Influence of Algae Supplementation on the Concentration of Glutathione and the Activity of Glutathione Enzymes in the Mice Liver and Kidney
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Pinnularia borealis
2.2. Animal Test Subjects and Tissue Processing
2.2.1. Animal Groups and Treatments
2.2.2. Tissue Sample Collection
2.2.3. Analytical Methods
2.3. Reduced Glutathione (GSH)
2.4. Glutathione Peroxidase (GPx)
2.5. Glutathione Transferase (GST)
2.6. Glutathione Reductase (GR)
2.7. Cholesterol Content
2.8. Triacylglycerols Content
2.9. Malondialdehyde (MDA) Concentration
2.10. Protein Content
2.11. Statistical Analysis
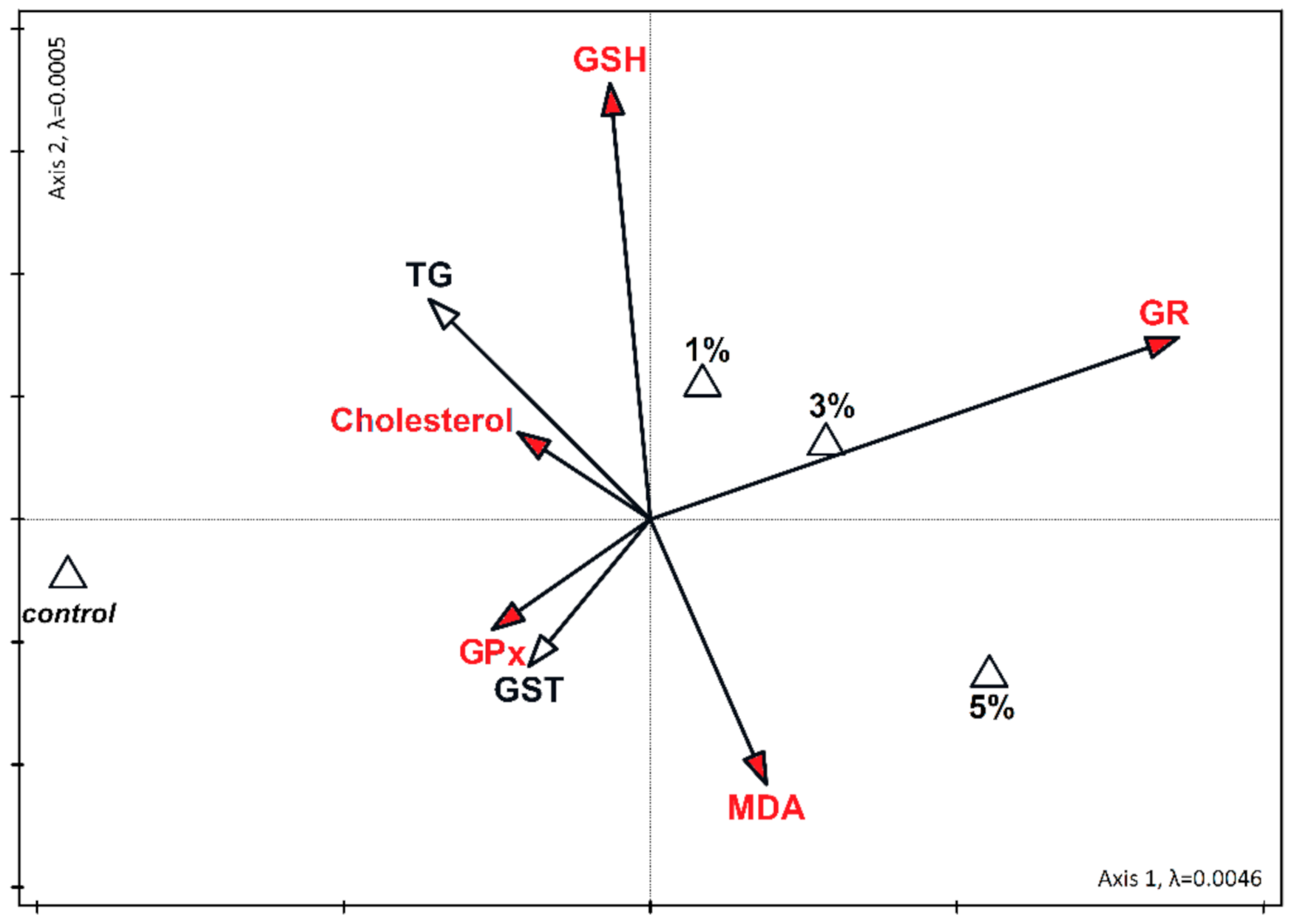
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015; pp. 1–905. [Google Scholar]
- Von Schacky, C. Omega-3 fatty acids: Antiarrhythmic, proarrhythmic or both? Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic potential of ω-3 polyunsaturated fatty acids in human autoimmune diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Wootton, S.A. Conversion of a-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Poja, S. Algae used as medical and food-a short review. J. Pharm. Sci. Res. 2011, 6, 33–35. [Google Scholar]
- Richardson, J.S. Limits to productivity in streams: Evidence from studies on macroinvertebrates. In Production of Juvenile Atlantic Salmon, Salmo salar, in Natural Waters; Gibson, R.J., Cutting, R.E., Eds.; Canadian Special Publications in Fisheries Aquatic Science; National Research Council of Canada and Department of Fisheries and Oceans: Ottawa, ON, Canada, 1993; Volume 118, pp. 9–15. [Google Scholar]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and method for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, A. Biological importance of marine algae. J. Pharm. Sci. 2010, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Xu, M.; Magnusdottir, M.; Brynjolfsson, S.; Fu, W. Photo-oxidative stress-driven mutagenesisi and adaptive evolution on the marine diatom Phaeodactylum tricornutum for enhanced carotenoid accumulation. Mar. Drugs 2015, 13, 6138–6151. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Sardo, A.; Paris, D.; Vella, F.M.; Adelfi, M.G.; Botte, P.; Gallo, C.; Fontana, A. Potential of lipid metabolism in marine diatoms for biofuel production. Biotechnol. Biofuels 2015, 8, 28. [Google Scholar] [CrossRef]
- Aditya, T.; Bitu, G.; Mercy, E. The role of algae in pharmaceutical development. J. Pharm. Pharm. 2016, 4, 82–89. [Google Scholar]
- Fu, W.; Wichuk, K.; Brynjolfsson, S. Developing diatoms for value-added products: Challenges and opportunities. N. Biotechnol. 2015, 32, 547–551. [Google Scholar] [CrossRef]
- Yi, Z.; Xu, M.; Di, X.; Brynjolfsson, S.; Fu, W. Exploring valuable lipids in diatoms. Front. Mar. Sci. 2017, 4, 17. [Google Scholar] [CrossRef]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 124. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.U.; Riaz, H.; Valavoor, S.; Zhao, D.; Vaughan, L.; Okunrintemi, V.; Riaz, I.B.; Khan, M.S.; Kaluski, E. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: An umrella review and evidence map. Ann. Int. Med. 2019, 171, 190–198. [Google Scholar] [CrossRef]
- Stock, W.; Blommaert, L.; De Troch, M.; Mangelinckx, S.; Willems, A.; Vyverman, W.; Sabbe, K. Host specificity in diatom—bacteria interactions alleviates antagonistic effects. FEMS Microbiol. Ecol. 2019, 95, fiz171. [Google Scholar] [CrossRef]
- Czerwik-Marcinkowska, J.; Mrozińska, T. Algae and cyanobacteria in caves of the Polish Jura. Pol. Bot. J. 2011, 56, 203–243. [Google Scholar]
- Peng, J.; Wu, C.; Wang, J.H.; Yuan, J.P. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 806–828. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Soundharrajan, I.; Srigopalram, S.; Yusoff, M.M.; Maniam, G.P.; Govindan, N.; Choi, K.C. Potential pharmaceutical and biomedical applications of diatoms microalgae—An overview. Ind. J. Geo Mar. Sci. 2017, 46, 663–667. [Google Scholar]
- Dobashi, K.; Ghosh, B.; Orak, J.K.; Singh, I.; Singh, A.K. Kidney ischemia-reperfusion: Modulation of antioxidant defens. Mol. Cell. Biochem. 2000, 205, 1–11. [Google Scholar] [CrossRef]
- Kouakou, C.R.C.; Poder, T.G. Economic impact of harmful algal blooms on human health: A systematic review. J. Water Health 2019, 17, 499–516. [Google Scholar] [CrossRef]
- Lenton, K.J.; Therriault, H.; Cantin, A.M.; Fülöp, T.; Payette, H.; Wagner, J.R. Direct correlation of glutathione and ascorbate and their dependence on age and season in human lymphocytes. Am. J. Clin. Nutr. 2000, 71, 1194–1200. [Google Scholar] [CrossRef]
- Thirupathi, A.; Pinho, R.A. Effects of reactive oxygen species and interplay of antioxidants during physical exercise in skeletal muscles. J. Physiol. Biochem. 2018, 74, 359–367. [Google Scholar] [CrossRef]
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 14, 5967–6026. [Google Scholar] [CrossRef]
- Hayes, S.C.; Luoma, J.B.; Bond, F.W.; Masuda, A.; Lillis, J. Acceptance and commitment therapy: Model, processes and outcomes. Behav. Res. Ther. 2006, 44, 1–25. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 2003, 20, 7369–7375. [Google Scholar] [CrossRef]
- Kim, S.Y.; Dietz, P.M.; England, L.; Morrow, B.; Callaghan, W.M. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obes. Res. J. 2007, 15, 986–993. [Google Scholar] [CrossRef]
- Czerwik-Marcinkowska, J.; Zwijacz-Kozica, T.; Pusz, W.; Wojciechowska, A. The relationship between presence of brown bear (Ursus arctos) and diversity of airbone algae and cyanobacteria in the Głowoniowa Nyża Cave, Tatra Mountains, Poland. J. Cave Karst Stud. 2018, 81, 57–67. [Google Scholar] [CrossRef]
- Massalski, A.; Mrozińska, T.; Olech, M. Lobococcus irregularis (Boye-Pet.) Reisigl var. antarcicus var.nov. (Chlorellales, Chlorophyta) from King George Island, South Shetland Islands, Antarctica and its ultrastructure. Nova Hedwig. 1995, 61, 199–206. [Google Scholar]
- Rindi, F.; Allali, H.A.; Lam, W.; Lopez-Bautista, J.M. An overview of the biodiversity and biogeography of terrestrial green algae. In Biodiversity Hotspots; Rescigno, V., Maletta, S., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2009; pp. 1–250. [Google Scholar]
- Guzmán, J.F.; Esteve, H.; Pablos, C.; Pablos, A.; Blasco, C.; Villegas, J. DHA-rich fish oil improves complex reaction time in female elite soccer players. J. Sports Sci. Med. 2011, 10, 301–305. [Google Scholar]
- Mateos, R.; Lecumberri, E.; Ramos, S.; Goya, L.; Bravo, L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress: Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J. Chrom. B 2005, 1, 76–82. [Google Scholar]
- Deplancke, B.; Gaskins, H.R. Redox control of the transsulfuration and glutathione biosynthesis pathways. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Searcy, R. Diagnostic Biochemistry; McGraw-Hill: New York, NY, USA, 1974; pp. 1–15. [Google Scholar]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- McHugh, M.L. Multiple comparison analysis testing in ANOVA. Comp. Stud. 2011, 21, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Peltomaa. E.T.; Aalto, S.L.; Vuorio, K.M.; Taipale, S.J. The importance of phytoplankton biomolecule availability for secondary production. Front. Ecol. Evol. 2017, 5, 128. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Goiris, K.; Muylaert, K.; Foubert, I. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem. 2014, 1, 393–400. [Google Scholar] [CrossRef]
- Chen, G.Q.; Jiang, Y.; Chen, F. Variation of lipid class composition in Nitzschia laevis as a response to growth temperature change. Food Chem. 2008, 109, 88–94. [Google Scholar] [CrossRef]
- Sano, T.; Kumamoto, Y.; Okuda, M.; Tanaka, Y. Effect of lipophilic extract of Chlorella vulgaris on alimentary hyperlipidemia in cholesterol-fed rats. Artery 1988, 15, 217–224. [Google Scholar]
- Fallah, A.A.; Sarmast, E.; Dehkordi, S.D.; Engardeh, J.; Mahmoodnia, L.; Khaledifar, A.; Jafari, T. Effect of Chlorella supplementation on cardiovascular risk factors: A meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 1892–1901. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Shih, M.F. Preventing dyslipidemia by Chlorella pyrenoidosa in rats and hamsters after chronic high fat diet treatment. Life Sci. 2005, 76, 3001–3013. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Douziech, A.; Levesque, I.; Varin, A.; Herbein, G. Cytokine receptor signalling and aging. Mech. Ageing Dev. 2006, 27, 526–537. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Chu, C.H.; Chang, W.H.; Hsu, T.C.; Lin, S.; Liu, C.; Yang, A.; Shih, S.C. Clinical analysis of multiple primary malignancies in the digestive system: A hospital-based study. World J. Gastroenterol. 2005, 11, 4215–4219. [Google Scholar] [CrossRef]
- Samane, S.; Christon, R.; Dombrowski, L.; Turcotte, S.; Charrouf, Z.; Lavigne, C.; Levy, E.; Bachelard, H.; Amarouch, H.; Marette, A.; et al. Fish oil and argan oil intake differently modulate insulin resistance and glucose intolerance in a rat model of dietary-induced obesity. Metabolism 2009, 58, 909–919. [Google Scholar] [CrossRef]
- Kajikawa, M.; Sasaki, K.; Wakimoto, Y.; Toyooka, M.; Motohashi, T.; Shimojima, T.; Takeda, S.; Park, E.Y.; Maenaka, K. Efficient silkworm expression of human GPCR (nociceptin receptor) by a Bombyx mori bacmid DNA system. Biochem. Biophys. Res. Commun. 2009, 31, 375–379. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, C.Y.; Cho, C.; Song, Y. Attenuating Effect of Chlorella Supplementation on Oxidative Stress and NF.KAPPA.B Activation in Peritoneal Macrophages and Liver of C57BL/6 Mice Fed on an Atherogenic Diet. Biosci. Biotechnol. Biochem. 2003, 67, 2083–2090. [Google Scholar] [CrossRef]
- Martins, J.G. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: Evidence from a meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 2009, 28, 525–542. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Valli, V.; Bordoni, A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HEPG2 cells. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 121–127. [Google Scholar] [CrossRef]
- Bond, L.M.; Miyazaki, M.; O’Neill, L.M.; Ding, F.; Ntambi, J.M. Fatty Acid Desaturation and Elongation in Mammals. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Ridgway, N.D., McLeod, R.S., Eds.; Elsevier: New York, NY, USA, 2016; pp. 185–208. [Google Scholar]
- Di Nunzio, M.; Valli, V.; Bordoni, A. PUFA and oxidative stress. Differential modulation of cell response by DHA. Int. J. Food Sci. Nutr. 2016, 67, 834–843. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Chapter 4—Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry: Recent Progress in Biotechnology; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar]
- Gaynor, P. Claim that the use of Mycell’s Emulsified Algal Oil in Select Food Categories is Generally Recognized as Safe (GRAS); Myell Technologies LLC: Paramus, NJ, USA, 2016; pp. 1–128. [Google Scholar]
- Bernstein, A.M.; Ding, E.L.; Willett, W.C.; Rimm, E.B. A meta-analysis shows that docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in persons without coronary heart disease. J. Nutr. 2012, 142, 99–104. [Google Scholar] [CrossRef]
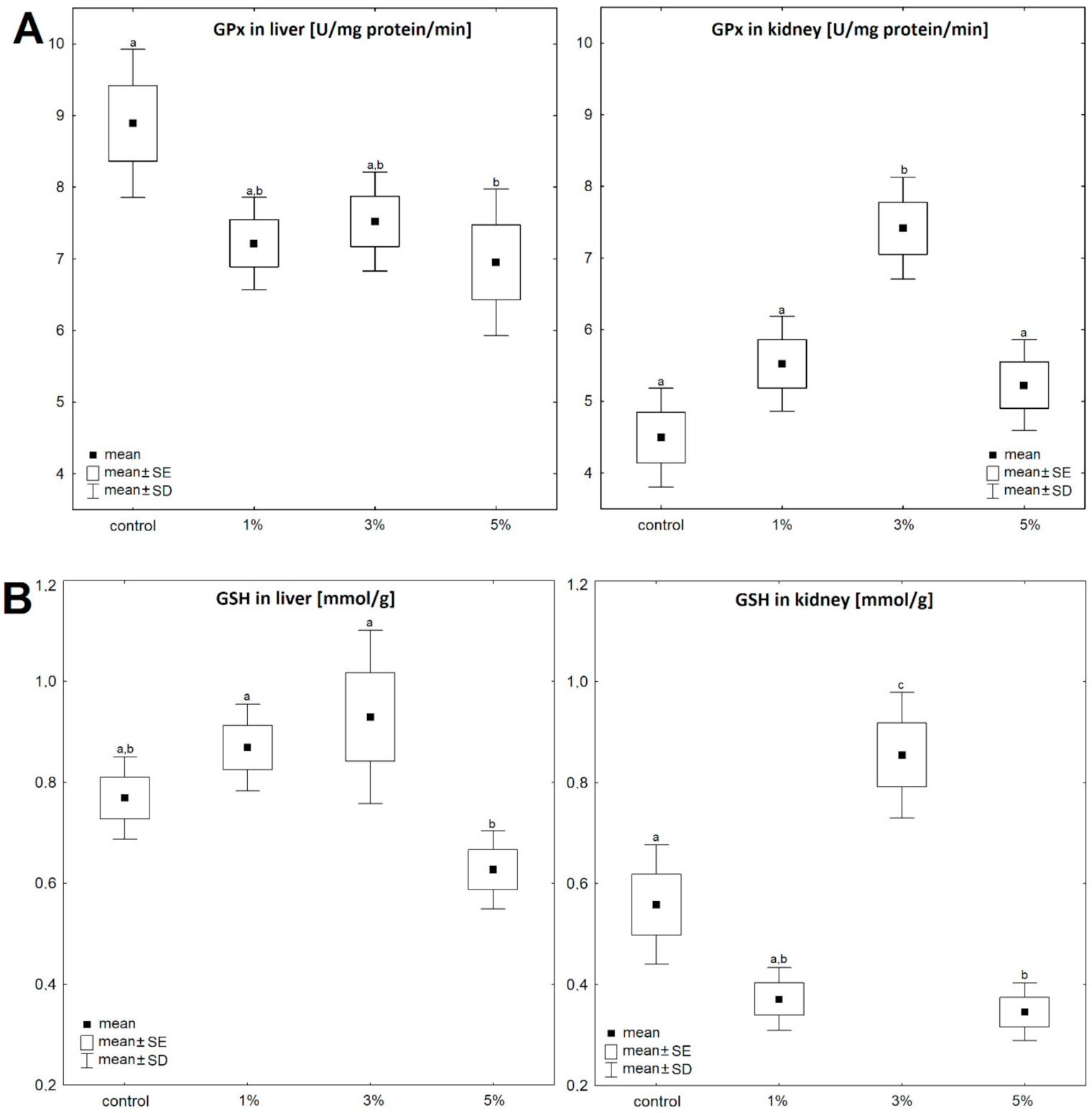
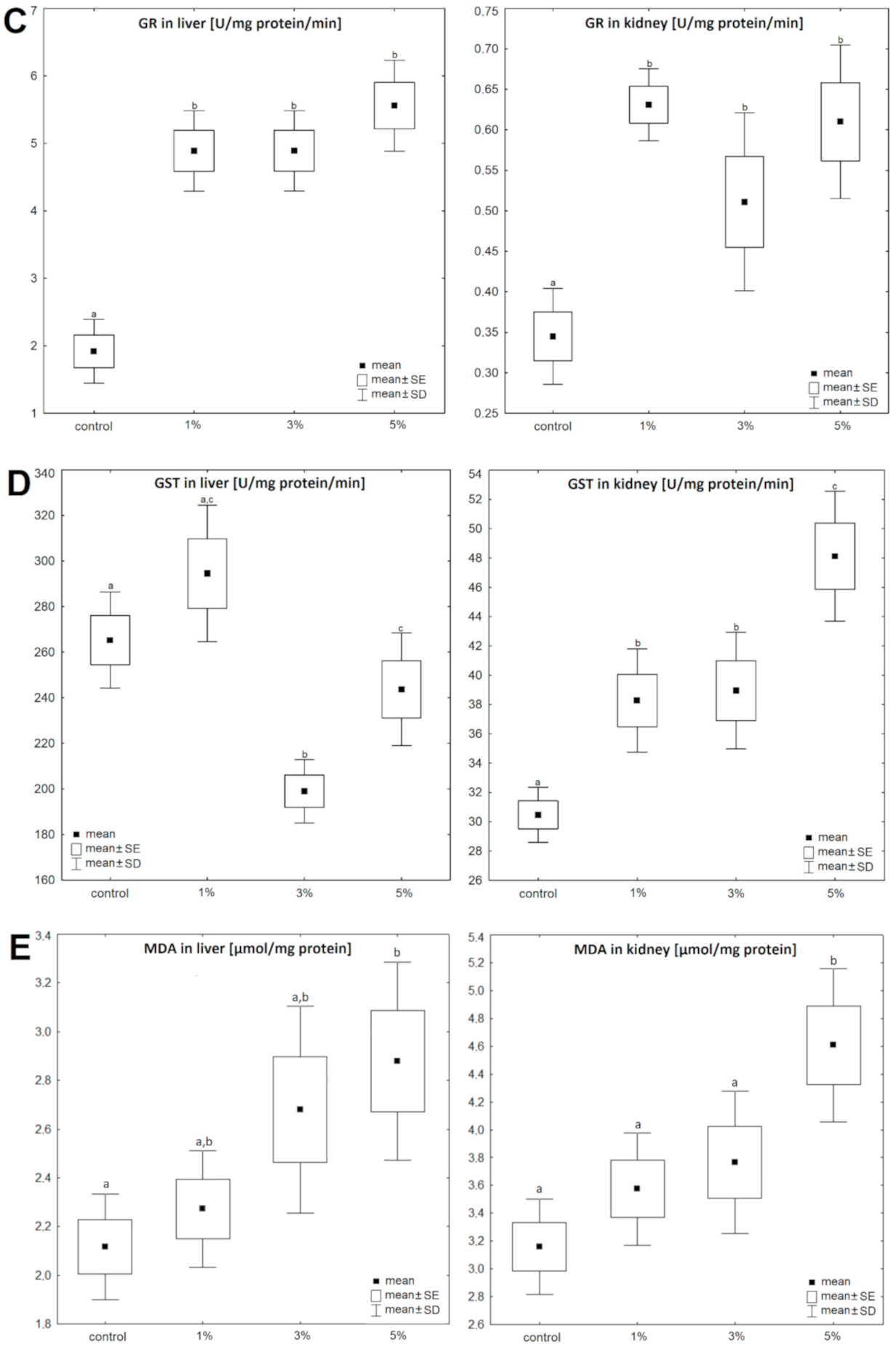


| GSH | GPx | GR | GST | MDA | Triacylglycerols | Cholesterol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x ± SD | % | x ± SD | % | x ± SD | % | x ± SD | % | x ± SD | % | x ± SD | % | x ± SD | % | ||
| liver | Control | 0.77 ± 0.17 | 100 | 8.89 ± 2.06 | 100 | 1.92 ± 0.28 | 100 | 265.9 ± 49.9 | 100 | 2.12 ± 0.11 | 100 | 17.21 ± 4.06 | 100 | 7.15 ± 2.84 | 100 |
| Diatom 1% | 0.87 ± 0.12 | 113 | 7.21 ± 1.68 | 81 | 4.89 ± 0.58 | 255 | 294.5 ± 43.1 | 111 | 2.27 ± 0.12 | 107 | 16.49 ± 2.41 | 96 | 6.67 ± 0.89 | 93 | |
| Diatom 3% | 0.93 ± 0.15 | 121 | 7.52 ± 0.76 | 85 | 4.87 ± 1.01 | 254 | 199.0 ± 38.2 | 75 | 2.68 ± 0.22 | 127 | 12.95 ± 3.71 | 75 | 6.28 ± 3.83 | 88 | |
| Diatom 5% | 0.63 ± 0.07 | 82 | 6.95 ± 1.01 | 78 | 5.56 ± 0.78 | 290 | 243.9 ± 33.4 | 92 | 2.88 ± 0.21 | 136 | 11.27 ± 5.08 | 66 | 5.50 ± 2.98 | 77 | |
| kidney | Control | 0.56 ± 0.17 | 100 | 4.49 ± 1.37 | 100 | 0.34 ± 0.09 | 100 | 30.47 ± 6.07 | 100 | 3.16 ± 0.17 | 100 | 16.47 ± 6.22 | 100 | 6.13 ± 2.78 | 100 |
| Diatom 1% | 0.37 ± 0.14 | 66 | 5.52 ± 1.12 | 123 | 0.63 ± 0.09 | 185 | 38.27 ± 5.82 | 126 | 3.57 ± 0.21 | 113 | 14.79 ± 5.16 | 90 | 5.49 ± 1.22 | 90 | |
| Diatom 3% | 0.86 ± 0.29 | 154 | 7.41 ± 1.54 | 165 | 0.51 ± 0.1 | 150 | 38.95 ± 6.13 | 128 | 3.77 ± 0.26 | 119 | 15.11 ± 1.91 | 92 | 5.55 ± 2.47 | 91 | |
| Diatom 5% | 0.33 ± 0.09 | 59 | 5.22 ± 1.51 | 116 | 0.61 ± 0.14 | 179 | 48.12 ± 5.41 | 158 | 4.61 ± 0.28 | 146 | 12.05 ± 5.37 | 73 | 4.23 ± 0.91 | 69 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świderska-Kołacz, G.; Jefimow, M.; Klusek, J.; Rączka, N.; Zmorzyński, S.; Wojciechowska, A.; Stanisławska, I.; Łyp, M.; Czerwik-Marcinkowska, J. Influence of Algae Supplementation on the Concentration of Glutathione and the Activity of Glutathione Enzymes in the Mice Liver and Kidney. Nutrients 2021, 13, 1996. https://doi.org/10.3390/nu13061996
Świderska-Kołacz G, Jefimow M, Klusek J, Rączka N, Zmorzyński S, Wojciechowska A, Stanisławska I, Łyp M, Czerwik-Marcinkowska J. Influence of Algae Supplementation on the Concentration of Glutathione and the Activity of Glutathione Enzymes in the Mice Liver and Kidney. Nutrients. 2021; 13(6):1996. https://doi.org/10.3390/nu13061996
Chicago/Turabian StyleŚwiderska-Kołacz, Grażyna, Małgorzata Jefimow, Jolanta Klusek, Norbert Rączka, Szymon Zmorzyński, Anna Wojciechowska, Iwona Stanisławska, Marek Łyp, and Joanna Czerwik-Marcinkowska. 2021. "Influence of Algae Supplementation on the Concentration of Glutathione and the Activity of Glutathione Enzymes in the Mice Liver and Kidney" Nutrients 13, no. 6: 1996. https://doi.org/10.3390/nu13061996
APA StyleŚwiderska-Kołacz, G., Jefimow, M., Klusek, J., Rączka, N., Zmorzyński, S., Wojciechowska, A., Stanisławska, I., Łyp, M., & Czerwik-Marcinkowska, J. (2021). Influence of Algae Supplementation on the Concentration of Glutathione and the Activity of Glutathione Enzymes in the Mice Liver and Kidney. Nutrients, 13(6), 1996. https://doi.org/10.3390/nu13061996






