H. The regulation of the release of ketone bodies by the liver. Adv. Enzym. Regul. 1966, 4, 339–353. [Google Scholar] [CrossRef]
- Reichard, G., Jr.; Owen, O.E.; Haff, A.C.; Bortz, W.M. Ketone-body production and oxidation in fasting obese humans. J. Clin. Investig. 1974, 53, 508. [Google Scholar] [CrossRef]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.; Phinney, S.D.; et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef]
- Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; McKenzie, A.L.; Bhanpuri, N.H.; Campbell, W.W.; Volek, J.S.; Phinney, S.D.; McCarter, J.P. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-Year Non-randomized Clinical Trial. Front. Endocrinol. 2019, 10, 348. [Google Scholar] [CrossRef]
- Strahlman, R.S. Can Ketosis Help Migraine Sufferers? A Case Report. Headache. J. Head Face Pain 2006, 46, 182. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Currà, A.; Sirianni, G.; Coppola, G.; Bracaglia, M.; Cardillo, A.; De Nardis, L.; Pierelli, F. Diet transiently improves migraine in two twin sisters: Possible role of ketogenesis? Diet Exerc. Cogn. Funct. Neurol. Dis. 2014, 28, 305–308. [Google Scholar]
- Di Lorenzo, C.; Coppola, G.; Sirianni, G.; Di Lorenzo, G.; Bracaglia, G.; Di Lenola, D.; Siracusano, A.; Rossi, P.; Pirelli, F. Migraine improvement during short lasting ketogenesis: A proof-of-concept study. Eur. J. Neurol. 2015, 22, 170–177. [Google Scholar] [CrossRef]
- Zuccoli, G.; Marcello, N.; Pisanello, A.; Servadei, F.; Vaccaro, S.; Mukherjee, P.; Seyfried, T.N. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr. Metab. 2010, 7, 33. [Google Scholar] [CrossRef]
- Van der Louw, E.; Olierman, J.F.; van den Dernt, P.M.L.A.; Bromberg, J.E.C.; de Hoop, E.O.; Neuteboorn, R.F.; Catsman-Berrevoets, C.E.; Vincent, A.J.P.E. Ketogenic diet treatment as adjuvant to standard treatment of glioblastoma multiforme: A feasibility and safety study. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L.; Bradshaw, P.C.; Clarke, K.; Curtis, W.; Pawlosky, R.; King, M.T. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life 2017, 69, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L.; Chaance, B.; Kashiwaya, Y.; Lardy, H.A.; Cahill, G.C., Jr. Ketone bodies, potential therapeutic uses. IUBMB Life 2001, 51, 241–247. [Google Scholar]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Poffé, C.; Ramaekers, M.; Bogaerts, S.; Hespel, P. Bicarbonate Unlocks the Ergogenic Action of Ketone Monoester Intake in Endurance Exercise. Med. Sci. Sports Exerc. 2021, 53, 431–441. [Google Scholar] [CrossRef]
- Poffé, C.; Ramaekers, M.; Bogaerts, S.; Hespel, P. Exogenous ketosis impacts neither performance nor muscle glycogen breakdown in prolonged endurance exercise. J. Appl. Physiol. 2020, 128, 1643–1653. [Google Scholar] [CrossRef]
- Cox, P.J.; Kirk, T.; Ashmore, T.; Willerton, K.; Evans, R.; Smith, A.; Murray, A.; Stubbs, B.; West, J.; McLure, S.W.; et al. Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metab. 2016, 24, 256–268. [Google Scholar] [CrossRef]
- Waldman, H.S.; Basham, S.A.; Price, F.G.; Smith, J.W.; Chander, H.; Knight, A.C.; Krings, B.M.; McAllister, M.J. Exogenous ketone salts do not improve cognitive responses after a high-intensity exercise protocol in healthy college-aged males. Appl. Physiol. Nutr. Metab. 2018, 43, 711–717. [Google Scholar] [CrossRef]
- Mujica-Parodi, L.R.; Amgalan, A.; Sultan, S.F.; Antal, B.; Sun, X.; Skiena, S.; Lithen, A.; Adra, N.; Ratai, E.-M.; Weistuch, C.; et al. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc. Natl. Acad. Sci. USA 2020, 117, 6170–6177. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Egan, B. Intermittent Running and Cognitive Performance after Ketone Ester Ingestion. Med. Sci. Sports Exerc. 2018, 50, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, I.K.; Egan, J.M.; Mattson, M.P.; Kapogiannis, D. Medium Chain Triglycerides induce mild ketosis and may improve cognition in Alzheimer’s disease. A systematic review and meta-analysis of human studies. Ageing Res. Rev. 2020, 58. [Google Scholar] [CrossRef]
- Walsh, J.J.; Neudorf, H.; Little, J.P. 14-Day Ketone Supplementation Lowers Glucose and Improves Vascular Function in Obesity: A Randomized Crossover Trial. J. Clin. Endocrinol. Metab. 2021, 106, e1738–e1754. [Google Scholar] [CrossRef]
- Myette-Côté, É.; Neudorf, H.; Rafiei, H.; Clarke, K.; Little, J.P. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J. Physiol. 2018, 596, 1385–1395. [Google Scholar] [CrossRef]
- Myette-Côté, É.; Caldwell, H.G.; Ainslie, P.N.; Clarke, K.; Little, J.P. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 1491–1501. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Norwitz, N.G.; Clarke, K. Why a d-beta-hydroxybutyrate monoester? Biochem. Soc. Trans. 2020, 48, 51–59. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Vansant, H.; Evans, R.D.; Clarke, K. Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul. Toxicol. Pharmacol. 2019, 109. [Google Scholar] [CrossRef]
- Stefan, M.; Sharp, M.; Gheith, R.; Lowery, R.; Wilson, J. The Effects of Exogenous Beta-Hydroxybutyrate Supplementation on Metrics of Safety and Health. Int. J. Nutr. Food Sci. 2020, 9, 154–162. [Google Scholar]
- Fortier, M.; Castellano, C.; St-Pierre, V.; Myette-Côté, É.; Langlois, F.; Roy, M.; Morin, M.; Bocti, C.; Fulop, T.; Godin, J.; et al. A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6-month RCT. Alzheimer’s Dement. 2021, 17, 543–552. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Kirk, T.; Evans, R.D.; Clarke, K. Gastrointestinal Effects of Exogenous Ketone Drinks are Infrequent, Mild, and Vary According to Ketone Compound and Dose. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 596–603. [Google Scholar] [CrossRef]
- Jeukendrup, E.A.; Thielen, J.J.; Wagenmakers, A.J.; Brouns, F.; Saris, W.H. Effect of medium-chain triacylglycerol and carbohydrate ingestion during exercise on substrate utilization and subsequent cycling performance. Am. J. Clin. Nutr. 1998, 67, 397–404. [Google Scholar] [CrossRef]
- Wibisono, C.; Rowe, N.; Beavis, E.; Kepreotes, H.; Mackie, F.E.; Lawson, J.A.; Cardamone, M. Ten-Year Single-Center Experience of the Ketogenic Diet: Factors Influencing Efficacy, Tolerability, and Compliance. J. Pediatr. 2015, 166, 1030.e1–1036.e1. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Blade, T.; Mills, S.; Thomas, J.; Yufei, X.; Nelson, F.R.; Higley, N.; Nikiforov, A.I.; Rhiner, M.O.; Verdin, E.; et al. In vitro stability and in vivo pharmacokinetics of the novel ketogenic ester, bis hexanoyl (R)-1,3-butanediol. Food Chem. Toxicol. 2021, 147. [Google Scholar] [CrossRef]
- Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. In Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and Amended by the: 59th WMA General Assembly, Seoul, October 2008; World Medical Association: Ferney-Voltaire, France, 2008. [Google Scholar]
- Boler, B.M.V.; Serao, M.C.R.; Bauer, L.L.; Staeger, M.A.; Boileau, T.W.; Swanson, K.; Fahey, G.C. Digestive physiological outcomes related to polydextrose and soluble maize fibre consumption by healthy adult men. Br. J. Nutr. 2011, 106, 1864–1871. [Google Scholar] [CrossRef]
- Maki, K.C.; Rains, T.M.; Kelley, K.M.; Cook, C.M.; Schild, A.L.; Gietl, E. Fibermalt is well tolerated in healthy men and women at intakes up to 60 g/d: A randomized, double-blind, crossover trial. Int. J. Food Sci. Nutr. 2012, 64, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.S.; Earleywine, M.; Musty, R.E.; Perrine, M.W.; Swift, R.M. Development and Validation of the Biphasic Alcohol Effects Scale. Alcohol. Clin. Exp. Res. 1993, 17, 140–146. [Google Scholar] [CrossRef]
- Rueger, S.Y.; King, A.C. Validation of the Brief Biphasic Alcohol Effects Scale (B-BAES). Alcohol. Clin. Exp. Res. 2012, 37, 470–476. [Google Scholar] [CrossRef]
- Peterson, J.I.; Young, D.S. Evaluation of the hexokinase-glucose-6-phosphate dehydrogenase method of determination of glucose in urine. Anal. Biochem. 1968, 23, 301–316. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Ivy, J.L.; Costill, D.L.; Fink, W.J.; Maglischo, E. Contribution of Medium and Long Chain Triglyceride Intake to Energy Metabolism during Prolonged Exercise. Int. J. Sports Med. 1980, 1, 15–20. [Google Scholar] [CrossRef]
- Shah, N.; Limketkai, B. The Use of Medium-Chain Triglycerides in Gastrointestinal Disorders. Pract. Gastroenterol. 2017, 41, 20–28. [Google Scholar]
- Liu, Y.-M.; Wang, H.-S. Medium-chain Triglyceride Ketogenic Diet, An Effective Treatment for Drug-resistant Epilepsy and A Comparison with Other Ketogenic Diets. Biomed. J. 2013, 36, 9–15. [Google Scholar] [CrossRef]
- Ohnuma, T.; Toda, A.; Kimoto, A.; Takebayashi, Y.; Higashiyama, R.; Tagata, Y.; Ito, M.; Ota, T.; Shibata, N.; Arai, H. Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer’s disease: A prospective, open-label pilot study. Clin. Interv. Aging 2016, 11, 29–36. [Google Scholar] [CrossRef]
- Liu, Y.-M.C. Medium-chain triglyceride (MCT) ketogenic therapy. Epilepsia 2008, 49, 33–36. [Google Scholar] [CrossRef]
- Goedecke, J.H.; Clark, V.R.; Noakes, T.D.; Lambert, E. The Effects of Medium-Chain Triacylglycerol and Carbohydrate Ingestion on Ultra-Endurance Exercise Performance. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 15–27. [Google Scholar] [CrossRef]
- Stubbs, B.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef]
- Rittig, N.; Svart, M.; Thomsen, H.H.; Vestergaard, E.T.; Rehfeld, J.F.; Hartmann, B.; Holst, J.J.; Johannsen, M.; Moller, N.; Jessen, N. Oral D/L-3-Hydroxybutyrate Stimulates Cholecystokinin and Insulin Secretion and Slows Gastric Emptying in Healthy Males. J. Clin. Endocrinol. Metab. 2020, 105, e3597–e3605. [Google Scholar] [CrossRef]
- Fischer, T.; Och, U.; Klawon, I.; Och, T.; Grüneberg, M.; Fobker, M.; Bordewick-Dell, U.; Marquardt, T. Effect of a Sodium and Calcium DL-β-Hydroxybutyrate Salt in Healthy Adults. J. Nutr. Metab. 2018, 2018, 9812806. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Patchett, E.; Nally, R.; Kearns, R.; Larney, M.; Egan, B. Effect of acute ingestion of β-hydroxybutyrate salts on the response to graded exercise in trained cyclists. Eur. J. Sport Sci. 2018, 18, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Chander, H.; McAllister, M.J.; Holland, A.M.; Waldman, H.S.; Krings, B.M.; Swain, J.C.; Turner, A.J.; Basham, S.A.; Smith, J.W.; Knight, A.C. Effects of 7-Day Ketone Ingestion and a Physiological Workload on Postural Stability, Cognitive, and Muscular Exertion Measures in Professional Firefighters. Safety 2019, 5, 15. [Google Scholar] [CrossRef]
- Leckey, J.J.; Ross, M.L.; Quod, M.; Hawley, J.A.; Burke, L.M. Ketone Diester Ingestion Impairs Time-Trial Performance in Professional Cyclists. Front. Physiol. 2017, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- David, M.S.; Merien, F.; Braakhuis, A.; Plews, D.; Laursen, P.; Dulson, D.K. The Effect of 1,3-Butanediol on Cycling Time-Trial Performance. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 466–473. [Google Scholar]
- Vandoorne, T.; De Smet, S.; Ramaekers, M.; Van Thienen, R.; De Bock, K.; Clarke, K.; Hespel, P. Intake of a Ketone Ester Drink during Recovery from Exercise Promotes mTORC1 Signaling but Not Glycogen Resynthesis in Human Muscle. Front. Physiol. 2017, 8, 310. [Google Scholar] [CrossRef]
- Evans, M.; Mcswiney, F.T.; Brady, A.J.; Egan, B. No Benefit of Ingestion of a Ketone Monoester Supplement on 10-km Running Performance. Med. Sci. Sports Exerc. 2019, 51, 2506–2515. [Google Scholar] [CrossRef]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; King, M.T.; Musa-Veloso, K.; Ho, M.; Roberts, A.; Robertson, J.; VanItallie, T.B.; et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 2012, 63, 401–408. [Google Scholar] [CrossRef]
- Schrieks, I.C.; Stafleu, A.; Kallen, V.L.; Grootjen, M.; Witkamp, R.; Hendriks, H.F.J. The Biphasic Effects of Moderate Alcohol Consumption with a Meal on Ambiance-Induced Mood and Autonomic Nervous System Balance: A Randomized Crossover Trial. PLoS ONE 2014, 9, e86199. [Google Scholar] [CrossRef]
- Fridberg, D.J.; Rueger, S.Y.; Smith, P.; King, A.C. Association of Anticipated and Laboratory-Derived Alcohol Stimulation, Sedation, and Reward. Alcohol. Clin. Exp. Res. 2017, 41, 1361–1369. [Google Scholar] [CrossRef]
- Greaves, G.; Xiang, R.; Rafiei, H.; Malas, A.; Little, J.P. Prior ingestion of a ketone monoester supplement reduces postprandial glycemic responses in young healthy-weight individuals. Appl. Physiol. Nutr. Metab. 2020, 1–9. [Google Scholar] [CrossRef]


| Day 0–7 BH-BD | Day 8–28 BH-BD | Placebo | |
|---|---|---|---|
| BH-BD (g) * | 12.5 | 25 | - |
| Canola oil (g) | 12.5 | - | 25 |
| Energy (kcal) | 225 | 210 | 246 |
| Total carbohydrate (g) | 1 | 2 | 2 |
| Total fat (g) | 13 | 0.5 | 25.5 |
| Protein (g) | 1 | 2 | 2 |
| Beverage Group | ||
|---|---|---|
| BH-BD (n = 30) | Placebo (n = 29) | |
| Age (years) | 43.9 (11.7) | 41.7 (15.0) |
| Sex | n (%) | |
| Female | 16 (53.3) | 16 (55.2) |
| Male | 14 (46.7) | 13 (44.8) |
| Race | n (%) | |
| Asian | 4 (13.3%) | 2 (6.9%) |
| Black/African American | 3 (10.0%) | 3 (10.3%) |
| Native Hawaiian or Other Pacific Islander | 1 (3.3%) | 0 (0%) |
| White | 22 (73.3%) | 23 (79.3%) |
| Multiracial | 0 (0%) | 1 (3.4%) |
| Anthropometrics | Mean (SD) | |
| Height (cm) | 168.5 (10.6) | 171.0 (11.6) |
| BMI (kg/m2) | 27.9 (3.8) | 27.7 (4.0) |
| Weight (kg) | 79.9 (17.8) | 8281.5 (16.9) |
| Days (BH-BD per Serving) | Timing | Beverage Group | Weekly Total Composite BTQ Score 1 | Change vs. Previous Week | ||
|---|---|---|---|---|---|---|
| Mean (SD) | p-Value 2 | Mean (SD) | p-Value 3 | |||
| 0–6 (12.5 g) | Before | BH-BD | 2.4 (6.2) | 0.47 | - | - |
| PLA | 0.9 (1.9) | - | - | |||
| After | BH-BD | 4.7 (7.9) | 0.16 | - | - | |
| PLA | 2.0 (4.2) | - | - | |||
| 7–13 (25 g) | Before | BH-BD | 2.7 (6.1) | 0.42 | 0.3 (4.6) | 0.29 |
| PLA | 1.5 (2.6) | 0.6 (2.3) | 0.27 | |||
| After | BH-BD | 5.6 (9.3) | 0.29 | 0.9 (6.9) | 0.57 | |
| PLA | 3.4 (6.9) | 1.3 (6.1) | 0.49 | |||
| 14–22 (25 g) | Before | BH-BD | 2.3 (5.4) | 0.40 | −0.4 (2.6) | 0.26 |
| PLA | 1.2 (3.3) | −0.3 (2.2) | 0.52 | |||
| After | BH-BD | 5.5 (10.0) | 0.32 | −0.1 (3.4) | 0.72 | |
| PLA | 2.4 (6.5) | −0.9 (3.4) | 0.18 | |||
| 21–27 (25 g) | Before | BH-BD | 2.2 (5.3) | 0.44 | −0.1 (2.4) | 0.85 |
| PLA | 2.0 (4.7) | 0.8 (2.7) | 0.13 | |||
| After | BH-BD | 6.2 (12.3) | 0.30 | 0.6 (5.0) | 0.43 | |
| PLA | 3.0 (6.7) | 0.6 (3.4) | 0.65 | |||
| Clinic Visit Day | Timing | Beverage Group | BTQ | B-BAES | ||||
|---|---|---|---|---|---|---|---|---|
| BTQ Composite n (%) | p-Value 1 | SED Score Mean (SD) | p-Value 2 | STIM Score Mean (SD) | p-Value 2 | |||
| 0 3 | Pre- | BH-BD | 4 (13.3) | 0.11 | 4.17 (5.11) | - | 19.73 (4.77) | - |
| PLA | 0 (0.0) | 4.55 (5.09) | 17.38 (6.73) | |||||
| 1-h post | BH-BD | 2 (6.7) | 1.00 | 5.57 (6.62) | - | 17.13 (7.04) | - | |
| PLA | 3 (10.3) | 3.59 (4.51) | 18.17 (6.80) | |||||
| △pre vs. 1-h post | BH-BD | 1 (3.3) | 0.35 | - | - | - | - | |
| PLA | 3 (10.3) | |||||||
| Model Estimate △ BH-BD vs. PLA | 2.22 | 0.021 | −2.77 | 0.011 | ||||
| 7 3 | Pre- | BH-BD | 3 (10) | 1.00 | 3.77 (5.20) | - | 17.07 (7.67) | - |
| PLA | 2 (6.9) | 3.10 (4.30) | 16.38 (7.36) | |||||
| 1-h post | BH-BD | 8 (26.7) | 0.33 | 3.73 (5.34) | - | 17.33 (7.49) | - | |
| PLA | 4 (13.8) | 2.03 (3.46) | 18.83 (6.16) | |||||
| △pre vs. 1-h post | BH-BD | 5 (16.7) | 0.71 | - | - | - | - | |
| PLA | 3 (10.3) | |||||||
| Model Estimate △ BH-BD vs. PLA | 1.35 | 0.16 | −1.91 | 0.075 | ||||
| 14 3 | Pre- | BH-BD | 2 (6.7) | 0.67 | 3.73 (5.23) | - | 17.30 (7.73) | - |
| PLA | 3 (10.3) | 3.07 (3.99) | 16.38 (6.83) | |||||
| 1-h post | BH-BD | 8 (26.7) | 0.33 | 3.70 (5.02) | - | 17.70 (7.84) | - | |
| PLA | 4 (13.8) | 2.21 (3.77) | 17.90 (7.04) | |||||
| △pre vs. 1-h post | BH-BD | 6 (20.0) | 0.25 | - | - | - | - | |
| PLA | 2 (6.9) | |||||||
| Model Estimate △ BH-BD vs. PLA | 1.15 | 0.23 | −0.80 | 0.46 | ||||
| Issue | Beverage Group | #Days (n) Issue Reported Mean (SD) | Proportion of Subjects with BTQ Issue | Proportion of Subjects with Moderate—Severe Issue | ||||
|---|---|---|---|---|---|---|---|---|
| At Least Once | At Least Twice | |||||||
| n (%) | Overall p-Value 1 | n (%) | Overall p-Value 1 | n (%) | Overall p-Value 1 | |||
| Burping | BH-BD | 1.4 (4.6) | 7 (23.3) | 1.00 | 4 (13.3) | 0.73 | 0 (0.0) | 0.24 |
| PLA | 2.8 (7.2) | 6 (20.7) | 5 (17.2) | 2 (6.9) | ||||
| Cramping | BH-BD | 1.2 (3.0) | 10 (33.3) | 0.78 | 4 (13.3) | 1.00 | 4 (13.3) | 0.11 |
| PLA | 0.5 (1.0) | 8 (27.6) | 3 (10.3) | 0 (0.0) | ||||
| Diarrhea | BH-BD | 1.4 (4.0) | 7 (23.3) | 0.57 | 4 (13.3) | 0.73 | 3 (10.0) | 0.47 |
| PLA | 0.9 (2.5) | 9 (31.0) | 5 (17.2) | 5 (17.2) | ||||
| Dizziness | BH-BD | 1.1 (3.0) | 7 (23.3) | 0.011 | 5 (16.7) | 0.052 | 2 (6.7) | 0.49 |
| PLA | 0.0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Gas | BH-BD | 2.7 (5.0) | 16 (53.3) | 1.00 | 12 (40.0) | 0.79 | 3 (10.0) | 0.47 |
| PLA | 2.4 (4.4) | 16 (55.2) | 13 (44.8) | 5 (17.2) | ||||
| Headache | BH-BD | 1.9 (4.3) | 14 (46.7) | 0.010 | 7 (23.3) | 0.011 | 3 (10.0) | 0.24 |
| PLA | 0.1 (0.4) | 4 (13.8) | 0 (0.0) | 0 (0.0) | ||||
| Nausea | BH-BD | 2.3 (4.3) | 14 (46.7) | 0.010 | 9 (30.0) | 0.042 | 6 (20.0) | 0.024 |
| PLA | 0.2 (0.6) | 4 (13.8) | 2 (6.9) | 0 (0.0) | ||||
| Reflux | BH-BD | 1.8 (4.7) | 6 (20.0) | 0.47 | 5 (16.7) | 0.42 | 5 (16.7) | 0.19 |
| PLA | 0.6 (2.8) | 3 (10.3) | 2 (6.9) | 1 (3.4) | ||||
| Rumbling | BH-BD | 2.9 (5.0) | 13 (43.3) | 0.79 | 11 (36.7) | 0.40 | 5 (16.7) | 0.19 |
| PLA | 1.6 (3.7) | 11 (37.9) | 7 (24.1) | 1 (3.4) | ||||
| Vomiting | BH-BD | 0.3 (1.6) | 2 (6.7) | 0.49 | 1 (3.3) | 1.00 | 1 (3.3) | 1.00 |
| PLA | 0.0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Clinic Visit Day | Timing | Beverage | BHB (mM) | Glucose (mg/dL) | ||
|---|---|---|---|---|---|---|
| Mean (SD) | p-Value 2 | Mean (SD) | p-Value 2 | |||
| 0 1 | Pre- | BH-BD | 0.102 (0.052) | 93.40 (7.84) | ||
| PLA | 0.128 (0.151) | 91.59 (10.47) | ||||
| 1-h post | BH-BD | 0.431 (0.240) | <0.001 | 96.97 (18.83) | 0.17 | |
| PLA | 0.083 (0.050) | 100.4 (24.85) | ||||
| △pre vs. 1-h post | BH-BD | 0.329 (0.219) | 3.57 (14.46) | |||
| PLA | −0.046 (0.140) | 8.79 (21.03) | ||||
| 7 1 | Pre- | BH-BD | 0.108 (0.077) | 90.97 (9.02) | ||
| PLA | 0.131 (0.106) | 88.52 (7.47) | ||||
| 1-h post | BH-BD | 0.985 (0.444) | <0.001 | 96.07 (28.24) | 0.38 | |
| PLA | 0.082 (0.030) | 97.00 (21.03) | ||||
| △pre vs. 1-h post | BH-BD | 0.876 (0.427) | 5.10 (25.31) | |||
| PLA | −0.049 (0.088) | 8.48 (17.16) | ||||
| 14 1 | Pre- | BH-BD | 0.104 (0.046) | 92.37 (11.62) | ||
| PLA | 0.134 (0.132) | 87.07 (8.15) | ||||
| 1-h post | BH-BD | 0.980 (0.392) | <0.001 | 93.80 (29.53) | 0.092 | |
| PLA | 0.086 (0.032) | 93.66 (22.98) | ||||
| △pre vs. 1-h post | BH-BD | 0.875 (0.365) | 1.43 (22.84) | |||
| PLA | −0.048 (0.119) | 6.59 (18.12) | ||||
| Exogenous Ketone Type | Reference(s) | Participant
Type (n) | Daily Serving Size Range | Symptoms Reported |
|---|---|---|---|---|
| MCT | [34,44,47,48,49] | Healthy adults before/after exercise (25) or adults with Alzheimer’s disease (260) | 20–85 g | GI distress, nausea, vomiting, abdominal pain, bloating, diarrhea, flatulence, dyspepsia, dizziness, headache |
| Ketone Salts | [22,33,50,51,52,53,54] | Healthy adults at rest (23) or before/after exercise (44) | 12–36.5 g | GI distress, nausea, diarrhea, vomiting, light-headedness, cramps |
| AcAc Ketone Diester | [55] | Healthy adults before exercise (11) | ~37 g | Vomiting, dizziness, nausea, reflux |
| 1,3 Butanediol | [56] | Healthy adults before exercise (9) | ~24 g | Nausea, euphoria, dizziness, belching, abdominal pain |
| BHB
Ketone Monoester | [24,33,50,57,58,59] | Healthy adults at rest (43) or before/after exercise (27) | 12–155 g | Vomiting, abdominal pain, nausea, flatulence, heartburn, headache, dizziness |
| BH-BD | Current study | Healthy adults | 12.5–25 g | Headache, nausea, dizziness |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, O.; Blonquist, T.M.; Mah, E.; Sanoshy, K.; Beckman, D.; Nieman, K.M.; Winters, B.L.; Anthony, J.C.; Verdin, E.; Newman, J.C.; et al. Tolerability and Safety of a Novel Ketogenic Ester, Bis-Hexanoyl (R)-1,3-Butanediol: A Randomized Controlled Trial in Healthy Adults. Nutrients 2021, 13, 2066. https://doi.org/10.3390/nu13062066
Chen O, Blonquist TM, Mah E, Sanoshy K, Beckman D, Nieman KM, Winters BL, Anthony JC, Verdin E, Newman JC, et al. Tolerability and Safety of a Novel Ketogenic Ester, Bis-Hexanoyl (R)-1,3-Butanediol: A Randomized Controlled Trial in Healthy Adults. Nutrients. 2021; 13(6):2066. https://doi.org/10.3390/nu13062066
Chicago/Turabian StyleChen, Oliver, Traci M. Blonquist, Eunice Mah, Kristen Sanoshy, Dawn Beckman, Kristin M. Nieman, Barbara L. Winters, Joshua C. Anthony, Eric Verdin, John C. Newman, and et al. 2021. "Tolerability and Safety of a Novel Ketogenic Ester, Bis-Hexanoyl (R)-1,3-Butanediol: A Randomized Controlled Trial in Healthy Adults" Nutrients 13, no. 6: 2066. https://doi.org/10.3390/nu13062066
APA StyleChen, O., Blonquist, T. M., Mah, E., Sanoshy, K., Beckman, D., Nieman, K. M., Winters, B. L., Anthony, J. C., Verdin, E., Newman, J. C., & Stubbs, B. J. (2021). Tolerability and Safety of a Novel Ketogenic Ester, Bis-Hexanoyl (R)-1,3-Butanediol: A Randomized Controlled Trial in Healthy Adults. Nutrients, 13(6), 2066. https://doi.org/10.3390/nu13062066







