Lipoic Acid Exacerbates Oxidative Stress and Lipid Accumulation in the Liver of Wistar Rats Fed a Hypercaloric Choline-Deficient Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Protocol
2.2. Metabolic Measurements
2.3. Liver Lipids Content
2.4. Total Antioxidant Activity (AOA)
2.5. Glutathione Content
2.6. CYP2E1 Activity
2.7. Antioxidant Enzyme Activities
2.8. Histopathological Examination
2.9. Statistical Analysis
3. Results
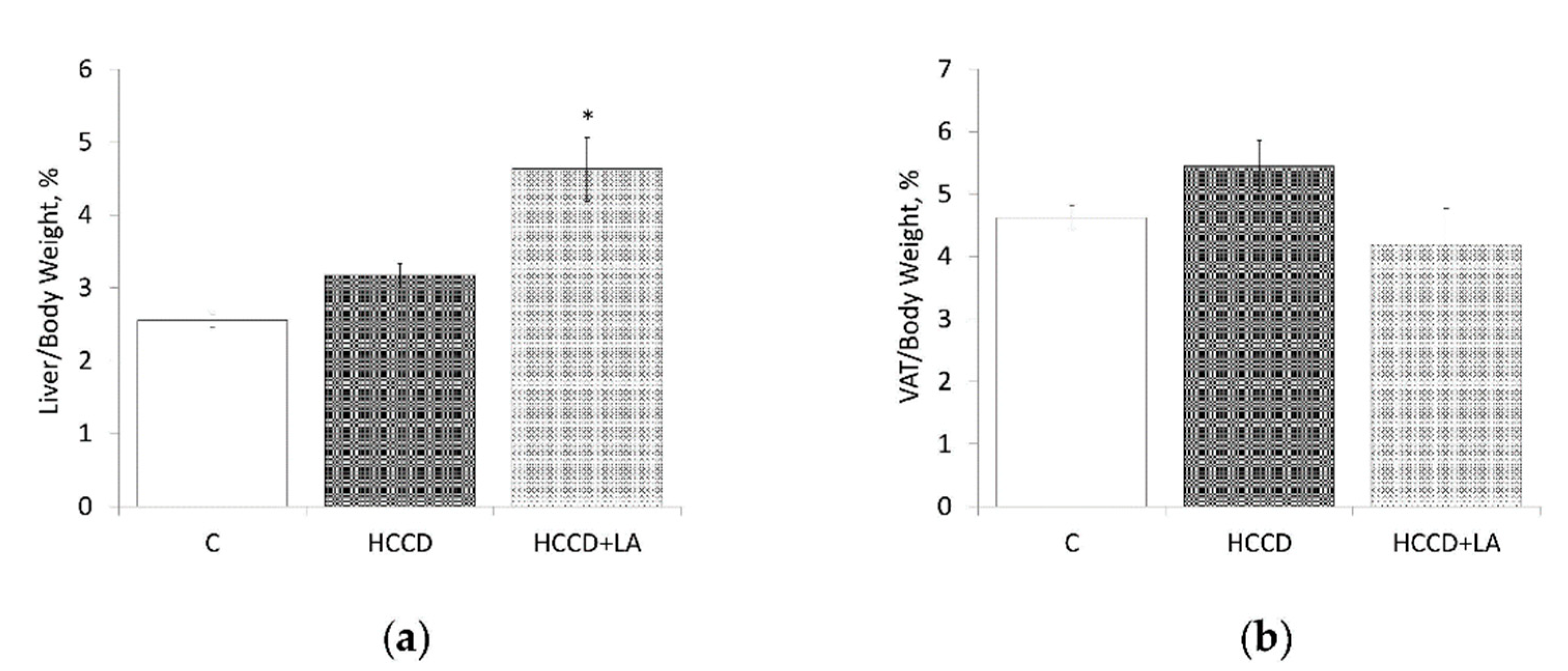
3.1. Weight Characteristics and Nutritional Profile
3.2. Liver Lipid Content
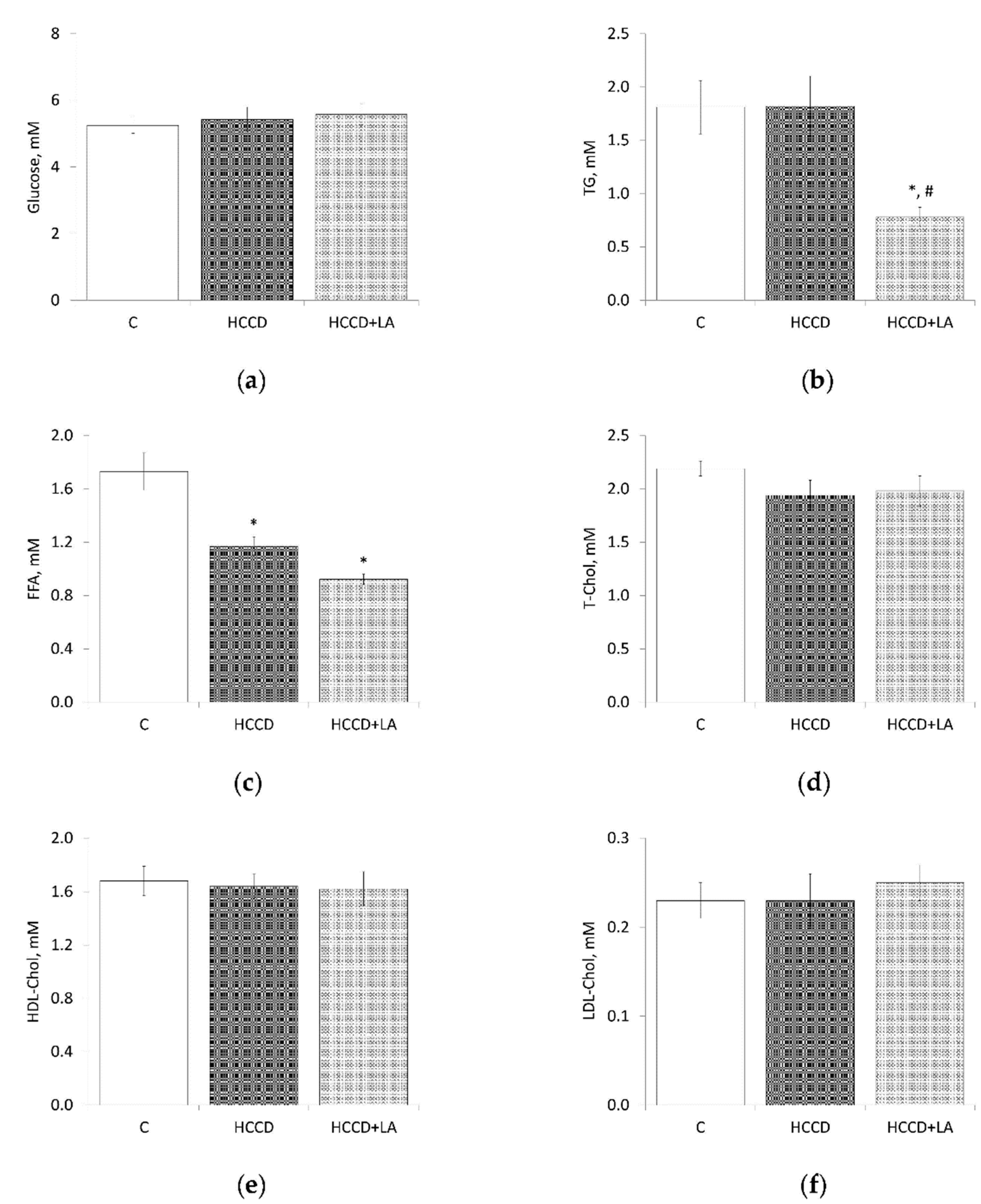
3.3. Metabolic Parameters
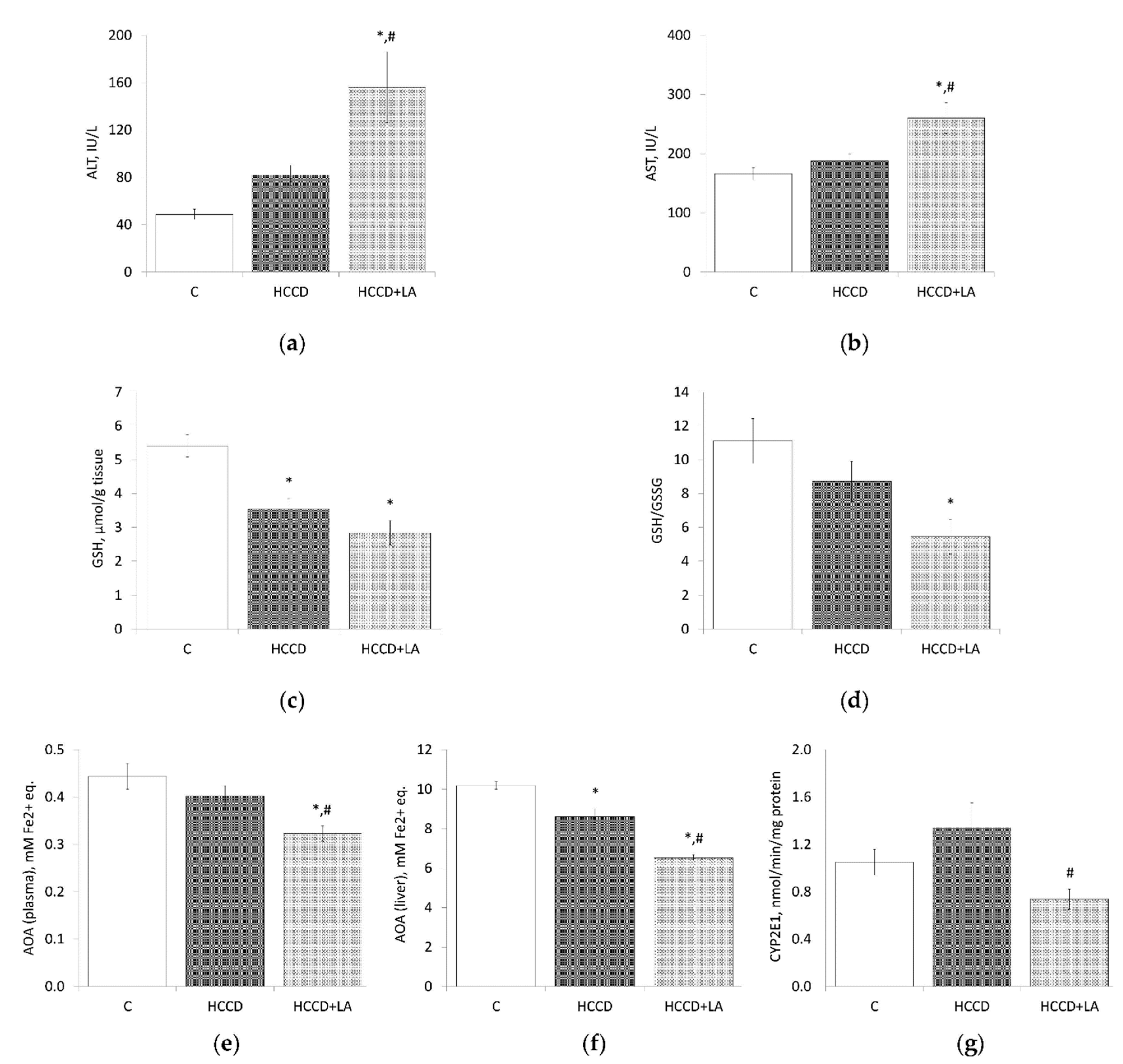
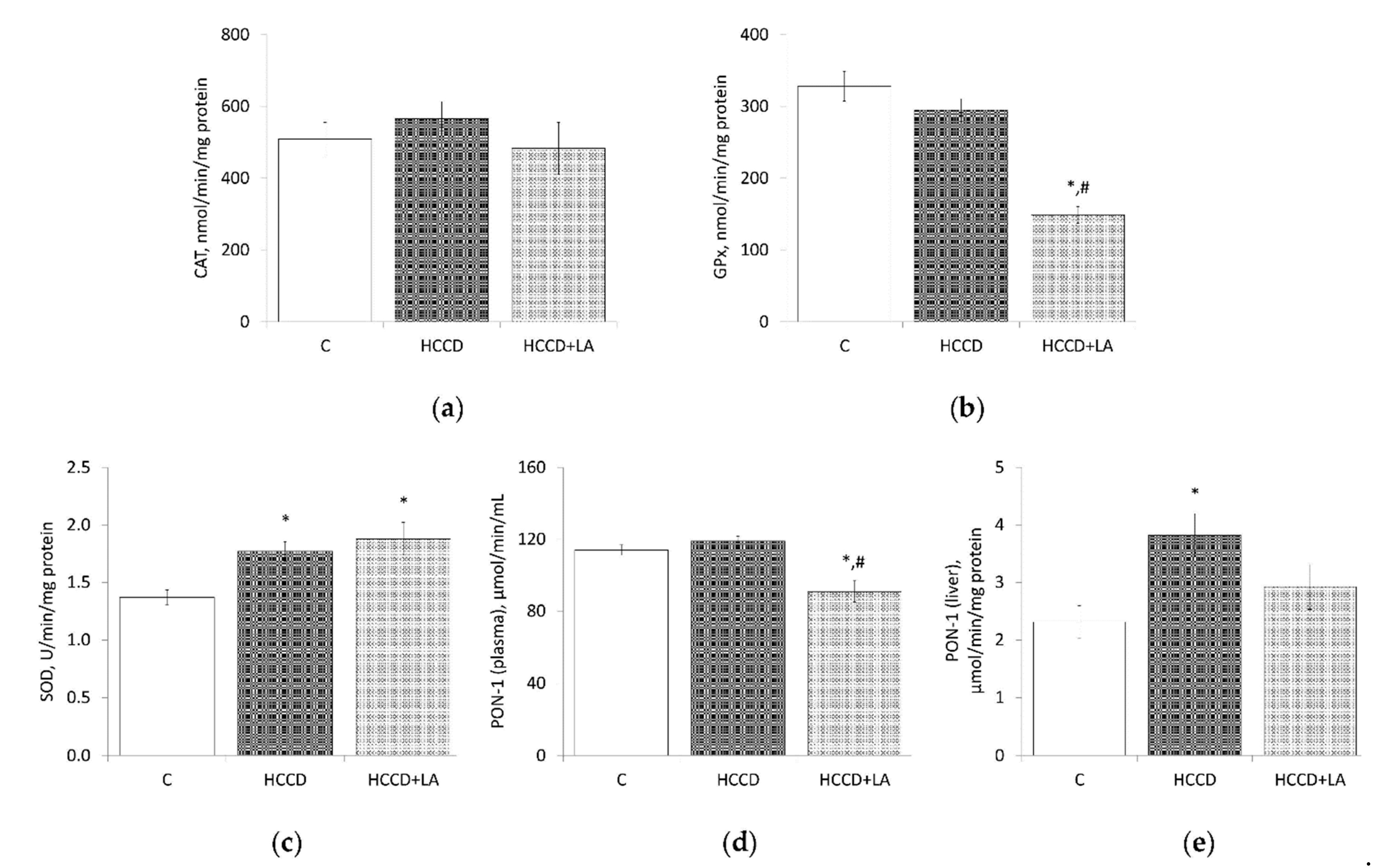
3.4. Oxidative Stress Markers
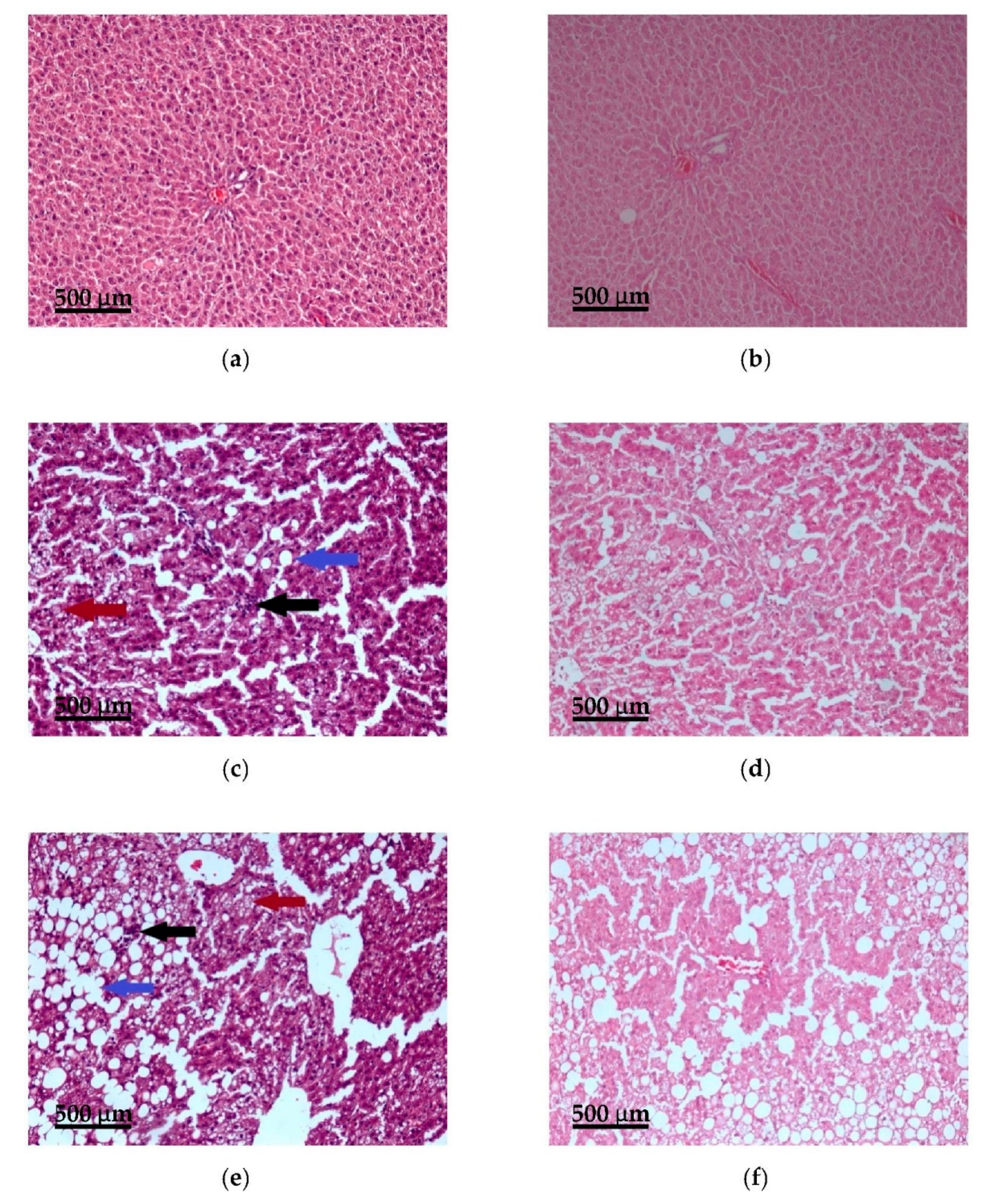
3.5. Histopathological Examination
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machado, M.; Marques-Vidal, P.; Cortez-Pinto, H. Liver histology in obese patients undergoing bariatric surgery. J. Hepatol. 2006, 454, 600–606. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Baker, S.S.; Baker, R.D.; Zhu, L. Antioxidant Mechanisms in Nonalcoholic Fatty Liver Disease. Curr. Drug Targets 2015, 16, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Jahn, D.; Kircher, S.; Hermanns, H.M.; Geier, A. Animal models of NAFLD from a hepatologist’s point of view. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Jarukamjorn, K.; Jearapong, N.; Pimson, C.; Chatuphonprasert, W. A High-Fat, High-Fructose Diet Induces Antioxidant Imbalance and Increases the Risk and Progression of Nonalcoholic Fatty Liver Disease in Mice. Scientifica 2016, 2016, 5029414. [Google Scholar] [CrossRef] [PubMed]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Hamedi-Shahraki, S.; Amirkhizi, F. Systemic redox imbalance in patients with nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2020, 50, e13211. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Mollica, M.P.; Donizzetti, I.; Gifuni, G.; Sica, R.; Pignalosa, A.; Cavaliere, G.; Gaita, M.; De Filippo, C.; Zorzano, A.; et al. High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat liver mitochondria. PLoS ONE 2014, 9, e92753. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Bae, J.H.; Im, S.S.; Hwang, I.; Ha, H.; Song, D.K. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflug. Arch. Eur. J. Physiol. 2019, 471, 829–843. [Google Scholar] [CrossRef]
- Robertson, G.; Leclercq, I.; Farrell, G.C. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1135–G1139. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Farrell, G.C.; Field, J.; Bell, D.R.; Gonzalez, F.J.; Robertson, G.R. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Investig. 2000, 105, 1067–1075. [Google Scholar] [CrossRef]
- Videla, L.A.; Rodrigo, R.; Orellana, M.; Fernandez, V.; Tapia, G.; Quiñones, L.; Varela, N.; Contreras, J.; Lazarte, R.; Csendes, A.; et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. 2004, 106, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, R.N.; Zhu, Y.R.; Xing, J.C.; Lou, X.W.; He, Y.J.; Ding, Q.L.; Zhang, M.Y.; Qiu, H. Involvement of xanthine oxidase and paraoxonase 1 in the process of oxidative stress in nonalcoholic fatty liver disease. Mol. Med. Rep. 2017, 15, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chang, R.; Zhang, T.; Zhou, X.; Wang, Q.; Gao, H.; Hou, L.; Loor, J.J.; Xu, C. Chinese Herbal Formula (CHF03) Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) Through Inhibiting Lipogenesis and Anti-Oxidation Mechanisms. Front. Pharmacol. 2019, 10, 1190. [Google Scholar] [CrossRef]
- Świderska, M.; Maciejczyk, M.; Zalewska, A.; Pogorzelska, J.; Flisiak, R.; Chabowski, A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic. Res. 2019, 53, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef]
- Sharma, R.S.; Harrison, D.J.; Kisielewski, D.; Cassidy, D.M.; McNeilly, A.D.; Gallagher, J.R.; Walsh, S.V.; Honda, T.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; et al. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2). Cell. Mol. Gastroenterol. Hepatol. 2017, 5, 367–398. [Google Scholar] [CrossRef] [PubMed]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 5–20. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Tibullo, D.; Li Volti, G.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. Off. J. Int. Assoc. Inflamm. Soc. Eur. Histamine Res. Soc. 2017, 66, 947–959. [Google Scholar] [CrossRef]
- Lake, B.G. Preparation and characterisation of microsomal fractions for studies on xenobiotic metabolism. In Biochemical Toxicology—A Practical Approach; Snell, K., Mullock, B., Eds.; IRL Press: Oxford, UK, 1987; pp. 183–215. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [CrossRef]
- Carlson, G.P. Influence of ethanol on microsomal p-nitrophenol hydroxylation and ethoxyresorufin deethylation in rat liver and lung. J. Toxicol. Environ. Health 1991, 32, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J.; Jamroz-Wiśniewska, A.; Borkowska, E.; Wójcicka, G. Differential effect of antioxidant treatment on plasma and tissue paraoxonase activity in hyperleptinemic rats. Pharmacol. Res. 2005, 51, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchill Livingstone: London, UK, 2002; 796p. [Google Scholar]
- Bedossa, P.; Poitou, C.; Veyrie, N.; Bouillot, J.L.; Basdevant, A.; Paradis, V.; Tordjman, J.; Clement, K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012, 56, 1751–1759. [Google Scholar] [CrossRef]
- Buettner, R.; Parhofer, K.G.; Woenckhaus, M.; Wrede, C.E.; Kunz-Schughart, L.A.; Schölmerich, J.; Bollheimer, L.C. Defining high-fat-diet rat models: Metabolic and molecular effects of different fat types. J. Mol. Endocrinol. 2006, 36, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Muniz, L.B.; Alves-Santos, A.M.; Camargo, F.; Martins, D.B.; Celes, M.; Naves, M. High-Lard and High-Cholesterol Diet, but not High-Lard Diet, Leads to Metabolic Disorders in a Modified Dyslipidemia Model. Arq. Bras. Cardiol. 2019, 113, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.M.; Estevam, W.M.; Coelho, P.M.; Haese, D.; Kobi, J.; Lima-Leopoldo, A.P.; Leopoldo, A.S. Differential Effects of High Sugar, High Lard or a Combination of Both on Nutritional, Hormonal and Cardiovascular Metabolic Profiles of Rodents. Nutrients 2018, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.Y.; Ha, A.W.; Kim, W.K. α-Lipoic acid reduced weight gain and improved the lipid profile in rats fed high fat diet. Nutr. Res. Pract. 2012, 6, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Valdecantos, M.P.; Pérez-Matute, P.; González-Muniesa, P.; Prieto-Hontoria, P.L.; Moreno-Aliaga, M.J.; Martínez, J.A. Lipoic acid improves mitochondrial function in nonalcoholic steatosis through the stimulation of sirtuin 1 and sirtuin 3. Obesisty 2012, 20, 1974–1983. [Google Scholar] [CrossRef]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Machado, M.V.; Michelotti, G.A.; Xie, G.; Almeida Pereira, T.; Boursier, J.; Bohnic, B.; Guy, C.D.; Diehl, A.M. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS ONE 2015, 10, e0127991. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Song, D.K. Catalase and nonalcoholic fatty liver disease. Pflug. Arch. Eur. J. Physiol. 2018, 470, 1721–1737. [Google Scholar] [CrossRef]
- Camps, J.; Marsillach, J.; Joven, J. Measurement of serum paraoxonase-1 activity in the evaluation of liver function. World J. Gastroenterol. 2009, 15, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T. Effect of dietary lipids on paraoxonase-1 activity and gene expression. Nutr. Metab. Cardiovasc. Dis. NMCD 2012, 22, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Ruiz, N.; Murillo-González, F.E.; Rojas-García, A.E.; Mackness, M.; Bernal-Hernández, Y.Y.; Barrón-Vivanco, B.S.; González-Arias, C.A.; Medina-Díaz, I.M. Transcriptional regulation of human Paraoxonase 1 by nuclear receptors. Chem. Biol. Interact. 2017, 268, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ferré, N.; Camps, J.; Prats, E.; Vilella, E.; Paul, A.; Figuera, L.; Joven, J. Serum paraoxonase activity: A new additional test for the improved evaluation of chronic liver damage. Clin. Chem. 2002, 48, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.R.; de Abreu, I.C.; Guerra, J.F.; Lage, N.N.; Lopes, J.M.; Silva, M.; de Lima, W.G.; Silva, M.E.; Pedrosa, M.L. Açai (Euterpe oleracea Mart.) Upregulates Paraoxonase 1 Gene Expression and Activity with Concomitant Reduction of Liver Steatosis in High-Fat Diet-Fed Rats. Oxidative Med. Cell. Longev. 2016, 2016, 8379105. [Google Scholar] [CrossRef]
- Perlemuter, G.; Davit-Spraul, A.; Cosson, C.; Conti, M.; Bigorgne, A.; Paradis, V.; Corre, M.P.; Prat, L.; Kuoch, V.; Basdevant, A.; et al. Increase in liver antioxidant enzyme activities in non-alcoholic fatty liver disease. Liver Int. Off. J. Int. Assoc. Study Liver 2005, 25, 946–953. [Google Scholar] [CrossRef]
- Min, A.K.; Kim, M.K.; Kim, H.S.; Seo, H.Y.; Lee, K.U.; Kim, J.G.; Park, K.G.; Lee, I.K. Alpha-lipoic acid attenuates methionine choline deficient diet-induced steatohepatitis in C57BL/6 mice. Life Sci. 2012, 90, 200–205. [Google Scholar] [CrossRef]
- Stanković, M.N.; Mladenović, D.; Ninković, M.; Ethuričić, I.; Sobajić, S.; Jorgačević, B.; de Luka, S.; Vukicevic, R.J.; Radosavljević, T.S. The effects of α-lipoic acid on liver oxidative stress and free fatty acid composition in methionine-choline deficient diet-induced NAFLD. J. Med. Food 2014, 17, 254–261. [Google Scholar] [CrossRef]
- Kucukgoncu, S.; Zhou, E.; Lucas, K.B.; Tek, C. Alpha-lipoic acid (ALA) as a supplementation for weight loss: Results from a meta-analysis of randomized controlled trials. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Vajdi, M.; Abbasalizad Farhangi, M. Alpha-lipoic acid supplementation significantly reduces the risk of obesity in an updated systematic review and dose response meta-analysis of randomised placebo-controlled clinical trials. Int. J. Clin. Pract. 2020, 74, e13493. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, J.Y.; Namkoong, C.; Jang, P.G.; Ryu, J.W.; Song, H.S.; Yun, J.Y.; Namgoong, I.S.; Ha, J.; Park, I.S.; et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 2004, 10, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, K.U. Role of hypothalamic 5′-AMP-activated protein kinase in the regulation of food intake and energy homeostasis. J. Mol. Med. 2005, 83, 514–520. [Google Scholar] [CrossRef] [PubMed]
- El Midaoui, A.; Fantus, I.G.; Ait Boughrous, A.; Couture, R. Beneficial Effects of Alpha-Lipoic Acid on Hypertension, Visceral Obesity, UCP-1 Expression and Oxidative Stress in Zucker Diabetic Fatty Rats. Antioxidants 2019, 8, 648. [Google Scholar] [CrossRef] [PubMed]
- Pashaj, A.; Xia, M.; Moreau, R. α-Lipoic acid as a triglyceride-lowering nutraceutical. Can. J. Physiol. Pharmacol. 2015, 93, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Al-Gayyar, M.M.; Shams, M.E.; Barakat, E.A. Fish oil improves lipid metabolism and ameliorates inflammation in patients with metabolic syndrome: Impact of nonalcoholic fatty liver disease. Pharm. Biol. 2012, 50, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, A.; Derbenev, M.; Shih, H.Y.; Vollmar, B. Prophylactic and abundant intake of α-lipoic acid causes liver steatosis and should be reconsidered in usage as an anti-aging drug. Biofactors 2016, 42, 179–189. [Google Scholar] [CrossRef]
- Cakatay, U.; Kayali, R. Plasma protein oxidation in aging rats after alpha-lipoic acid administration. Biogerontology 2005, 6, 87–93. [Google Scholar] [CrossRef]
- Cakatay, U. Pro-oxidant actions of alpha-lipoic acid and dihydrolipoic acid. Med. Hypotheses 2006, 66, 110–117. [Google Scholar] [CrossRef]
| Ingredient(g/kg Diet) | Diets | |
|---|---|---|
| C | HCCD | |
| Casein (>85% protein) | 140 | 140 |
| Cornstarch | 721.2 | 271.2 |
| Fructose | - | 246 |
| Sunflower Oil | 20 | 20 |
| Lard | 20 | 226 |
| Fiber (Cellulose Powder) | 50 | 50 |
| Mineral Mix (AIN-93M-MX) [22] | 35 | 35 |
| Vitamin Mix (AIN-93-VX) [22] | 10 | 10 |
| Choline Chloride (52% choline) | 2.0 | - |
| L-Cysteine | 1.8 | 1.8 |
| Energy Density (kcal/g) | 3.89 | 4.92 |
| Parameter | C | HCCD | HCCD+LA |
|---|---|---|---|
| Initial Body Weight, g | 215 ± 1 | 207 ± 5 | 204 ± 6 |
| Final Body Weight, g | 364 ± 5 | 414 ± 13 * | 404 ± 20 |
| Body Weight Gain, g | 149 ± 5 | 207 ± 14 * | 199 ± 19 * |
| Total Food Consumed, g | 870 ± 21 | 881 ± 27 | 882 ± 10 |
| Food Intake, g/day | 16.0 ± 0.4 | 16.3 ± 0.5 | 16.0 ± 0 |
| Calorie Intake, kcal/day | 62.3 ± 1.8 | 79.5 ± 2.6 * | 79.3 ± 1.1 * |
| Feed Efficiency Ratio | 0.172 ± 0.006 | 0.235 ± 0.017 | 0.226 ± 0.028 |
| Parameter (mg/g Liver Tissue) | C | HCCD | HCCD+LA |
|---|---|---|---|
| Total Lipids | 70 ± 3 | 237 ± 21 * | 343 ± 21 *,# |
| TG | 17 ± 2 | 140 ± 18 * | 215 ± 19 *,# |
| T-Chol | 3.3 ± 0.4 | 9.6 ± 1.1 * | 12.5 ± 1.1 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravchenko, L.V.; Aksenov, I.V.; Nikitin, N.S.; Guseva, G.V.; Avrenyeva, L.I.; Trusov, N.V.; Balakina, A.S.; Tutelyan, V.A. Lipoic Acid Exacerbates Oxidative Stress and Lipid Accumulation in the Liver of Wistar Rats Fed a Hypercaloric Choline-Deficient Diet. Nutrients 2021, 13, 1999. https://doi.org/10.3390/nu13061999
Kravchenko LV, Aksenov IV, Nikitin NS, Guseva GV, Avrenyeva LI, Trusov NV, Balakina AS, Tutelyan VA. Lipoic Acid Exacerbates Oxidative Stress and Lipid Accumulation in the Liver of Wistar Rats Fed a Hypercaloric Choline-Deficient Diet. Nutrients. 2021; 13(6):1999. https://doi.org/10.3390/nu13061999
Chicago/Turabian StyleKravchenko, Lidia V., Ilya V. Aksenov, Nikolay S. Nikitin, Galina V. Guseva, Ludmila I. Avrenyeva, Nikita V. Trusov, Anastasia S. Balakina, and Victor A. Tutelyan. 2021. "Lipoic Acid Exacerbates Oxidative Stress and Lipid Accumulation in the Liver of Wistar Rats Fed a Hypercaloric Choline-Deficient Diet" Nutrients 13, no. 6: 1999. https://doi.org/10.3390/nu13061999
APA StyleKravchenko, L. V., Aksenov, I. V., Nikitin, N. S., Guseva, G. V., Avrenyeva, L. I., Trusov, N. V., Balakina, A. S., & Tutelyan, V. A. (2021). Lipoic Acid Exacerbates Oxidative Stress and Lipid Accumulation in the Liver of Wistar Rats Fed a Hypercaloric Choline-Deficient Diet. Nutrients, 13(6), 1999. https://doi.org/10.3390/nu13061999







