Investigation of Sensitization Potential of the Soybean Allergen Gly m 4 by Using Caco-2/Immune Cells Co-Culture Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Heterologous Expression of Gly m 4 in E. coli
2.2. Ligand-Binding Fluorescence Assay
2.3. Bioinformatic Approach to Study Interaction of Que-3,4′-di-Glc with Gly m 4
2.4. Simulation of Gastrointestinal Digestion In Vitro
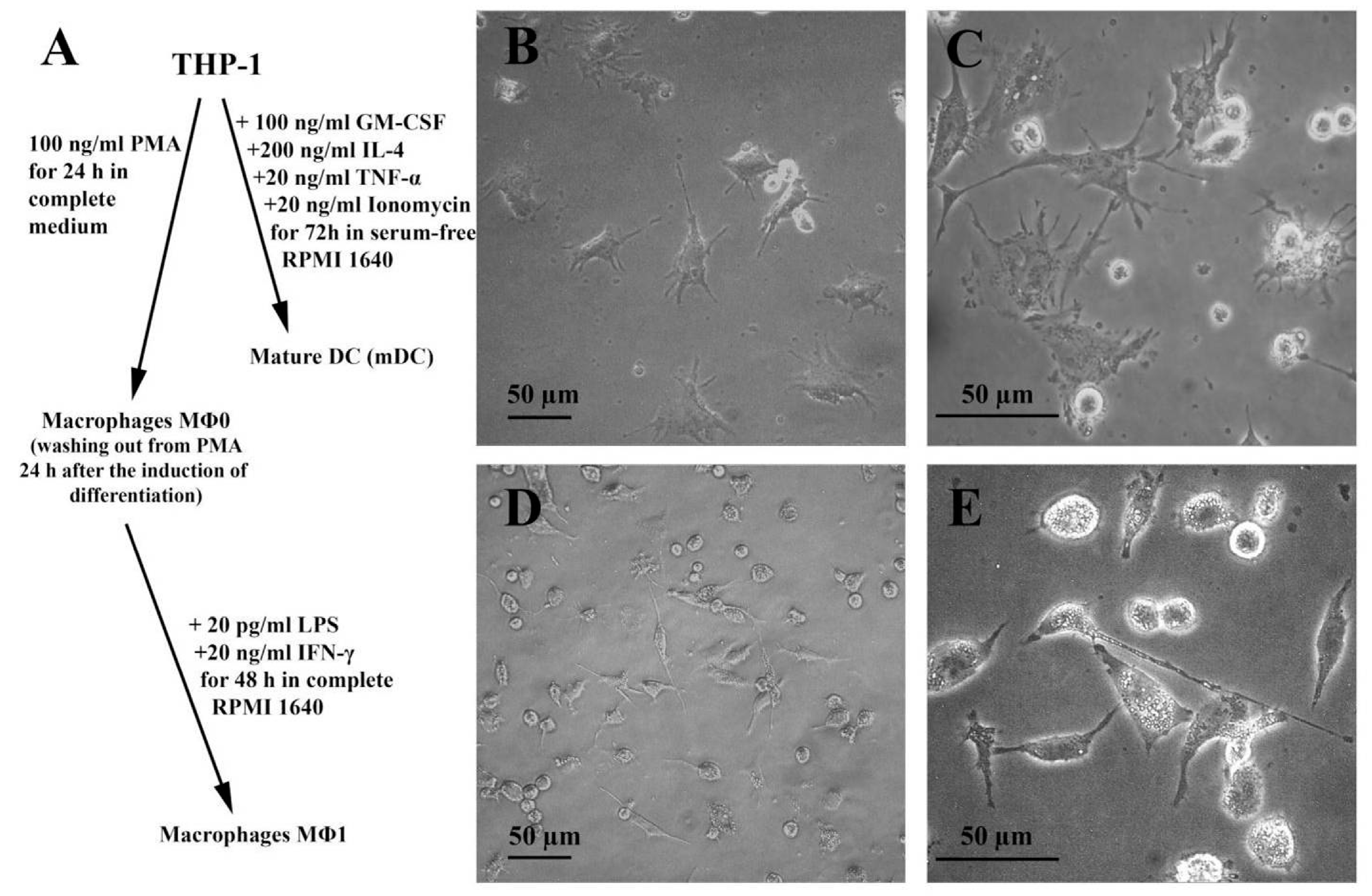
2.5. Human Cell Lines and Cultures
2.6. Labeling of Gly m 4 with FITC
2.7. Transport of FITC-Gly m 4 across the Caco-2 Epithelial Barrier
2.8. LC-MS/MS
2.9. Cytokines/Chemokines/Growth Factors Production by Cell Cultures
2.10. Assessment of Absolute Levels of Cytokines, Chemokines, and Growth Factors in Cell Cultures
2.11. Statistics
3. Results
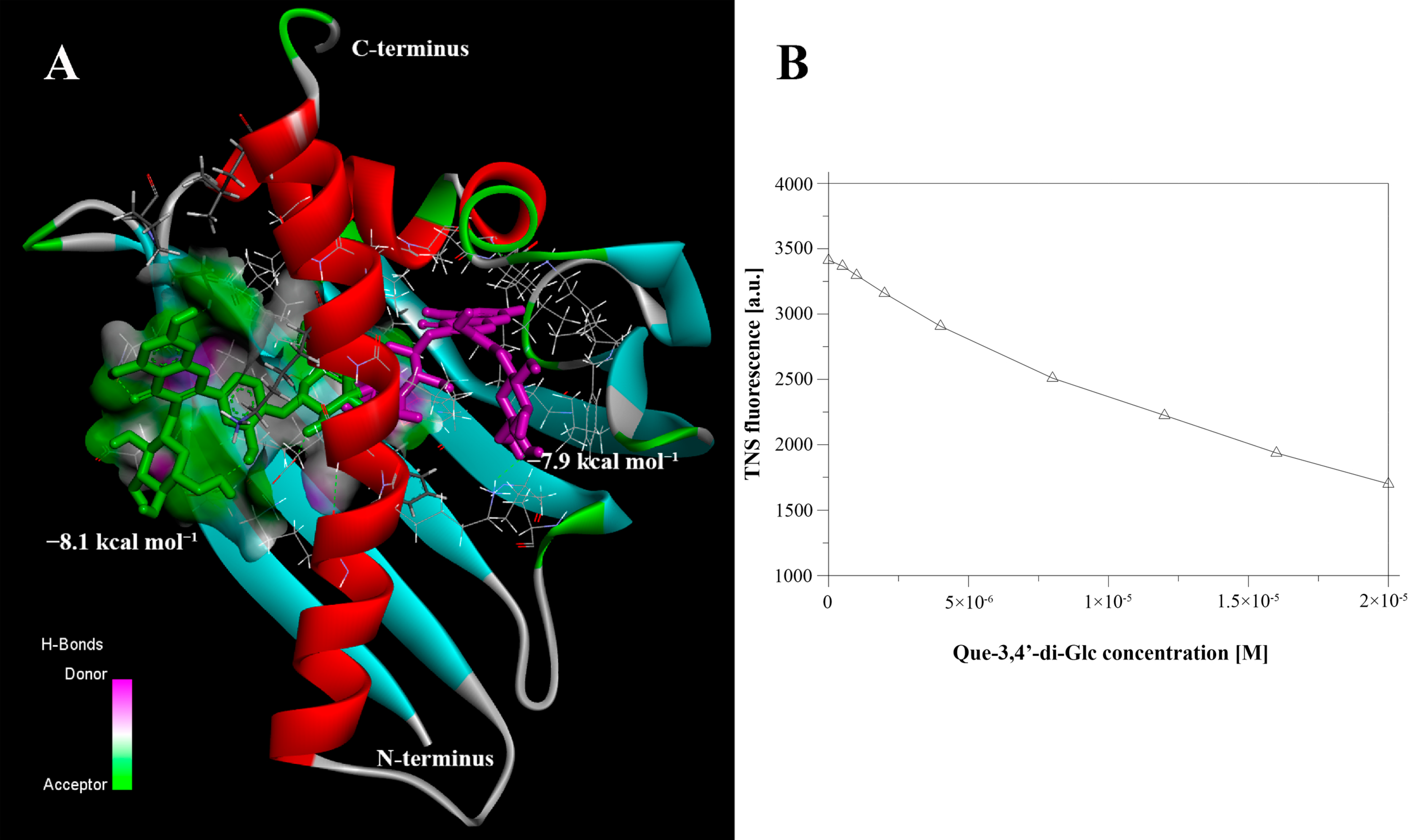
3.1. Gly m 4 Is Able to Bind Quercetin-3,4′-Diglucoside
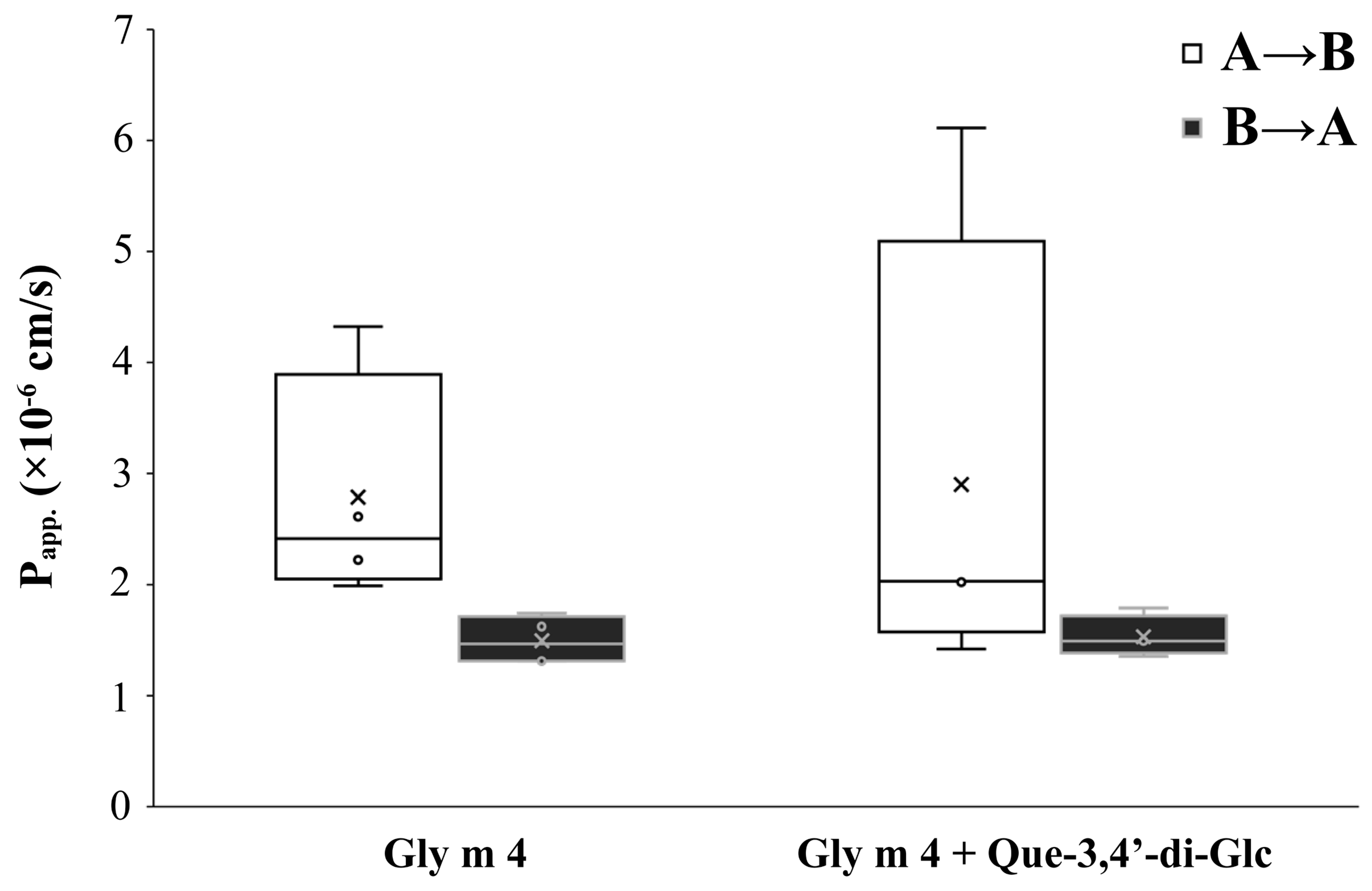
3.2. Gly m 4 Can Effectively cross the Caco-2 Epithelial Barrier
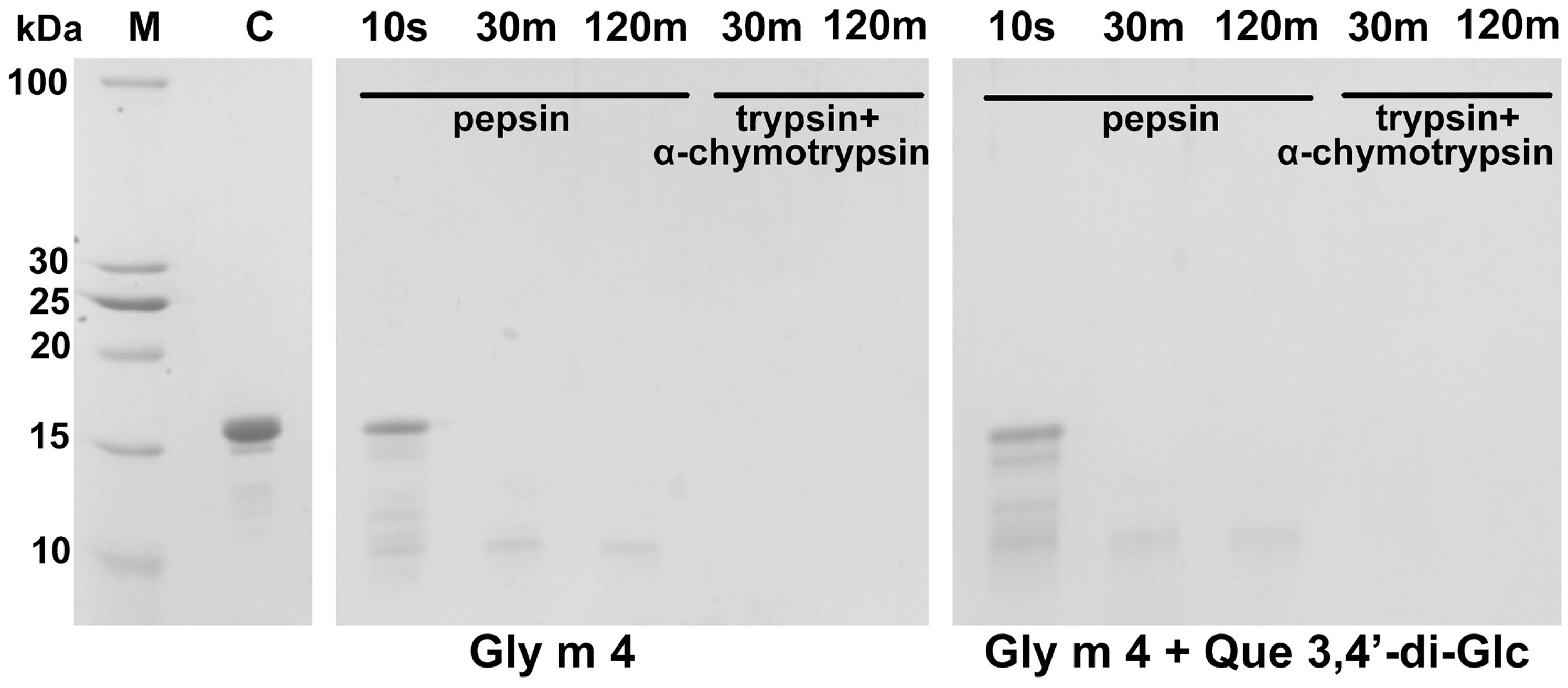
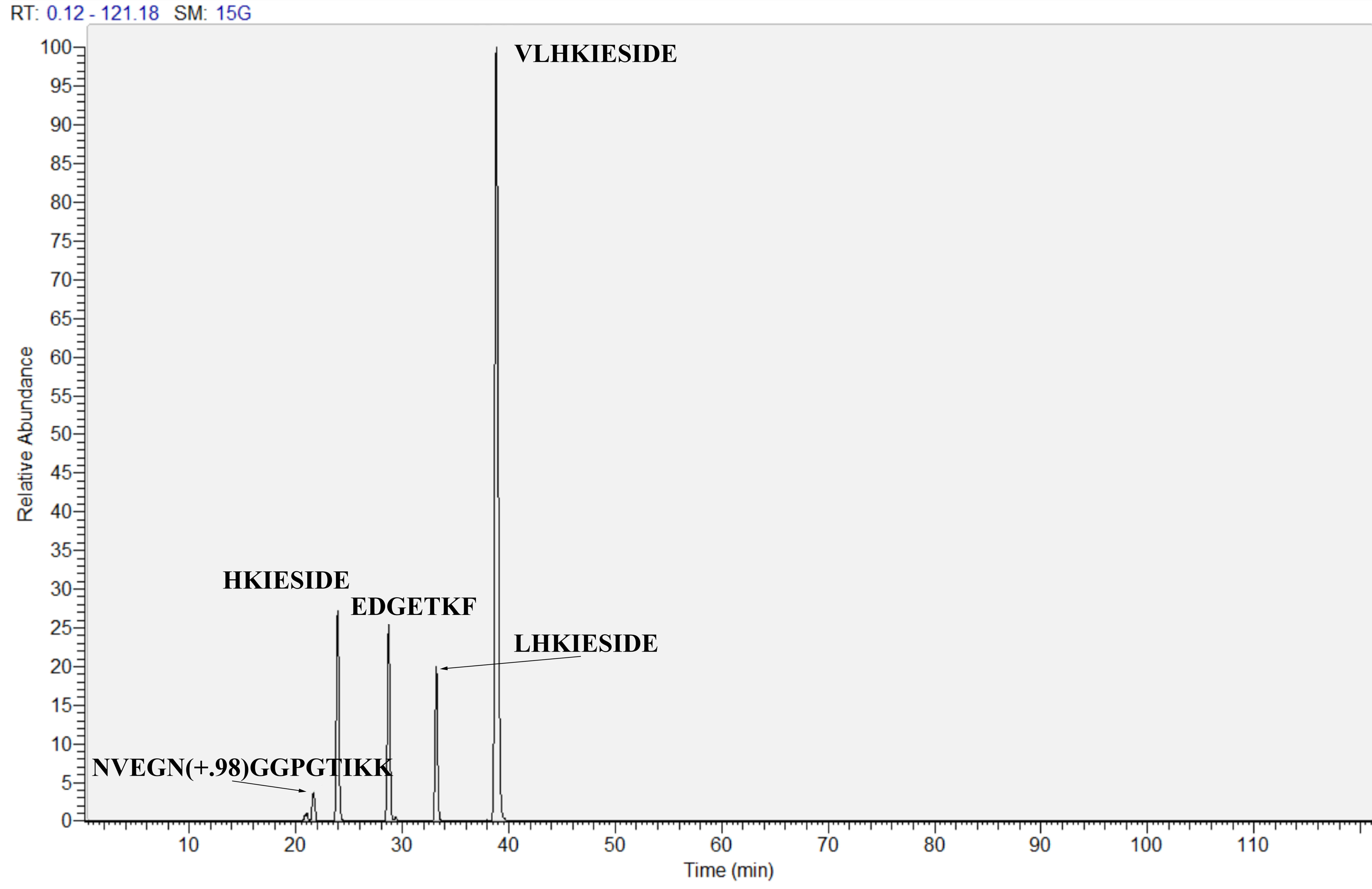
3.3. Gly m 4 Is Susceptible to Proteolytic Cleavage Mimicking Gastrointestinal Digestion In Vitro
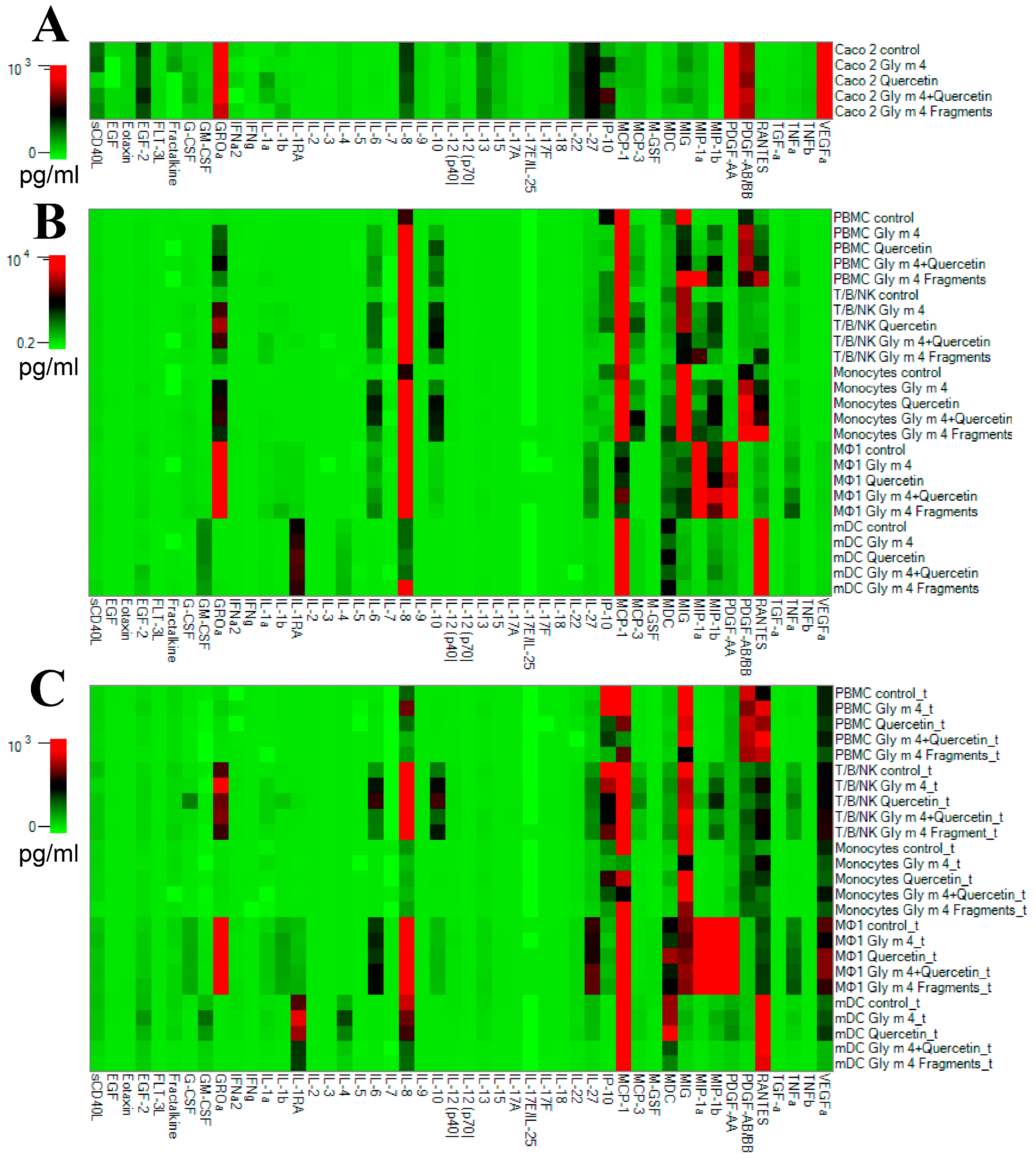
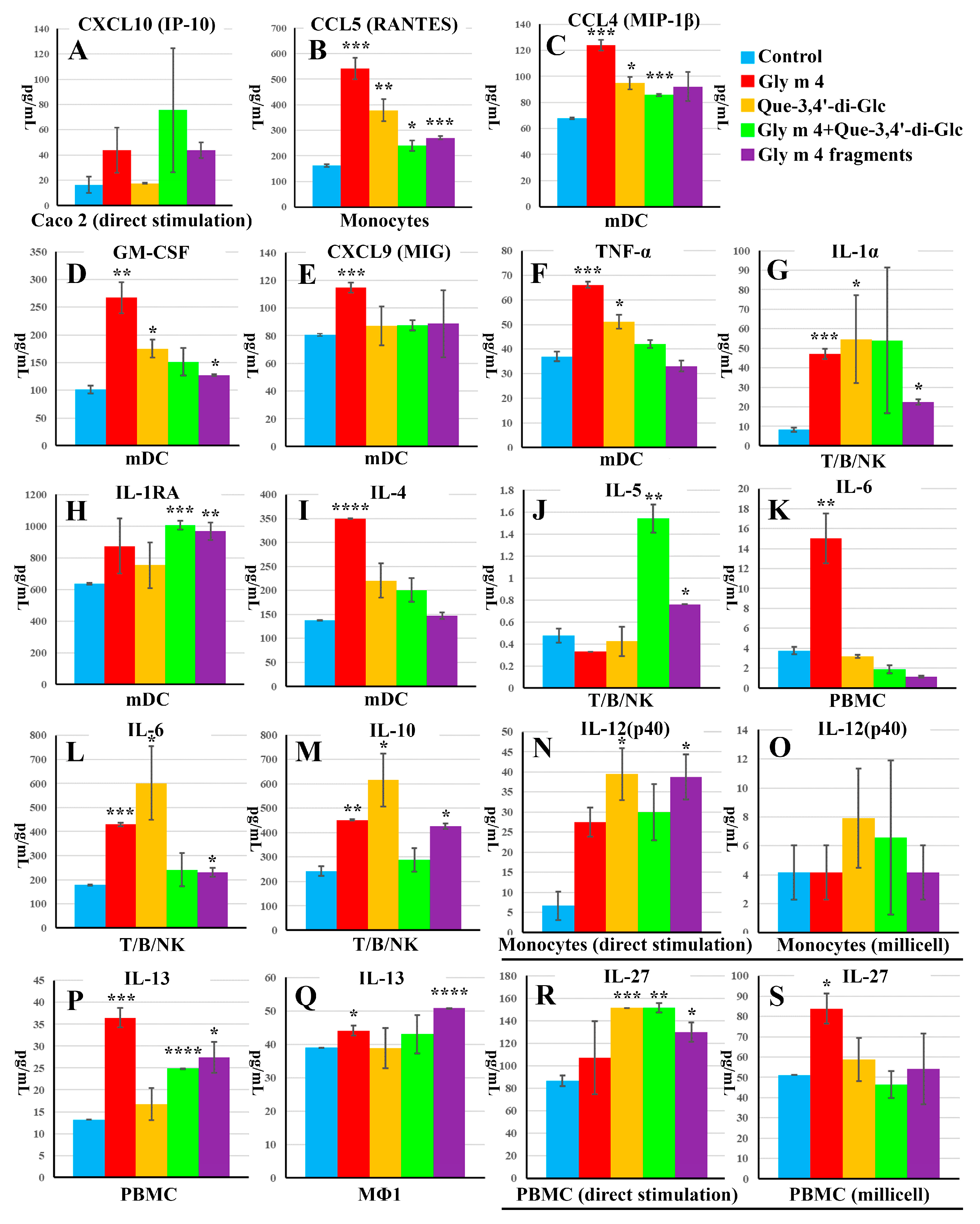
3.4. Intercommunication between Epithelial and Immune Cells Changes Cytokine Production in Response to the Intact Gly m 4 and Its Proteolytic Fragments
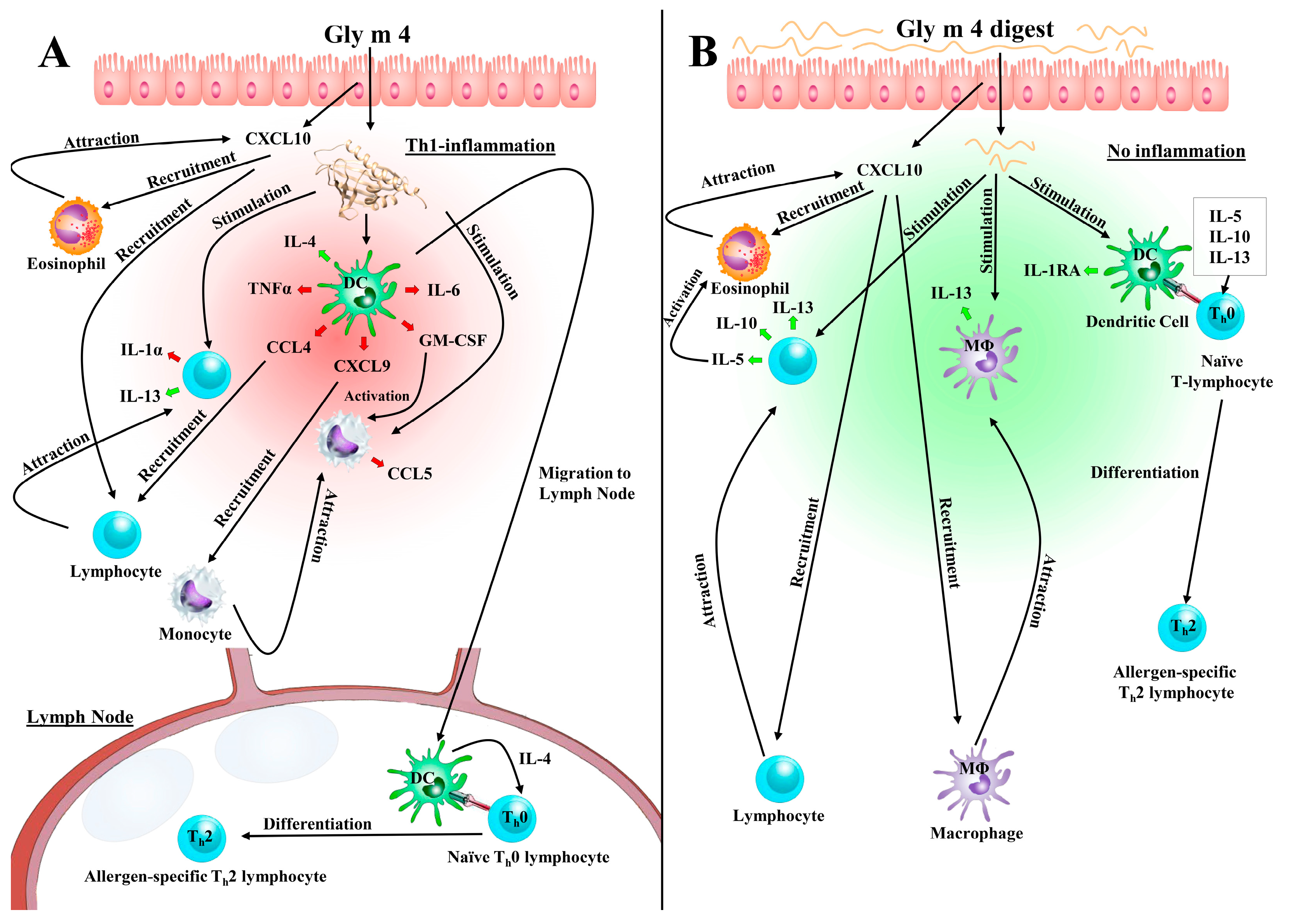
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kosma, P.; Sjölander, S.; Landgren, E.; Borres, M.P.; Hedlin, G. Severe reactions after the intake of soy drink in birch pollen-allergic children sensitized to Gly m 4. Acta Paediatr. 2011, 100, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, M.; Brostedt, P.; Sjölander, S.; Sato, S.; Borres, M.P.; Ito, K. Gly m 2S albumin is a major allergen with a high diagnostic value in soybean-allergic children. J. Allergy Clin. Immunol. 2013, 132, 976–978.e5. [Google Scholar] [CrossRef] [PubMed]
- Treudler, R.; Simon, J.-C. Pollen-related food allergy: An update. Allergo J. Int. 2017, 26, 273–282. [Google Scholar] [CrossRef]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Plant pathogenesis-related proteins PR-10 and PR-14 as components of innate immunity system and ubiquitous allergens. Curr. Med. Chem. 2017, 24, 1772–1787. [Google Scholar] [CrossRef]
- Berneder, M.; Bublin, M.; Hoffmann-Sommergruber, K.; Hawranek, T.; Lang, R. Allergen chip diagnosis for soy-allergic patients: Gly m 4 as a marker for severe food-allergic reactions to soy. Int. Arch. Allergy Immunol. 2013, 161, 229–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tordesillas, L.; Cubells-Baeza, N.; Gómez-Casado, C.; Berin, C.; Esteban, V.; Barcik, W.; O’Mahony, L.; Ramirez, C.; Pacios, L.F.; Garrido-Arandia, M.; et al. Mechanisms underlying induction of allergic sensitization by Pru p 3. Clin. Exp. Allergy 2017, 47, 1398–1408. [Google Scholar] [CrossRef]
- Jacob, T.; von Loetzen, C.S.; Reuter, A.; Lacher, U.; Schiller, D.; Schobert, R.; Mahler, V.; Vieths, S.; Rösch, P.; Schweimer, K.; et al. Identification of a natural ligand of the hazel allergen Cor a 1. Sci. Rep. 2019, 9, 8714. [Google Scholar] [CrossRef]
- Von Loetzen, C.S.; Hoffmann, T.; Hartl, M.J.; Schweimer, K.; Schwab, W.; Rösch, P.; Hartl-Spiegelhauer, O. Secret of the major birch pollen allergen Bet v 1: Identification of the physiological ligand. Biochem. J. 2014, 457, 379–390. [Google Scholar] [CrossRef]
- Mogensen, J.E.; Wimmer, R.; Larsen, J.N.; Spangfort, M.D.; Otzen, D.E. The major birch allergen, Bet v 1, shows affinity for a broad spectrum of physiological ligands. J. Biol. Chem. 2002, 277, 23684–23692. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Dassault Systèmes BIOVIA. Discovery Studio Visualizer; v20.1.0.19295; Dassault Systèmes: San Diego, CA, USA, 2020. [Google Scholar]
- Bogdanov, I.V.; Shenkarev, Z.O.; Finkina, E.I.; Melnikova, D.N.; Rumynskiy, E.I.; Arseniev, A.S.; Ovchinnikova, T.V. A novel lipid transfer protein from the pea Pisum sativum: Isolation, recombinant expression, solution structure, antifungal activity, lipid binding, and allergenic roperties. BMC Plant Biol. 2016, 16, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backman, L.; Persson, K. The No-Nonsens SDS-PAGE. Methods Mol. Biol. 2018, 1721, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berges, C.; Naujokat, C.; Tinapp, S.; Wieczorek, H.; Höh, A.; Sadeghi, M.; Opelz, G.; Daniel, V. A cell line model for the differentiation of human dendritic cells. Biochem. Biophys. Res. Commun. 2005, 333, 896–907. [Google Scholar] [CrossRef]
- Kovalchuk, S.I.; Jensen, O.N.; Rogowska-Wrzesinska, A. FlashPack: Fast and simple preparation of ultrahigh-performance capillary columns for LC-MS. Mol. Cell Proteom. 2019, 18, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating effects of physical Activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.K.; Cheng, H.; Maleki, S.J.; Hurlburt, B.K. Purification and Characterization of Pathogenesis Related Class 10 Panallergens. Foods 2019, 8, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lea, T. Caco-2 cell line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 103–111. [Google Scholar]
- Liu, X.; Zheng, S.; Qin, Y.; Ding, W.; Tu, Y.; Chen, X.; Wu, Y.; Yanhua, L.; Cai, X. Experimental Evaluation of the Transport Mechanisms of PoIFN-α in Caco-2 Cells. Front. Pharmacol. 2017, 8, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tordesillas, L.; Gómez-Casado, C.; Garrido-Arandia, M.; Murua-García, A.; Palacín, A.; Varela, J.; Konieczna, P.; Cuesta-Herranz, J.; Akdis, C.A.; O’Mahony, L.; et al. Transport of Pru p 3 across gastrointestinal epithelium—An essential step towards the induction of food allergy? Clin. Exp. Allergy 2013, 43, 1374–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Press, B. Optimization of the Caco-2 permeability assay to screen drug compounds for intestinal absorption and efflux. Methods Mol. Biol. 2011, 763, 139–154. [Google Scholar] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Schimek, E.M.; Zwölfer, B.; Briza, P.; Jahn-Schmid, B.; Vogel, L.; Vieths, S.; Ebner, C.; Bohle, B. Gastrointestinal digestion of Bet v 1-homologous food allergens destroys their mediator-releasing, but not T cell-activating, capacity. J. Allergy Clin. Immunol. 2005, 116, 1327–1333. [Google Scholar] [CrossRef]
- Jahn-Schmid, B.; Radakovics, A.; Lüttkopf, D.; Scheurer, S.; Vieths, S.; Ebner, C. Bohle, B. Bet v 1142-156 is the dominant T-cell epitope of the major birch pollen allergen and important for cross-reactivity with Bet v 1-related food allergens. J. Allergy Clin. Immunol. 2005, 116, 213–219. [Google Scholar] [CrossRef]
- Kleiveland, C.R. Co-culture Caco-2/Immune Cells. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 197–205. [Google Scholar]
- Tworek, D.; Kuna, P.; Młynarski, W.; Górski, P.; Pietras, T.; Antczak, A. MIG (CXCL9), IP-10 (CXCL10) and I-TAC (CXCL11) concentrations after nasal allergen challenge in patients with allergic rhinitis. Arch. Med. Sci. 2013, 9, 849–853. [Google Scholar] [CrossRef]
- König, K.; Klemens, C.; Eder, K.; Nicoló, M.S.; Becker, S.; Kramer, M.F.; Gröger, M. Cytokine profiles in nasal fluid of patients with seasonal or persistent allergic rhinitis. Allergy Asthma Clin. Immunol. 2015, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Holgate, S.T.; Bodey, K.S.; Janezic, A.; Frew, A.J.; Kaplan, A.P.; Teran, L.M. Release of RANTES, MIP-1 alpha, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am. J. Respir. Crit. Care Med. 1997, 156, 1377–1383. [Google Scholar] [CrossRef]
- Bieber, T. Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Nagase, H.; Ueki, S.; Fujieda, S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol. Int. 2020, 69, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Pekar, J.; Ret, D.; Untersmayr, E. Stability of allergens. Mol. Immunol. 2018, 100, 14–20. [Google Scholar] [CrossRef]
- Wills-Karp, M. IL-12/IL-13 axis in allergic asthma. J. Allergy Clin. Immunol. 2001, 107, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Deng, R.; Chi, W.; Hua, X.; Lu, F.; Bian, F.; Gao, N.; Li, Z.; Pflugfelder, S.C.; de Paiva, C.S.; et al. IL-27 signaling deficiency develops Th17-enhanced Th2-dominant inflammation in murine allergic conjunctivitis model. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 910–921. [Google Scholar] [CrossRef] [PubMed]

| Stimulation Way | Control | Gly m 4 Alone | Que-3,4′-di-Glc Alone | Gly m 4 + Que-3,4′-di-Glc | Gly m 4 Digest |
|---|---|---|---|---|---|
| Direct stimulation (into 96-well plate) | − | 5 μM | 2.5 μM | 5 μM + 2.5 μM | 5 μM |
| Transepithelial stimulation (into 24-well plate with Caco-2 inserts) | − | 5 μM | 5 μM | 5 μM + 5 μM | 5 μM |
| Peptide | −10lgP | Molecular Mass | ppm | m/z | RT | Peak Area |
|---|---|---|---|---|---|---|
| V66LHKIESIDE75 | 33.48 | 1181.6292 | 0.9 | 591.8224 | 38.81 | 1.50 × 108 |
| H68KIESIDE75 | 22.38 | 969.4767 | 0.6 | 485.7459 | 23.92 | 2.07 × 107 |
| L67HKIESIDE75 | 27.23 | 1082.5608 | 1.6 | 542.2885 | 33.2 | 1.76 × 107 |
| E59DGETKF65 | 23.74 | 824.3552 | 2.1 | 413.1857 | 28.72 | 1.51 × 107 |
| N42VEGN(+0.98)GGPGTIKK54 | 33.21 | 1270.6517 | 1.9 | 636.3344 | 21.63 | 1.05 × 107 |
| Y119ETKGDAEPNQDELKTGK136 | 48.5 | 2021.9541 | 3.5 | 1011.988 | 27.7 | 9.75 × 106 |
| K38SVENVEGN(+0.98)GGPGTIKK54 | 45.73 | 1713.8896 | 2.6 | 857.9543 | 25.6 | 9.26 × 106 |
| S39VENVEGN(+0.98)GGPGTIKK54 | 34.66 | 1585.7947 | 2.2 | 529.6067 | 30.94 | 8.78 × 106 |
| N42VEGN(+0.98)GGPGTIK53 | 27.83 | 1142.5568 | 2.5 | 572.2871 | 28.81 | 3.99 × 106 |
| T116VKYETKGDAEPNQDELKTGK136 | 35.94 | 2350.1653 | 4 | 784.3989 | 29.17 | 2.32 × 106 |
| S39VENVEGNGGPGTIKK54 | 31.09 | 1584.8107 | 3.1 | 793.415 | 29.33 | 2.30 × 106 |
| F37KSVENVEGN(+0.98)GGPGTIKK54 | 39.96 | 1860.9581 | −0.7 | 466.2465 | 38.79 | 2.07 × 106 |
| N108(+0.98)GGSAGKL115 | 22.71 | 703.35 | 1.2 | 352.6827 | 23.07 | 1.86 × 106 |
| N42VEGNGGPGTIKK54 | 26.72 | 1269.6677 | 1 | 424.2303 | 18.9 | 1.47 × 106 |
| K38SVENVEGN(+0.98)GGPGTIK53 | 41.56 | 1585.7947 | 3.3 | 793.9072 | 32.29 | 1.31 × 106 |
| N42VEGNGGPGTIK53 | 24.76 | 1141.5728 | 1.8 | 571.7947 | 26.04 | 1.01 × 106 |
| V40ENVEGN(+0.98)GGPGTIK53 | 22.11 | 1370.6677 | 3.3 | 686.3434 | 35.55 | 9.07 × 105 |
| E120TKGDAEPNQDELKTGK136 | 46.16 | 1858.8907 | 1.4 | 930.4539 | 22.67 | 8.76 × 105 |
| E41NVEGN(+0.98)GGPGTIK53 | 30.24 | 1271.5994 | 1.8 | 636.8081 | 30.92 | 8.76 × 105 |
| V66LHKIESID74 | 20.04 | 1052.5865 | 1.6 | 527.3014 | 36.84 | 8.58 × 105 |
| N128QDELKTGK136 | 22.69 | 1031.5247 | 3.4 | 516.7714 | 15.08 | 7.29 × 105 |
| V40ENVEGN(+0.98)GGPGTIKK54 | 24.92 | 1498.7627 | 3.2 | 750.391 | 27.38 | 7.26 × 105 |
| T4FEDEINSPVAPATL18 | 36.32 | 1602.7777 | 3.6 | 802.399 | 97.49 | 5.50 × 105 |
| G123DAEPNQDELKTGK136 | 35.57 | 1500.7056 | 1.7 | 751.3613 | 25.69 | 5.18 × 105 |
| S39VENVEGNGGPGTIK53 | 23.14 | 1456.7157 | 5.1 | 729.3688 | 38.54 | 5.00 × 105 |
| P91DTAEKITF99 | 30.33 | 1020.5128 | 2.5 | 511.265 | 64.51 | 3.78 × 105 |
| S39VENVEGN(+0.98)GGPGTIK53 | 27.36 | 1457.6997 | 1.6 | 729.8583 | 40.55 | 3.75 × 105 |
| V66LHKIESIDEANL78 | 33.81 | 1479.7932 | 0.9 | 740.9045 | 64.96 | 2.07 × 105 |
| E120TKGDAEPNQDELK133 | 34.3 | 1572.7267 | 1.7 | 787.3719 | 24.41 | 1.43 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanov, I.V.; Finkina, E.I.; Melnikova, D.N.; Ziganshin, R.H.; Ovchinnikova, T.V. Investigation of Sensitization Potential of the Soybean Allergen Gly m 4 by Using Caco-2/Immune Cells Co-Culture Model. Nutrients 2021, 13, 2058. https://doi.org/10.3390/nu13062058
Bogdanov IV, Finkina EI, Melnikova DN, Ziganshin RH, Ovchinnikova TV. Investigation of Sensitization Potential of the Soybean Allergen Gly m 4 by Using Caco-2/Immune Cells Co-Culture Model. Nutrients. 2021; 13(6):2058. https://doi.org/10.3390/nu13062058
Chicago/Turabian StyleBogdanov, Ivan V., Ekaterina I. Finkina, Daria N. Melnikova, Rustam H. Ziganshin, and Tatiana V. Ovchinnikova. 2021. "Investigation of Sensitization Potential of the Soybean Allergen Gly m 4 by Using Caco-2/Immune Cells Co-Culture Model" Nutrients 13, no. 6: 2058. https://doi.org/10.3390/nu13062058
APA StyleBogdanov, I. V., Finkina, E. I., Melnikova, D. N., Ziganshin, R. H., & Ovchinnikova, T. V. (2021). Investigation of Sensitization Potential of the Soybean Allergen Gly m 4 by Using Caco-2/Immune Cells Co-Culture Model. Nutrients, 13(6), 2058. https://doi.org/10.3390/nu13062058










