Human Milk Oligosaccharide Profiles over 12 Months of Lactation: The Ulm SPATZ Health Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Measurements
2.3. Analysis of HMOs
2.4. Method Validation
2.5. Secretor Phenotyping and Milk Group Assignment
2.6. Statistical Analysis
3. Results
3.1. HMO Concentrations at Different Timepoints of Lactation
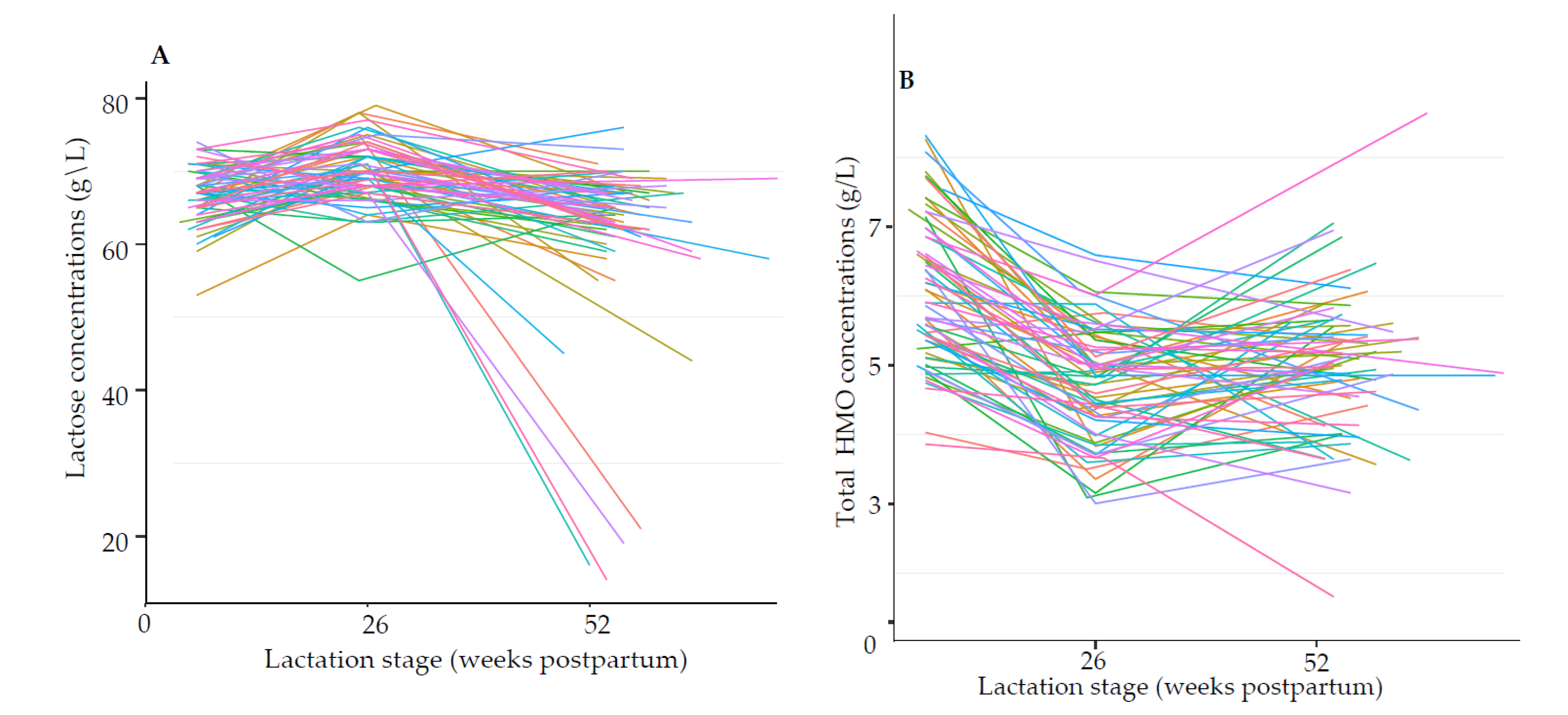
3.1.1. Absolute HMO Concentrations
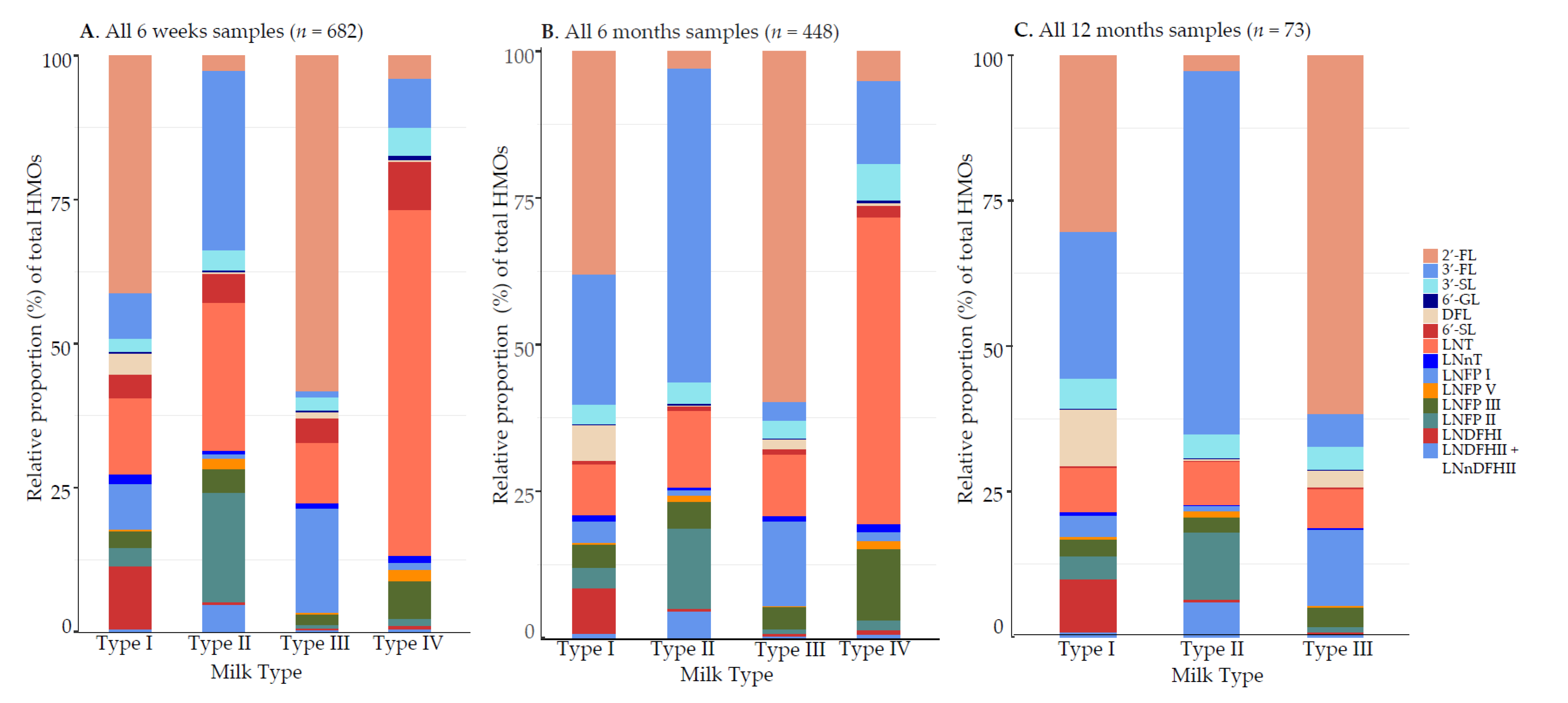
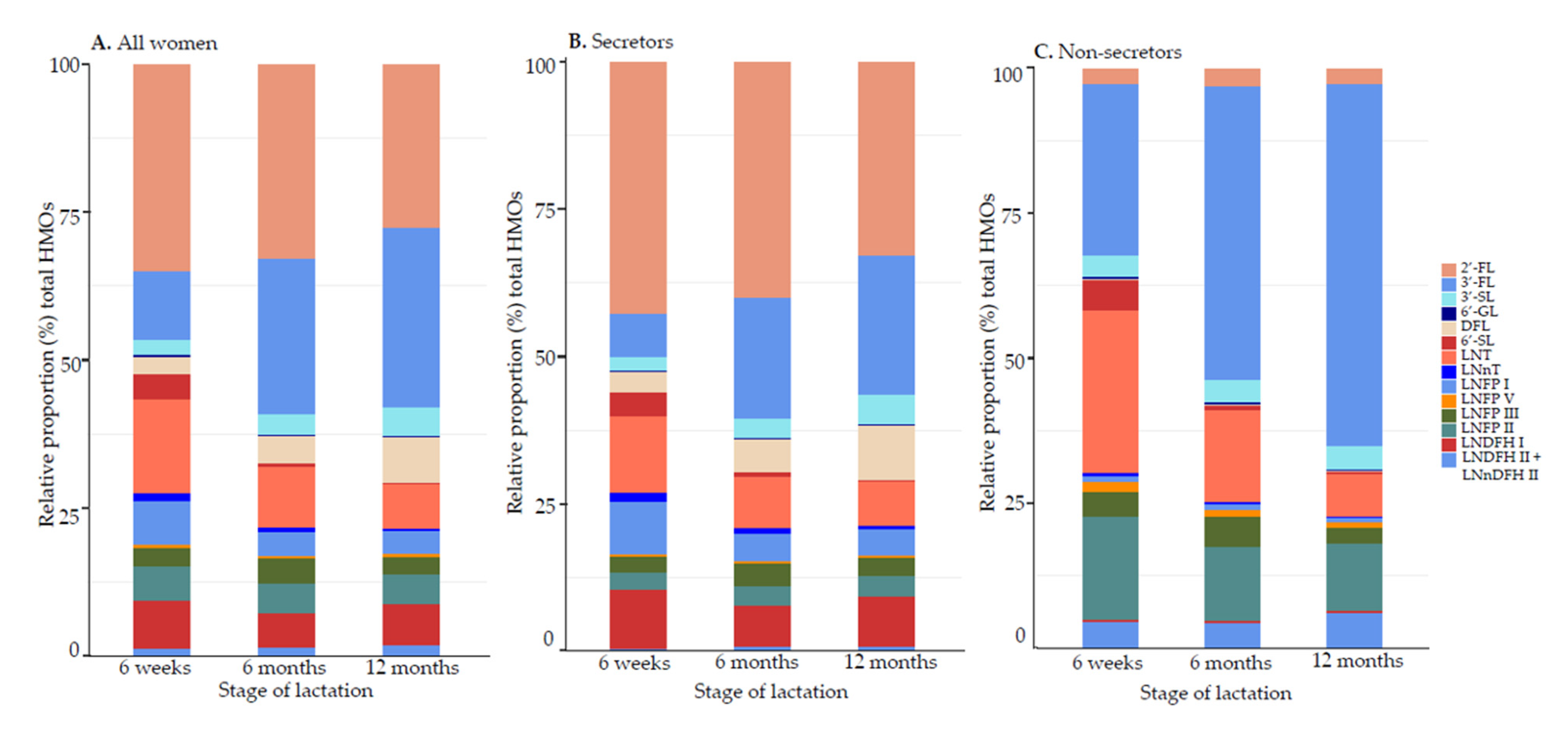
3.1.2. Relative Proportions (%) of HMO Concentrations
3.1.3. Trajectory of Lactose and HMOs during Lactation
3.1.4. Effect of Time, Secretor Status, and Milk Group during Lactation
3.2. Factors Associated with HMO Concentrations
3.2.1. At 6 Weeks of Lactation
3.2.2. Longitudinal Changes within Milk Groups I and II
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Coppa, G.; Pierani, P.; Zampini, L.; Carloni, I.; Carlucci, A.; Gabrielli, O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr. 1999, 88, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Taufik, E. Oligosaccharides in Milk: Their Benefits and Future Utilization. Media Peternak. 2010, 33, 189. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Henker, J.; Siegel, M.; Tovar, K.; Sawatzki, G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj. J. 1997, 14, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Oriol, R.; Mollicone, R.; Cailleau, A.; Balanzino, L.; Breton, C. Divergent evolution of fucosyltransferase genes from vertebrates, invertebrates, and bacteria. Glycobiology 1999, 9, 323–334. [Google Scholar] [CrossRef]
- Wu, J.; Wu, S.; Huo, J.; Ruan, H.; Xu, X.; Hao, Z.; Wei, Y.A. Systematic Characterization and Longitudinal Study Reveal Distinguishing Features of Human Milk Oligosaccharides in China. Curr. Dev. Nutr. 2020, 4. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.; Alves, R.; Figueiredo, A.; Alves-Santos, N.; Freitas-Costa, N.; Batalha, M.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L.; et al. Human Milk Oligosaccharide Profile Variation Throughout Postpartum in Healthy Women in a Brazilian Cohort. Nutrients 2020, 12, 790. [Google Scholar] [CrossRef]
- Plows, J.F.; Berger, P.K.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Najera, J.A.; Khwajazada, S.; Bode, L.; Goran, M.I. Longitudinal Changes in Human Milk Oligosaccharides (HMOs) Over the Course of 24 Months of Lactation. J. Nutr. 2021, 151, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Derraik, J.G.B.; Binia, A.; Sprenger, N.; Vickers, M.H.; Cutfield, W.S. Maternal and Infant Factors Influencing Human Milk Oligosaccharide Composition: Beyond Maternal Genetics. J. Nutr. 2021, 151, 1383–1393. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm Milk Oligosaccharides During the First Month of Lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef]
- Austin, S.; De Castro, C.A.; Sprenger, N.; Binia, A.; Affolter, M.; Garcia-Rodenas, C.L.; Beauport, L.; Tolsa, J.F.; Fumeaux, C.J. Human Milk Oligosaccharides in the Milk of Mothers Delivering Term versus Preterm Infants. Nutrients 2019, 11, 1282. [Google Scholar] [CrossRef]
- Tonon, M.K.; de Morais, B.M.; Abrão, F.V.A.C.; Miranda, A.; Morais, B.T. Maternal and Infant Factors Associated with Human Milk Oligosaccharides Concentrations According to Secretor and Lewis Phenotypes. Nutrients 2019, 11, 1358. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Kunz, C.; Meyer, C.; Collado, M.; Geiger, L.; García-Mantrana, I.; Bertua-Ríos, B.; Martínez-Costa, C.; Borsch, C.; Rudloff, S. Influence of Gestational Age, Secretor, and Lewis Blood Group Status on the Oligosaccharide Content of Human Milk. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef]
- Lefebvre, G.; Shevlyakova, M.; Charpagne, A.; Marquis, J.; Vogel, M.; Kirsten, T.; Kiess, W.; Austin, S.; Sprenger, N.; Binia, A. Time of Lactation and Maternal Fucosyltransferase Genetic Polymorphisms Determine the Variability in Human Milk Oligosaccharides. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef]
- Erney, R.M.; Malone, W.T.; Skelding, M.B.; Marcon, A.A.; Kleman–Leyer, K.M.; O’Ryan, M.L.; Ruiz–Palacios, G.; Hilty, M.D.; Pickering, L.K.; Prieto, P.A. Variability of Human Milk Neutral Oligosaccharides in a Diverse Population. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 181. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Chichlowski, M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. The Influence of Milk Oligosaccharides on Microbiota of Infants: Opportunities for Formulas. Annu. Rev. Food Sci. Technol. 2011, 2, 331–351. [Google Scholar] [CrossRef]
- Lin, A.E.; Autran, C.A.; Szyszka, A.; Escajadillo, T.; Huang, M.; Godula, K.; Prudden, A.R.; Boons, G.J.; Lewis, A.L.; Doran, K.S.; et al. Human milk oligosaccharides inhibit growth of group B Streptococcus. J. Biol. Chem. 2017, 292, 11243–11249. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Morrow, A.L. Human Milk Glycans Protect Infants Against Enteric Pathogens. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab 2016, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Zuurveld, M. Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Front. Immunol. 2020, 11, 17. [Google Scholar] [CrossRef]
- Ayechu-Muruzabal, V.; van Stigt, A.H.; Mank, M.; Willemsen, L.E.M.; Stahl, B.; Garssen, J.; Van’t Land, B. Diversity of Human Milk Oligosaccharides and Effects on Early Life Immune Development. Front. Pediatr. 2018, 6. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. Sialylated Oligosaccharides and Glycoconjugates of Human Milk. The Impact on Infant and Newborn Protection, Development and Well-Being. Nutrients 2019, 11, 306. [Google Scholar] [CrossRef]
- Kuntz, S.; Kunz, C.; Borsch, C.; Vazquez, E.; Buck, R.; Reutzel, M.; Eckert, G.P.; Rudloff, S. Metabolic Fate and Distribution of 2´-Fucosyllactose: Direct Influence on Gut Microbial Activity but not on Brain. Mol. Nutr. Food Res. 2019, 63, 1900035. [Google Scholar] [CrossRef]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human milk oligosaccharide 2’-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS ONE 2020, 15, e0228323. [Google Scholar] [CrossRef]
- Tonon, K.M.; Miranda, A.; Abrão, A.C.F.V.; de Morais, M.B.; Morais, T.B. Validation and application of a method for the simultaneous absolute quantification of 16 neutral and acidic human milk oligosaccharides by graphitized carbon liquid chromatography—electrospray ionization—mass spectrometry. Food Chem. 2019, 274, 691–697. [Google Scholar] [CrossRef]
- Bao, Y.; Chen, C.; Newburg, D.S. Quantification of neutral human milk oligosaccharides by graphitic carbon high-performance liquid chromatography with tandem mass spectrometry. Anal. Biochem. 2013, 433, 28–35. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Chen, X.; Pang, X.; Zhang, S.; Obaroakpo, J.U.; Shilong, J.; Lu, J.; Lv, J. Absolute quantification of twelve oligosaccharides in human milk using a targeted mass spectrometry-based approach. Carbohydr. Polym. 2019, 219, 328–333. [Google Scholar] [CrossRef]
- Xu, G.; Davis, J.C.; Goonatilleke, E.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Absolute Quantitation of Human Milk Oligosaccharides Reveals Phenotypic Variations during Lactation123. J. Nutr. 2017, 147, 117–124. [Google Scholar] [CrossRef]
- Mank, M.; Welsch, P.; Heck, A.J.R.; Stahl, B. Label-free targeted LC-ESI-MS2 analysis of human milk oligosaccharides (HMOS) and related human milk groups with enhanced structural selectivity. Anal. Bioanal. Chem. 2019, 411, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, W.; Urashima, T.; Nakamura, T.; Arai, I.; Saito, T.; Tsumura, N.; Wang, B.; Brand-Miller, J.; Watanabe, Y.; Kimura, K. Determination of each neutral oligosaccharide in the milk of Japanese women during the course of lactation. Br. J. Nutr. 2003, 89, 61–69. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, N.; Lee, L.Y.; De Castro, C.A.; Steenhout, P.; Thakkar, S.K. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; De Castro, C.A.; Bénet, T.; Hou, Y.; Sun, H.; Thakkar, S.K.; Vinyes-Pares, G.; Zhang, Y.; Wang, P. Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers. Nutrients 2016, 8, 346. [Google Scholar] [CrossRef]
- Logan, C.; Zittel, T.; Striebel, S.; Reister, F.; Brenner, H.; Rothenbacher, D.; Genuneit, J. Changing Societal and Lifestyle Factors and Breastfeeding Patterns Over Time. Pediatrics 2016, 137, e20154473. [Google Scholar] [CrossRef]
- Blom, G. Statistical Estimates and Transformed Beta-Variables; Almqvist & Wiksell: Uppsala, Sweden, 1958. [Google Scholar]
- Elwakiel, M.; Hageman, J.A.; Wang, W.; Szeto, I.M.; van Goudoever, J.B.; Hettinga, K.A.; Schols, H.A. Human Milk Oligosaccharides in Colostrum and Mature Milk of Chinese Mothers: Lewis Positive Secretor Subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. [Google Scholar] [CrossRef]
- De Leoz, M.L.A.; Gaerlan, S.C.; Strum, J.S.; Dimapasoc, L.M.; Mirmiran, M.; Tancredi, D.J.; Smilowitz, J.T.; Kalanetra, K.M.; Mills, D.A.; German, J.B.; et al. Lacto N Tetraose, Fucosylation, and Secretor Status are Highly Variable in Human Milk Oligosaccharides From Women Delivering Preterm. J. Proteome Res. 2012, 11, 4662–4672. [Google Scholar] [CrossRef]
- van Leeuwen, S.S. Challenges and Pitfalls in Human Milk Oligosaccharide Analysis. Nutrients 2019, 11, 2684. [Google Scholar] [CrossRef]
- Goehring, K.C.; Kennedy, A.D.; Prieto, P.A.; Buck, R.H. Direct Evidence for the Presence of Human Milk Oligosaccharides in the Circulation of Breastfed Infants. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Dotz, V.; Rudloff, S.; Blank, D.; Lochnit, G.; Geyer, R.; Kunz, C. 13C-labeled oligosaccharides in breastfed infants’ urine: Individual-, structure- and time-dependent differences in the excretion. Glycobiology 2014, 24, 185–194. [Google Scholar] [CrossRef]
- Marriage, B.J.; Buck, R.H.; Goehring, K.C.; Oliver, J.S.; Williams, J.A. Infants Fed a Lower Calorie Formula With 2′FL Show Growth and 2′FL Uptake Like Breast-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 649–658. [Google Scholar] [CrossRef]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Altaye, M.; Jiang, X.; Lourdes Guerrero, M.; Meinzen-Derr, J.K.; Farkas, T.; Chaturvedi, P.; Pickering, L.K.; Newburg, D.S. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J. Pediatr. 2004, 145, 297–303. [Google Scholar] [CrossRef]
- Williams, J.E.; McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Brooker, S.L.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; Prentice, A.M.; et al. Key genetic variants associated with variation of milk oligosaccharides from diverse human populations. Genomics 2021, 113, 1867–1875. [Google Scholar] [CrossRef]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Galeotti, F.; Coppa, G.V.; Zampini, L.; Maccari, F.; Galeazzi, T.; Padella, L.; Santoro, L.; Gabrielli, O.; Volpi, N. Capillary electrophoresis separation of human milk neutral and acidic oligosaccharides derivatized with 2-aminoacridone. Electrophoresis 2014, 35, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, J.T.; O’Sullivan, A.; Barile, D.; German, J.B.; Lönnerdal, B.; Slupsky, C.M. The Human Milk Metabolome Reveals Diverse Oligosaccharide Profiles123. J. Nutr. 2013, 143, 1709–1718. [Google Scholar] [CrossRef]
- Sprenger, N.; Odenwald, H.; Kukkonen, A.K.; Kuitunen, M.; Savilahti, E.; Kunz, C. FUT2-dependent breast milk oligosaccharides and allergy at 2 and 5 years of age in infants with high hereditary allergy risk. Eur. J. Nutr. 2017, 56, 1293–1301. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; et al. Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef]
- Chouraqui, J.-P. Does the contribution of human milk oligosaccharides to the beneficial effects of breast milk allow us to hope for an improvement in infant formulas? Crit. Rev. Food Sci. Nutr. 2021, 61, 1503–1514. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yeung, C.-Y. Recent advance in infant nutrition: Human milk oligosaccharides. Pediatr. Neonatol. 2021. [Google Scholar] [CrossRef]
| Characteristics | All 6 Weeks Samples (n = 682) | All 6 Months Samples (n = 448) | All 12 Months Samples (n = 73) | ||||
|---|---|---|---|---|---|---|---|
| n | % or Mean | n | % or Mean | n | % or Mean | ||
| Mother | |||||||
| Age | 681 | 33.1 | 447 | 33.5 | 72 | 34.3 | |
| Maternal body weight (6 weeks) (kg) | 587 | 70.5 | 385 | 69.9 | 58 | 70.8 | |
| Maternal BMI (6 weeks) (kg) | 662 | 24.7 | 438 | 24.8 | 72 | 25.1 | |
| Parity | |||||||
| 0 births | 368 | 54 | 243 | 54 | 42 | 57.5 | |
| ≥1 births | 314 | 46 | 205 | 46 | 31 | 42.5 | |
| Milk group | |||||||
| I | 502 | 74 | 330 | 74 | 55 | 75 | |
| II | 122 | 18 | 80 | 18 | 13 | 18 | |
| III | 49 | 7 | 32 | 7 | 5 | 7 | |
| IV | 9 | 1 | 6 | 1 | 0 | 0 | |
| Infant | |||||||
| Female | 339 | 50 | 216 | 48 | 29 | 40 | |
| Male | 343 | 50 | 232 | 52 | 44 | 60 | |
| Gestation weeks | 682 | 38.7 | 448 | 38.8 | 73 | 38.6 | |
| Birth weight (g) | 682 | 3272 | 448 | 3282 | 73 | 3292 | |
| Delivery mode | |||||||
| Vaginal spontaneous | 432 | 63 | 294 | 65 | 48 | 66 | |
| Elective caesarean | 80 | 12 | 52 | 11 | 8 | 11 | |
| Emergency caesarean | 118 | 17 | 66 | 14 | 13 | 18 | |
| Vaginal assisted | 52 | 8 | 36 | 8 | 4 | 5 | |
| HMO | 6 Weeks (n = 66) | 6 Months (n = 66) | 12 Months (n = 66) | p 1 | p 2 | p 3 |
|---|---|---|---|---|---|---|
| 2′-FL | ||||||
| Mean (SD) | 2.45 (1.32) | 1.65 (1.08) | 1.43 (0.85) | <0.001 | 0.305 | <0.001 |
| Median (min, max) | 2.60 (0.13, 6.00) | 1.65 (0.13, 4.30) | 1.45 (0.13, 3.30) | |||
| 3-FL | ||||||
| Mean (SD) | 0.64 (0.57) | 1.24 (0.74) | 1.52 (1.26) | <0.001 | 0.446 | <0.001 |
| Median (min, max) | 0.44 (0.04, 2.40) | 1.05 (0.08, 3.10) | 1.05 (0.12, 6.90) | |||
| 3′-SL | ||||||
| Mean (SD) | 0.12 (0.03) | 0.14 (0.04) | 0.25 (0.12) | 0.470 | <0.001 | <0.001 |
| Median (min, max) | 0.13 (0.08, 0.24) | 0.14 (0.07, 0.34) | 0.22 (0.09, 0.58) | |||
| 6′-GL | ||||||
| Mean (SD) | 0.02 (0.01) | 0.01 (0.00) | 0.01 (0.01) | <0.001 | 0.188 | <0.001 |
| Median (min, max) | 0.01 (0.01, 0.04) | 0.01 (0.00, 0.02) | 0.01 (0.00, 0.03) | |||
| DFL | ||||||
| Mean (SD) | 0.18 (0.14) | 0.20 (0.14) | 0.40 (0.41) | 0.198 | 0.010 | <0.001 |
| Median (min, max) | 0.15 (0.01, 0.56) | 0.20 (0.01, 0.51) | 0.27 (0.01, 2.20) | |||
| 6′-SL | ||||||
| Mean (SD) | 0.24 (0.09) | 0.04 (0.02) | 0.01 (0.00) | <0.001 | <0.001 | <0.001 |
| Median (min, max) | 0.22 (0.08, 0.53) | 0.03 (0.01, 0.12) | 0.01 (0.01, 0.03) | |||
| LNT | ||||||
| Mean (SD) | 0.86 (0.44) | 0.44 (0.28) | 0.36 (0.24) | <0.001 | 0.038 | <0.001 |
| Median (min, max) | 0.75 (0.15, 1.90) | 0.41 (0.10, 1.50) | 0.32 (0.05, 1.20) | |||
| LNnT | ||||||
| Mean (SD) | 0.08 (0.05) | 0.05 (0.05) | 0.02 (0.02) | <0.001 | <0.001 | <0.001 |
| Median (min, max) | 0.07 (0.01, 0.23) | 0.03 (0.01, 0.23) | 0.02 (0.01, 0.13) | |||
| LNFP I | ||||||
| Mean (SD) | 0.43 (0.37) | 0.19 (0.21) | 0.20 (0.21) | <0.001 | 0.696 | <0.001 |
| Median (min, max) | 0.31 (0.04, 1.60) | 0.09 (0.04, 1.10) | 0.12 (0.04, 1.00) | |||
| LNFP V | ||||||
| Mean (SD) | 0.03 (0.03) | 0.02 (0.02) | 0.02 (0.02) | 0.641 | 0.996 | 0.598 |
| Median (min, max) | 0.02 (0.01, 0.14) | 0.02 (0.01, 0.10) | 0.02 (0.01, 0.08) | |||
| LNFP III | ||||||
| Mean (SD) | 0.17 (0.07) | 0.19 (0.07) | 0.14 (0.05) | 0.089 | <0.001 | 0.002 |
| Median (min, max) | 0.18 (0.05, 0.31) | 0.18 (0.07, 0.36) | 0.13 (0.04, 0.30) | |||
| LNFP II | ||||||
| Mean (SD) | 0.30 (0.40) | 0.24 (0.24) | 0.24 (0.24) | 0.567 | 0.800 | 0.376 |
| Median (min, max) | 0.13 (0.04, 1.70) | 0.15 (0.04, 1.40) | 0.17 (0.04, 1.20) | |||
| LNDFH I | ||||||
| Mean (SD) | 0.50 (0.34) | 0.27 (0.19) | 0.36 (0.26) | <0.001 | 0.033 | 0.012 |
| Median (min, max) | 0.54 (0.02, 1.30) | 0.27 (0.02, 0.70) | 0.35 (0.02, 0.90) | |||
| LNDFH II + LNnDFH II | ||||||
| Mean (SD) | 0.06 (0.12) | 0.06 (0.08) | 0.08 (0.11) | 0.002 | 0.441 | <0.001 |
| Median (min, max) | 0.01 (0.01, 0.70) | 0.03 (0.01, 0.30) | 0.03 (0.01, 0.46) | |||
| Total HMOs | ||||||
| Mean (SD) | 6.09 (1.05) | 4.74 (0.84) | 5.03 (1.05) | <0.001 | 0.093 | <0.001 |
| Median (min, max) | 5.91 (3.86, 8.32) | 4.84 (3.01, 6.59) | 5.07 (1.66, 8.64) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siziba, L.P.; Mank, M.; Stahl, B.; Gonsalves, J.; Blijenberg, B.; Rothenbacher, D.; Genuneit, J. Human Milk Oligosaccharide Profiles over 12 Months of Lactation: The Ulm SPATZ Health Study. Nutrients 2021, 13, 1973. https://doi.org/10.3390/nu13061973
Siziba LP, Mank M, Stahl B, Gonsalves J, Blijenberg B, Rothenbacher D, Genuneit J. Human Milk Oligosaccharide Profiles over 12 Months of Lactation: The Ulm SPATZ Health Study. Nutrients. 2021; 13(6):1973. https://doi.org/10.3390/nu13061973
Chicago/Turabian StyleSiziba, Linda P., Marko Mank, Bernd Stahl, John Gonsalves, Bernadet Blijenberg, Dietrich Rothenbacher, and Jon Genuneit. 2021. "Human Milk Oligosaccharide Profiles over 12 Months of Lactation: The Ulm SPATZ Health Study" Nutrients 13, no. 6: 1973. https://doi.org/10.3390/nu13061973
APA StyleSiziba, L. P., Mank, M., Stahl, B., Gonsalves, J., Blijenberg, B., Rothenbacher, D., & Genuneit, J. (2021). Human Milk Oligosaccharide Profiles over 12 Months of Lactation: The Ulm SPATZ Health Study. Nutrients, 13(6), 1973. https://doi.org/10.3390/nu13061973






