Plasma Lutein, a Nutritional Biomarker for Development of Advanced Age-Related Macular Degeneration: The Alienor Study
Abstract
1. Introduction
2. Materials and Methods
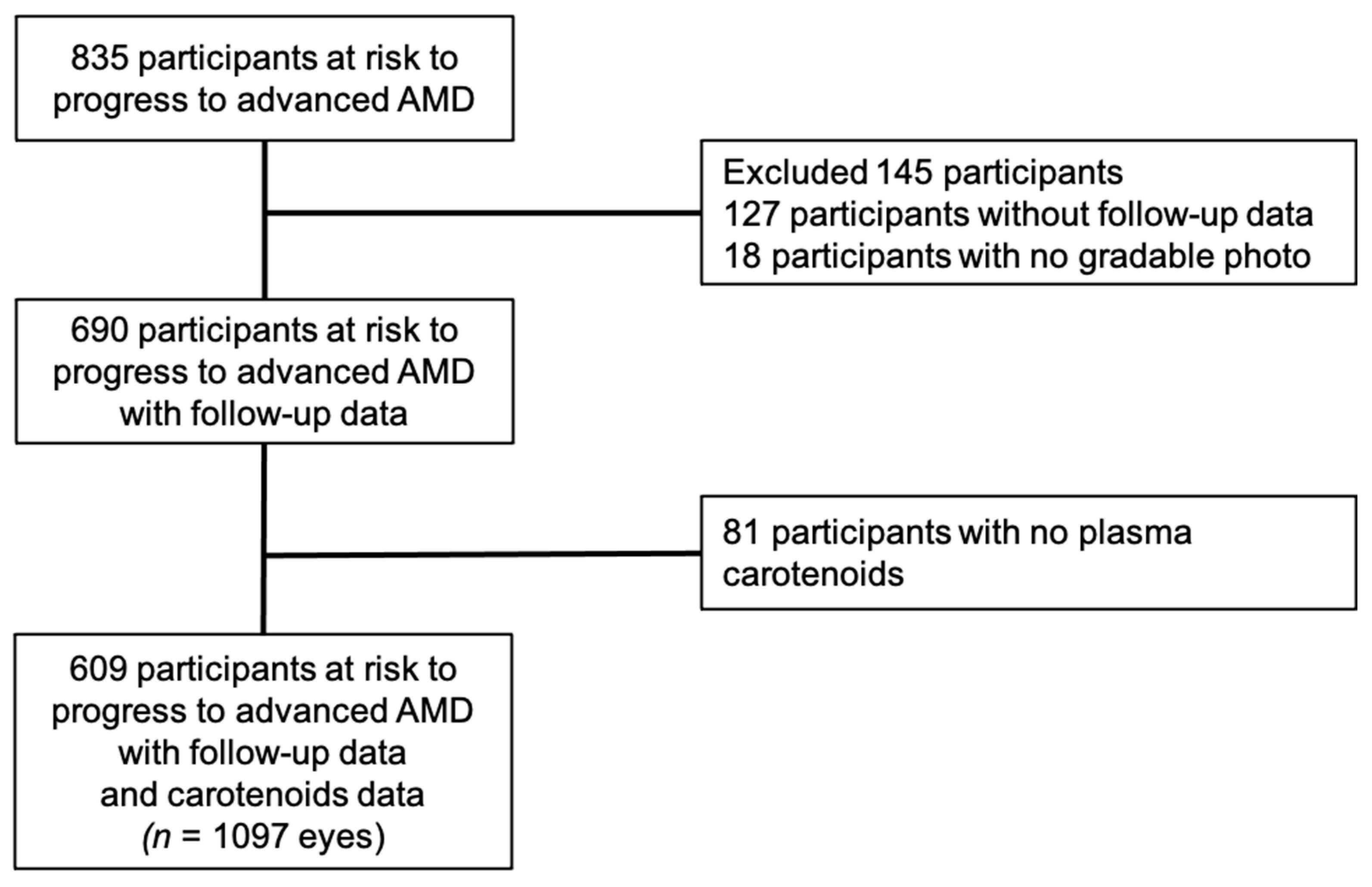
2.1. Study Population
2.2. Eye Examination
2.3. AMD Classification
2.4. Incidence of AMD
2.5. Plasma Carotenoids and Lipids Assessment
2.6. Other Variables
2.7. Statistical Analysis
Secondary Analyses
3. Results
3.1. Characteristics of the SAMPLE
3.2. Multivariate Associations Between Plasma Lutein and Zeaxanthin and Risk of AMD
3.3. Multivariate Associations Between Plasma Lutein and Zeaxanthin and Risk of Neovascular and Atrophic AMD
3.4. Multivariate Associations Between Other Carotenoids and Risk of AMD
3.5. Secondary Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.; Chung, C.Y.; Kim, R.Y. Ranibizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.-F.; Kaiser, P.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Sobrin, L.; Seddon, J.M. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Prog. Retin. Eye Res. 2014, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994, 272, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Marse-Perlman, J.A.; Fisher, A.I.; Klein, R.; Palta, M.; Block, G.; Millen, A.E.; Wright, J.D. Lutein and Zeaxanthin in the Diet and Serum and Their Relation to Age-related Maculopathy in the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2001, 153, 424–432. [Google Scholar] [CrossRef]
- Delcourt, C.; Carrie‘re, I.; Delage, M.; Barberger-Gateau, P.; Schalch, W. Plasma Lutein and Zeaxanthin and Other Carotenoids as Modifiable Risk Factors for Age-Related Maculopathy and Cataract: The POLA Study. Investig. Opthalmol. Vis. Sci. 2006, 47, 2329–2335. [Google Scholar] [CrossRef]
- Cho, E.; Hankinson, S.E.; Rosner, B.; Willett, W.C.; Colditz, G.A. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration2. Am. J. Clin. Nutr. 2008, 87, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Dou, H.-L.; Wu, Y.-Q.; Huang, Y.-M.; Huang, Y.-B.; Xu, X.-R.; Zou, Z.; Lin, X.-M. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, 350–359. [Google Scholar] [CrossRef]
- Van Leeuwen, E.M.; Emri, E.; Merle, B.M.; Colijn, J.M.; Kersten, E.; Cougnard-Gregoire, A.; Dammeier, S.; Meester-Smoor, M.; Pool, F.M.; de Jong, E.K.; et al. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 2018, 67, 56–86. [Google Scholar] [CrossRef]
- Merle, B.M.; Colijn, J.M.; Cougnard-Grégoire, A.; De Koning-Backus, A.P.; Delyfer, M.-N.; Jong, J.C.K.-D.; Meester-Smoor, M.; Féart, C.; Verzijden, T.; Samieri, C.; et al. Mediterranean Diet and Incidence of Advanced Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2019, 126, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef]
- Whitehead, A.J.; Mares, J.A.; Danis, R.P. Macular Pigment. Arch. Ophthalmol. 2006, 124, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; McCullough, M.L.; Newby, P.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Jones, D.P.; Goldberg, J.; Ziegler, T.R.; Bostick, R.M.; Wilson, P.W.; Manatunga, A.K.; Shallenberger, L.; Jones, L.; Vaccarino, V. Association between adherence to the Mediterranean diet and oxidative stress. Am. J. Clin. Nutr. 2008, 88, 1364–1370. [Google Scholar]
- Hammond, B.R.; Wooten, B.R. CFF thresholds: Relation to macular pigment optical density. Ophthalmic Physiol. Opt. 2005, 25, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Renzi, L.M.; Hammond, B.R. The relation between the macular carotenoids, lutein and zeaxanthin, and temporal vision. Ophthalmic Physiol. Opt. 2010, 30, 351–357. [Google Scholar] [CrossRef]
- Craft, N.E.; Haitema, T.B.; Garnett, K.M.; Fitch, K.A.; Dorey, C.K. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J. Nutr. Health Aging 2004, 8, 156–162. [Google Scholar]
- Mohn, E.S.; Erdman, J.W.; Kuchan, M.J.; Neuringer, M.; Johnson, E.J. Lutein accumulates in subcellular membranes of brain regions in adult rhesus macaques: Relationship to DHA oxidation products. PLoS ONE 2017, 12, e0186767. [Google Scholar] [CrossRef]
- Vandenlangenberg, G.M.; Mares-Perlman, J.A.; Klein, R.; Klein, B.E.K.; Brady, W.E.; Palta, M. Associations between Antioxidant and Zinc Intake and the 5-Year Incidence of Early Age-related Maculopathy in the Beaver Dam Eye Study. Am. J. Epidemiol. 1998, 148, 204–214. [Google Scholar] [CrossRef]
- Moeller, S.M.; Parekh, N.; Tinker, L.; Ritenbaugh, C.; Blodi, B.; Wallace, R.B.; Mares, J.A. Associations Between Intermediate Age-Related Macular Degeneration and Lutein and Zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Arch. Ophthalmol. 2006, 124, 1151–1162. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Rd, F.F.; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The Relationship of Dietary Carotenoid and Vitamin A, E, and C Intake with Age-Related Macular Degeneration in a Case-Control Study. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar] [CrossRef]
- Tan, J.S.; Wang, J.J.; Flood, V.; Rochtchina, E.; Smith, W.; Mitchell, P. Dietary Antioxidants and the Long-term Incidence of Age-Related Macular Degeneration. Ophthalmology 2008, 115, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-up. JAMA Ophthalmol. 2015, 133, 1415–1424. [Google Scholar] [CrossRef]
- Aoki, A.; Inoue, M.; Nguyen, E.; Obata, R.; Kadonosono, K.; Shinkai, S.; Hashimoto, H.; Sasaki, S.; Yanagi, Y. Dietary n-3 Fatty Acid, α-Tocopherol, Zinc, vitamin D, vitamin C and β-carotene are Associated with Age-Related Macular Degeneration in Japan. Sci. Rep. 2016, 6, 20723. [Google Scholar] [CrossRef]
- Antioxidant Status and Neovascular Age-Related Macular Degeneration. Arch. Ophthalmol. 1993, 111, 104–109. [CrossRef] [PubMed]
- Mares-Perlman, J.A.; Brady, W.E.; Klein, R.; Klein, B.E.K.; Bowen, P.; Stacewicz-Sapuntzakis, M.; Palta, M. Serum Antioxidants and Age-Related Macular Degeneration in a Population-Based Case-Control Study. Arch. Ophthalmol. 1995, 113, 1518–1523. [Google Scholar] [CrossRef]
- Gale, C.R.; Hall, N.F.; Phillips, D.I.W.; Martyn, C.N. Lutein and zeaxanthin status and risk of age-related macular degeneration. Investig. Opthalmol. Vis. Sci. 2003, 44, 2461–2465. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, T.; Ishida, S.; Nishiwaki, Y.; Kikuchi, Y.; Tsuboi, T.; Hosoda, K.; Ishigami, A.; Iwasawa, S.; Nakano, M.; Takebayashi, T. Serum antioxidants and age-related macular degeneration among older Japanese. Asia Pac. J. Clin. Nutr. 2009, 18, 1–7. [Google Scholar] [PubMed]
- Delcourt, C.; Korobelnik, J.-F.; Barberger-Gateau, P.; Delyfer, M.-N.; Rougier, M.-B.; Le Goff, M.; Malet, F.; Colin, J.; Dartigues, J.-F. Nutrition and age-related eye diseases: The Alienor (Antioxydants, lipides essentiels, nutrition et maladies oculaires) study. J. Nutr. Health Aging 2010, 14, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, M.; Pugliatti, M.; Hubbard, R.; Britton, J.; Sotgiu, S.; Sadovnick, A.D.; Yee, I.M.; Cumsille, M.A.; Bevilacqua, J.A.; Burdett, S.; et al. Vascular factors and risk of dementia: Design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology 2003, 22, 316–325. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Knudtson, M.D.; Wong, T.Y.; Cotch, M.F.; Liu, K.; Burke, G.; Saad, M.F.; Jacobs, D.R. Prevalence of Age-Related Macular Degeneration in 4 Racial/Ethnic Groups in the Multi-ethnic Study of Atherosclerosis. Ophthalmology 2006, 113, 373–380. [Google Scholar] [CrossRef]
- Bird, A.; Bressler, N.; Bressler, S.; Chisholm, I.; Coscas, G.; Davis, M.; de Jong, P.; Klaver, C.; Klein, B.; Klein, R.; et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv. Ophthalmol. 1995, 39, 367–374. [Google Scholar] [CrossRef]
- Saunier, V.; Merle, B.M.J.; Delyfer, M.-N.; Cougnard-Grégoire, A.; Rougier, M.-B.; Amouyel, P.; Lambert, J.-C.; Dartigues, J.-F.; Korobelnik, J.-F.; Delcourt, C. Incidence of and Risk Factors Associated with Age-Related Macular Degeneration. JAMA Ophthalmol. 2018, 136, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.; Thürmann, P.A.; Spitzer, V.; Schalch, W.; Manner, B.; Cohn, W. Plasma kinetics of zeaxanthin and 3′-dehydro-lutein after multiple oral doses of synthetic zeaxanthin. Am. J. Clin. Nutr. 2004, 79, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Beck, M.; Elliott, J.; Etheve, S.; Roberts, R.; Schalch, W. Effects of formulation on the bioavailability of lutein and zeaxanthin: A randomized, double-blind, cross-over, comparative, single-dose study in healthy subjects. Eur. J. Nutr. 2013, 52, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W. Bioavailability and metabolism. Mol. Asp. Med. 2002, 23, 39–100. [Google Scholar] [CrossRef]
- Feart, C.; Letenneur, L.; Helmer, C.; Samieri, C.; Schalch, W.; Etheve, S.; Delcourt, C.; Dartigues, J.-F.; Barberger-Gateau, P. Plasma Carotenoids Are Inversely Associated with Dementia Risk in an Elderly French Cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, G.; Forti, P.; Lucicesare, A.; Pisacane, N.; Rietti, E.; Mangialasche, F.; Cecchetti, R.; Patterson, C.; Mecocci, P. Plasma tocopherols and risk of cognitive impairment in an elderly Italian cohort. Am. J. Clin. Nutr. 2008, 87, 1306–1313. [Google Scholar] [CrossRef]
- Lambert, J.C.; Heath, S.; Even, G.; Campion, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Zelenika, D.; Bullido, M.J.; Tavernier, B. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1094–1099. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.C.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef]
- Delcourt, C.; Boniol, M.; Carriere, I.; Delyfer, M.-N.; Rougier, M.-B.; Le Goff, M.; Dartigues, J.-F.; Barberger-Gateau, P.; Korobelnik, J.-F.; Cougnard-Grégroire, A.; et al. Lifetime Exposure to Ambient Ultraviolet Radiation and the Risk for Cataract Extraction and Age-Related Macular Degeneration: The Alienor Study. Investig. Opthalmol. Vis. Sci. 2014, 55, 7619–7627. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, F.E. Sample Criteria for Testing Outlying Observations. Ann. Math. Stat. 1950, 21, 27–58. [Google Scholar] [CrossRef]
- Lamarca, R.; Alonso, J.; Gómez, G.; Muñoz, Á. Left-truncated Data with Age as Time Scale: An Alternative for Survival Analysis in the Elderly Population. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1998, 53, M337–M343. [Google Scholar] [CrossRef]
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical Damage of the Retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Hammond, B.; Yeum, K.-J.; Qin, J.; Wang, X.D.; Castaneda, C.; Snodderly, D.; Russell, R.M. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am. J. Clin. Nutr. 2000, 71, 1555–1562. [Google Scholar] [CrossRef]
- Curran-Celentano, J.; Hammond, B.R.; Ciulla, T.A.; Cooper, D.A.; Pratt, L.M.; Danis, R.B. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am. J. Clin. Nutr. 2001, 74, 796–802. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Guerra, L.H.; Ruiz, C.A. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J. Nutr. 2003, 133, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Korobelnik, J.-F.; Rougier, M.-B.; Delyfer, M.-N.; Bron, A.; Merle, B.M.J.; Savel, H.; Chêne, G.; Delcourt, C.; Creuzot-Garcher, C. Effect of Dietary Supplementation with Lutein, Zeaxanthin, and ω-3 on Macular Pigment: A Randomized Clinical Trial. JAMA Ophthalmol. 2017, 135, 1259–1266. [Google Scholar] [CrossRef]
- Tanito, M.; Obana, A.; Gohto, Y.; Okazaki, S.; Gellermann, W.; Ohira, A. Macular pigment density changes in Japanese individuals supplemented with lutein or zeaxanthin: Quantification via resonance Raman spectrophotometry and autofluorescence imaging. Jpn. J. Ophthalmol. 2012, 56, 488–496. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, Zeaxanthin and Meso-zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef]
- Dawczynski, J.; Jentsch, S.; Schweitzer, D.; Hammer, M.; Lang, G.E.; Strobel, J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: The LUTEGA study. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, R.; Boekhoorn, S.; Vingerling, J.R.; Witteman, J.C.M.; Klaver, C.C.W.; Hofman, A.; De Jong, P.T.V.M. Dietary Intake of Antioxidants and Risk of Age-Related Macular Degeneration. JAMA 2005, 294, 3101. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lin, X.-M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Heijer, T.D.; Launer, L.J.; De Groot, J.C.; De Leeuw, F.-E.; Oudkerk, M.; Van Gijn, J.; Hofman, A.; Breteler, M.M.B. Serum Carotenoids and Cerebral White Matter Lesions: The Rotterdam Scan Study. J. Am. Geriatr. Soc. 2001, 49, 642–646. [Google Scholar] [CrossRef]
- Stuerenburg, H.J.; Ganzer, S.; Müller-Thomsen, T. Plasma beta carotene in Alzheimer’s disease. Association with cerebrospinal fluid beta-amyloid 1-40, (Abeta40), beta-amyloid 1-42 (Abeta42) and total Tau. Neuro Endocrinol. Lett. 2005, 26, 696–698. [Google Scholar] [PubMed]
- Merle, B.M.J.; Maubaret, C.; Korobelnik, J.-F.; Delyfer, M.-N.; Rougier, M.-B.; Lambert, J.-C.; Amouyel, P.; Malet, F.; Le Goff, M.; Dartigues, J.-F.; et al. Association of HDL-Related Loci with Age-Related Macular Degeneration and Plasma Lutein and Zeaxanthin: The Alienor Study. PLoS ONE 2013, 8, e79848. [Google Scholar] [CrossRef]
- Clevidence, B.A.; Bieri, J.G. [4] Association of carotenoids with human plasma lipoproteins. Methods Enzymol. 1993, 214, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 Research Group. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.P.; Ferris, F.L.; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary Analyses of the Effects of Lutein/Zeaxanthin on Age-Related Macular Degeneration Progression. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Van Kappel, A.L.; Steghens, J.-P.; Zeleniuch-Jacquotte, A.; Chajès, V.; Toniolo, P.; Riboli, E. Serum carotenoids as biomarkers of fruit and vegetable consumption in the New York Women’s Health Study. Public Health Nutr. 2001, 4, 829–835. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Included (n = 609) | Excluded (n = 226) | p-Value |
|---|---|---|---|
| Mean ± SD or n (%) | Mean ± SD or n (%) | ||
| Age (years) | 79.7 ± 4.4 | 80.3 ± 4.2 | 0.07 |
| Sex | N = 609 | N = 226 | 0.23 |
| Men | 223 (36.6) | 93 (41.2) | |
| Women | 386 (63.4) | 133 (58.8) | |
| Marital status | N = 609 | N = 226 | 0.40 |
| Married | 369 (60.6) | 144 (63.7) | |
| Not married | 240 (39.4) | 82 (36.3) | |
| Smoking (pack-year) | N = 605 | N = 222 | 0.97 |
| Never smoker | 395 (65.3) | 143 (64.4) | |
| <20 | 106 (17.5) | 40 (18.0) | |
| ≥20 | 104 (17.2) | 39 (17.6) | |
| Physical activity | N = 609 | N = 226 | <0.0001 |
| None | 336 (55.1) | 102 (45.1) | |
| Medium | 124 (20.4) | 42 (18.6) | |
| High | 65 (10.7) | 19 (8.4) | |
| No answered | 84 (13.8) | 63 (27.9) | |
| Dietary intake | N = 593 | N = 201 | |
| Alcohol (g/d) | 12.7 ± 16.9 | 14.2 ± 6.3 | 0.29 |
| Carotenes (µg/d) | 3894 ± 5705 | 3383 ± 4650 | 0.44 |
| Lutein and zeaxanthin (µg/d) | 1022 ± 2852 | 658 ± 1263 | 0.20 |
| Total energy intake (kcal/d) | 1709 ± 546 | 1727 ± 500 | 0.91 |
| Mediterranean diet score | N = 573 | N = 197 | 0.35 |
| Low (0–3) | 183 (31.9) | 74 (37.6) | |
| Medium (4–5) | 251 (43.8) | 79 (40.1) | |
| High (6–9) | 139 (24.3) | 44 (22.3) | |
| AMD nutritional supplement during the study period | N = 609 | N = 226 | 0.002 |
| No | 536 (88.0) | 216 (95.6) | |
| Yes | 73 (12.0) | 10 (4.4) | |
| Body mass index (kg/m²) | N = 608 | N = 222 | 0.67 |
| <25 | 237 (39.0) | 79 (35.6) | |
| (25–30) | 282 (46.4) | 108 (48.6) | |
| ≥30 | 89 (14.6) | 35 (15.8) | |
| Diabetes | N = 604 | N = 172 | 0.34 |
| No | 558 (92.4) | 155 (90.1) | |
| Yes | 46 (7.6) | 17 (9.9) | |
| Plasma lipids (mmol/L) | N = 609 | N = 175 | |
| Total cholesterol | 5.81 ± 0.96 | 5.73 ± 0.98 | 0.48 |
| LDL | 3.67 ± 0.84 | 3.57 ± 0.84 | 0.43 |
| HDL | 1.59 ± 0.39 | 1.59 ± 0.37 | 0.94 |
| Triglycerides | 1.22 ± 0.58 | 1.23 ± 0.60 | 0.93 |
| Genetic risk score | N = 530 | N = 157 | 0.82 |
| 0.25 ± 1.23 | 0.28 ± 1.04 | ||
| AMD grade at baseline | N = 609 | N = 226 | 0.36 |
| No AMD | 415 (68.1) | 165 (73.0) | |
| Early AMD1 | 121 (19.9) | 40 (17.7) | |
| Early AMD2 | 73 (12.0) | 21 (9.3) | |
| Plasma carotenoids (mmol/L) | N = 609 | N = 138 | |
| Lutein | 0.30 ± 0.15 | 0.28 ± 0.15 | 0.15 |
| Zeaxanthin | 0.07 ± 0.04 | 0.07 ± 0.04 | 0.66 |
| Beta-cryptoxanthin | 0.31 ± 0.24 | 0.28 ± 0.22 | 0.22 |
| Alpha-carotene | 0.19 ± 0.15 | 0.19 ± 0.22 | 0.81 |
| Beta-carotene | 0.76 ± 0.55 | 0.75 ± 0.72 | 0.83 |
| Lycopene | 0.47 ± 0.32 | 0.46 ± 0.31 | 0.83 |
| Total carotenoids | 2.09 ± 1.02 | 2.05 ± 1.28 | 0.69 |
| Plasma Carotenoids | Non-Incident AMD | Incident AMD | Model 1 a | Model 2 b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n Cases/Total (Eye) | HR (95% CI) | p-Values | n Cases/Total (Eye) | HR (95% CI) | p-Values | |
| Lutein, (mmol/L) | 552 | 0.30 ± 0.15 | 53 | 0.29 ± 0.14 | 79/1089 | 0.71 (0.52, 0.98) | 0.037 | 66/869 | 0.63 (0.41, 0.97) | 0.03 |
| Zeaxanthin, (mmol/L) | 551 | 0.07 ± 0.04 | 53 | 0.07 ± 0.04 | 78/1087 | 0.69 (0.50, 0.95) | 0.02 | 65/867 | 0.82 (0.56, 1.21) | 0.33 |
| Lutein/(TC + TG) c | 552 | 0.04 ± 0.02 | 53 | 0.04 ± 0.02 | 79/1089 | 0.65 (0.47, 0.89) | 0.007 | 66/869 | 0.59 (0.39, 0.90) | 0.01 |
| Zeaxanthin/(TC + TG) c | 551 | 0.01 ± 0.006 | 53 | 0.01 ± 0.006 | 78/1087 | 0.69 (0.51, 0.94) | 0.02 | 65/867 | 0.80 (0.55, 1.17) | 0.25 |
| Plasma Carotenoids | Neovascular AMD | Atrophic AMD | ||||
|---|---|---|---|---|---|---|
| n Cases/Total (Eye) | HR a (95% CI) | p-Values | n Cases/Total (Eye) | HR a (95% CI) | p-Values | |
| Lutein, (mmol/L) | 34/869 | 0.64 (0.30, 1.34) | 0.23 | 38/869 | 0.64 (0.41, 1.01) | 0.05 |
| Zeaxanthin, (mmol/L) | 33/867 | 0.91 (0.54, 1.54) | 0.73 | 38/867 | 0.78 (0.45, 1.38) | 0.40 |
| Lutein/(TC + TG) b | 34/869 | 0.55 (0.25, 1.18) | 0.12 | 38/869 | 0.65 (0.41, 1.05) | 0.08 |
| Zeaxanthin/(TC + TG) b | 33/867 | 0.81 (0.49, 1.33) | 0.40 | 38/867 | 0.85 (0.51, 1.44) | 0.56 |
| Plasma Carotenoids (mmol/L) | Non-Incident AMD | Incident AMD | Model 1 a | Model 2 b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n Cases/Total (Eye) | HR (95% CI) | p-Values | n Cases/Total (Eye) | HR (95% CI) | p-Values | |
| Alpha-carotene | 543 | 0.18 ± 0.13 | 52 | 0.17 ± 0.14 | 80/1097 | 0.82 (0.53, 1.26) | 0.36 | 67/877 | 0.85 (0.55, 1.29) | 0.44 |
| Beta-carotene | 543 | 0.75 ± 0.51 | 54 | 0.70 ± 0.38 | 80/1097 | 0.83 (0.63, 1.09) | 0.18 | 67/877 | 0.92 (0.64, 1.31) | 0.63 |
| Lycopene | 548 | 0.46 ± 0.29 | 54 | 0.41 ± 0.23 | 80/1097 | 0.79 (0.60, 1.05) | 0.11 | 67/877 | 0.74 (0.51, 1.09) | 0.12 |
| Beta-cryptoxanthin | 539 | 0.30 ± 0.20 | 53 | 0.29 ± 0.19 | 80/1097 | 0.99 (0.81, 1.23) | 0.99 | 67/877 | 1.32 (1.00,1.72) | 0.05 |
| Total carotenoids | 542 | 2.08 ± 0.96 | 54 | 1.96 ± 0.81 | 80/1097 | 0.78 (0.58, 1.03) | 0.08 | 67/877 | 0.84 (0.55, 1.28) | 0.41 |
| Plasma carotenoids/(TC + TG) ratios c | ||||||||||
| Alpha-carotene | 543 | 0.03 ± 0.02 | 52 | 0.02 ± 0.02 | 80/1097 | 0.83 (0.53, 1.32) | 0.44 | 67/877 | 0.89 (0.60, 1.31) | 0.55 |
| Beta-carotene | 543 | 0.11 ± 0.08 | 54 | 0.10 ± 0.06 | 80/1097 | 0.84 (0.63, 1.11) | 0.21 | 67/877 | 0.94 (0.67, 1.33) | 0.74 |
| Lycopene | 548 | 0.07 ± 0.04 | 54 | 0.06 ± 0.03 | 80/1097 | 0.79 (0.59, 1,02) | 0.07 | 67/877 | 0.78 (0.54, 1.12) | 0.18 |
| Beta-cryptoxanthin | 539 | 0.04 ± 0.03 | 53 | 0.04 ± 0.03 | 80/1097 | 1.04 (0.82, 1.32) | 0.76 | 67/877 | 1.32 (1.00, 1.73) | 0.05 |
| Total carotenoids | 542 | 0.30 ± 0.14 | 54 | 0.28 ± 0.12 | 80/1097 | 0.78 (0.58, 1.04) | 0.09 | 67/877 | 0.88 (0.59, 1.31) | 0.52 |
| Plasma lipids (mmol/L) c | ||||||||||
| Total cholesterol | 555 | 5.80 ± 0.94 | 54 | 5.89 ± 1.11 | 80/1097 | 1.00 (0.78, 1.29) | 0.99 | 67/877 | 0.99 (0.71, 1.38) | 0.96 |
| Triglycerides | 555 | 1.22 ± 0.58 | 54 | 1.24 ± 0.53 | 80/1097 | 1.25 (1.00, 1.56) | 0.05 | 67/877 | 1.25 (0.89, 1.77) | 0.21 |
| Plasma Carotenoids | Advanced AMD | Neovascular AMD | Atrophic AMD | |||
|---|---|---|---|---|---|---|
| HR (95% CI) a | p-Value | HR (95% CI) a | p-Value | HR (95% CI) a | p-Value | |
| Lutein | 0.54 (0.36, 0.82) | 0.003 | 0.50 (0.29, 0.88) | 0.02 | 0.62 (0.39, 1.00) | 0.05 |
| Zeaxanthin | 0.77 (0.53, 1.14) | 0.19 | 0.87 (0.47, 1.61) | 0.66 | 0.71 (0.39, 1.27) | 0.24 |
| Alpha-carotene | 0.82 (0.53, 1.27) | 0.38 | 1.26 (0.87, 1.83) | 0.22 | 0.37 (0.07, 1.96) | 0.24 |
| Beta-carotene | 0.86 (0.59, 1.24) | 0.42 | 1.23 (0.74, 2.03) | 0.42 | 0.55 (0.21, 1.41) | 0.21 |
| Lycopene | 0.72 (0.49, 1.07) | 0.10 | 0.69 (0.41, 1.17) | 0.17 | 0.77 (0.42, 1.41) | 0.40 |
| Beta-cryptoxanthin | 1.25 (0.94, 1.68) | 0.13 | 1.84 (1.27, 2.69) | 0.002 | 0.90 (0.48, 1.67) | 0.73 |
| Total carotenoids | 0.76 (0.49, 1.16) | 0.20 | 1.24 (0.67, 2.31) | 0.50 | 0.48 (0.20, 1.16) | 0.10 |
| Plasma carotenoids/(TC + TG) ratio b | ||||||
| Lutein | 0.51 (0.33, 0.77) | 0.002 | 0.44 (0.24, 0.80) | 0.007 | 0.63 (0.39, 1.03) | 0.07 |
| Zeaxanthin | 0.75 (0.52, 1.09) | 0.13 | 0.77 (0.43, 1.39) | 0.39 | 0.78 (0.47, 1.31) | 0.35 |
| Alpha-carotene | 0.87 (0.58, 1.30) | 0.49 | 1.24 (0.88, 1.74) | 0.23 | 0.45 (0.08, 2.66) | 0.38 |
| Beta-carotene | 0.89 (0.62, 1.27) | 0.52 | 1.26 (0.80, 1.99) | 0.32 | 0.60 (0.26, 1.41) | 0.25 |
| Lycopene | 0.77 (0.53, 1.11) | 0.16 | 0.77 (0.45, 1.29) | 0.32 | 0.81 (0.47, 1.38) | 0.44 |
| Beta-cryptoxanthin | 1.27 (0.94, 1.70) | 0.11 | 1.69 (1.14, 2.49) | 0.009 | 1.05 (0.58, 1.91) | 0.86 |
| Total carotenoids | 0.80 (0.53, 1.20) | 0.28 | 1.23 (0.70, 2.14) | 0.47 | 0.57 (0.25, 1.28) | 0.17 |
| Plasma lipids b | ||||||
| Total cholesterol | 0.96 (0.69, 1.33) | 0.79 | 1.33 (0.71, 2.47) | 0.37 | 0.81 (0.56, 1.19) | 0.29 |
| Triglycerides | 1.26 (0.88, 1.80) | 0.21 | 1.39 (0.87, 2.23) | 0.17 | 1.09 (0.55, 2.16) | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merle, B.M.J.; Cougnard-Grégoire, A.; Korobelnik, J.-F.; Schalch, W.; Etheve, S.; Rougier, M.-B.; Féart, C.; Samieri, C.; Delyfer, M.-N.; Delcourt, C. Plasma Lutein, a Nutritional Biomarker for Development of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients 2021, 13, 2047. https://doi.org/10.3390/nu13062047
Merle BMJ, Cougnard-Grégoire A, Korobelnik J-F, Schalch W, Etheve S, Rougier M-B, Féart C, Samieri C, Delyfer M-N, Delcourt C. Plasma Lutein, a Nutritional Biomarker for Development of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients. 2021; 13(6):2047. https://doi.org/10.3390/nu13062047
Chicago/Turabian StyleMerle, Bénédicte M. J., Audrey Cougnard-Grégoire, Jean-François Korobelnik, Wolfgang Schalch, Stéphane Etheve, Marie-Bénédicte Rougier, Catherine Féart, Cécilia Samieri, Marie-Noëlle Delyfer, and Cécile Delcourt. 2021. "Plasma Lutein, a Nutritional Biomarker for Development of Advanced Age-Related Macular Degeneration: The Alienor Study" Nutrients 13, no. 6: 2047. https://doi.org/10.3390/nu13062047
APA StyleMerle, B. M. J., Cougnard-Grégoire, A., Korobelnik, J.-F., Schalch, W., Etheve, S., Rougier, M.-B., Féart, C., Samieri, C., Delyfer, M.-N., & Delcourt, C. (2021). Plasma Lutein, a Nutritional Biomarker for Development of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients, 13(6), 2047. https://doi.org/10.3390/nu13062047







