Characterizing the Effects of Calcium and Prebiotic Fiber on Human Gut Microbiota Composition and Function Using a Randomized Crossover Design—A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
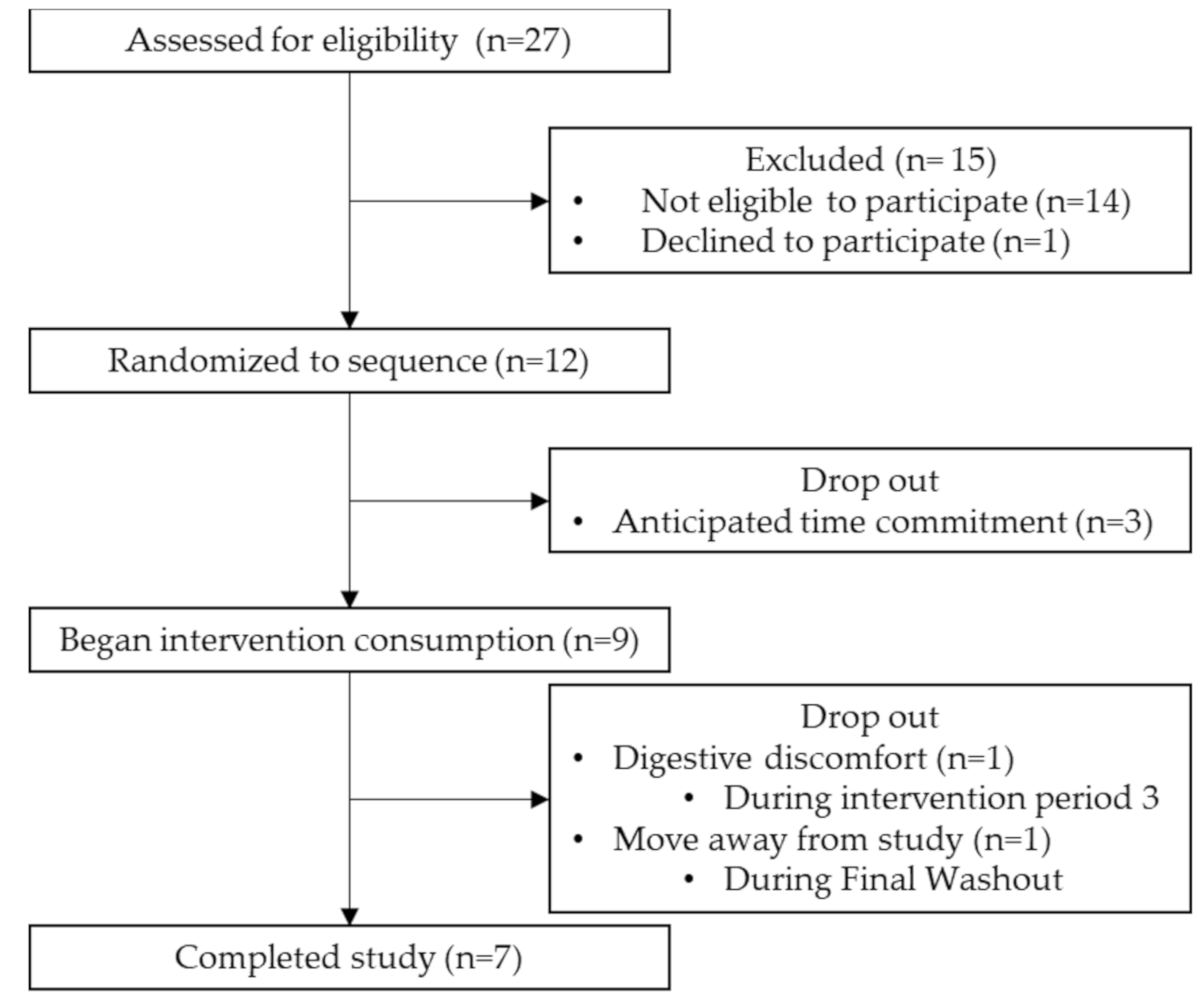
2.1. Participant Eligibility and Recruitment
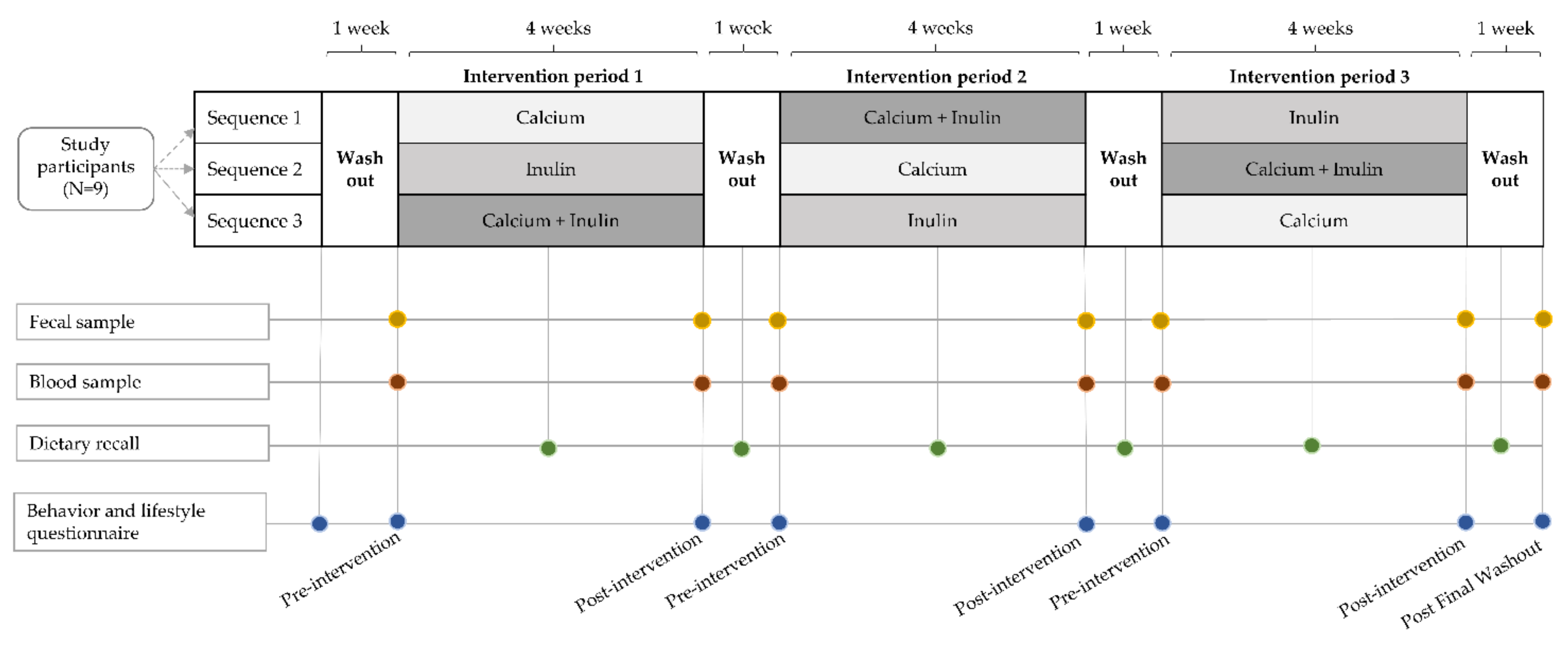
2.2. Experimental Design and Dietary Interventions
2.3. Questionnaires
2.4. Sample Collection
2.5. 16S rRNA Sequencing
2.6. Short-Chain Fatty Acid, Lipopolysaccharide Binding Protein, and Zonulin Analyseis
2.7. Statistical Analyses
3. Results
3.1. Baseline Cohort Characteristics
3.2. Feasibility and Post-Study Assessments
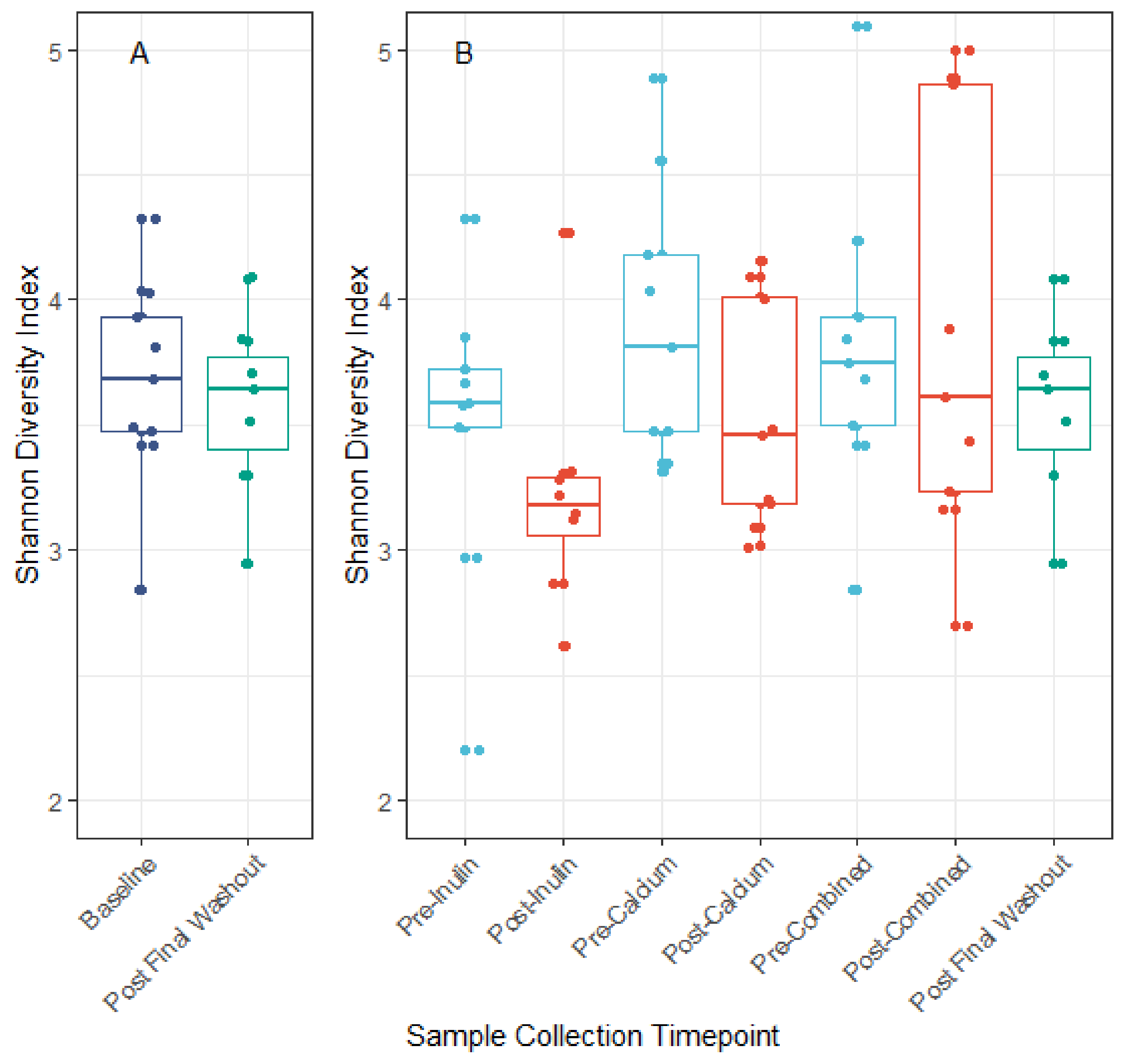
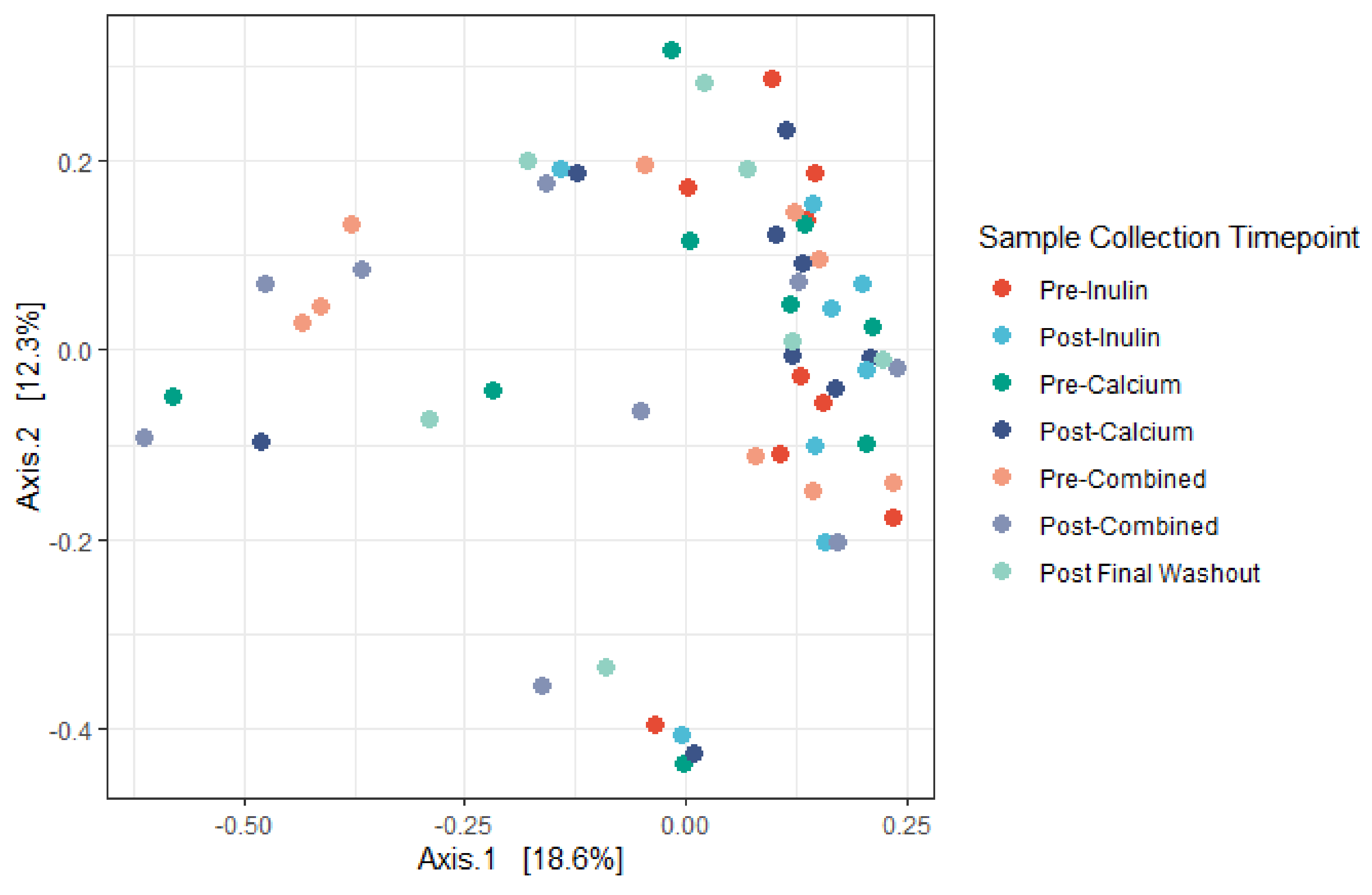
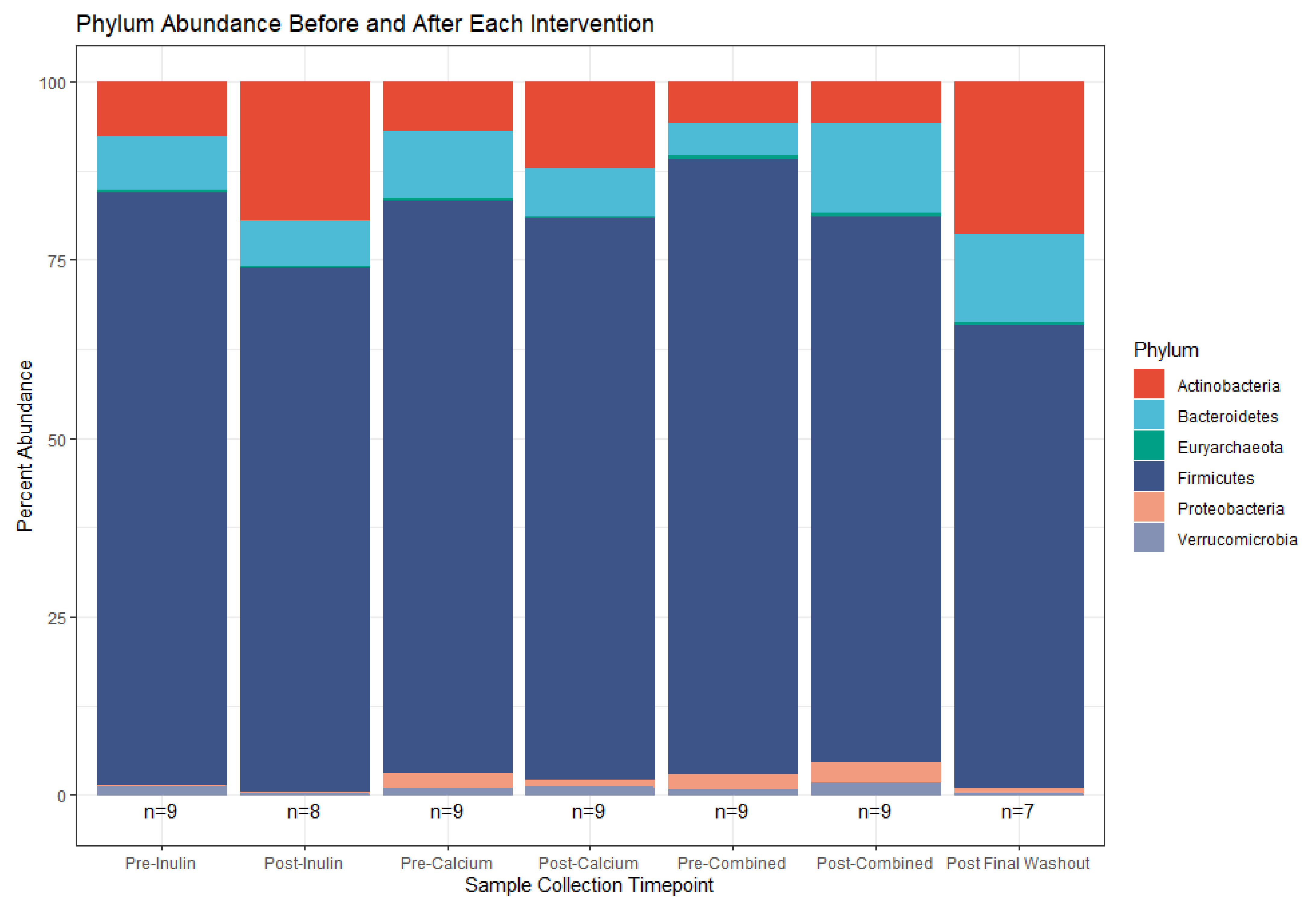
3.3. Microbiota Compositional Changes Associated with Calcium and Inulin Consumption
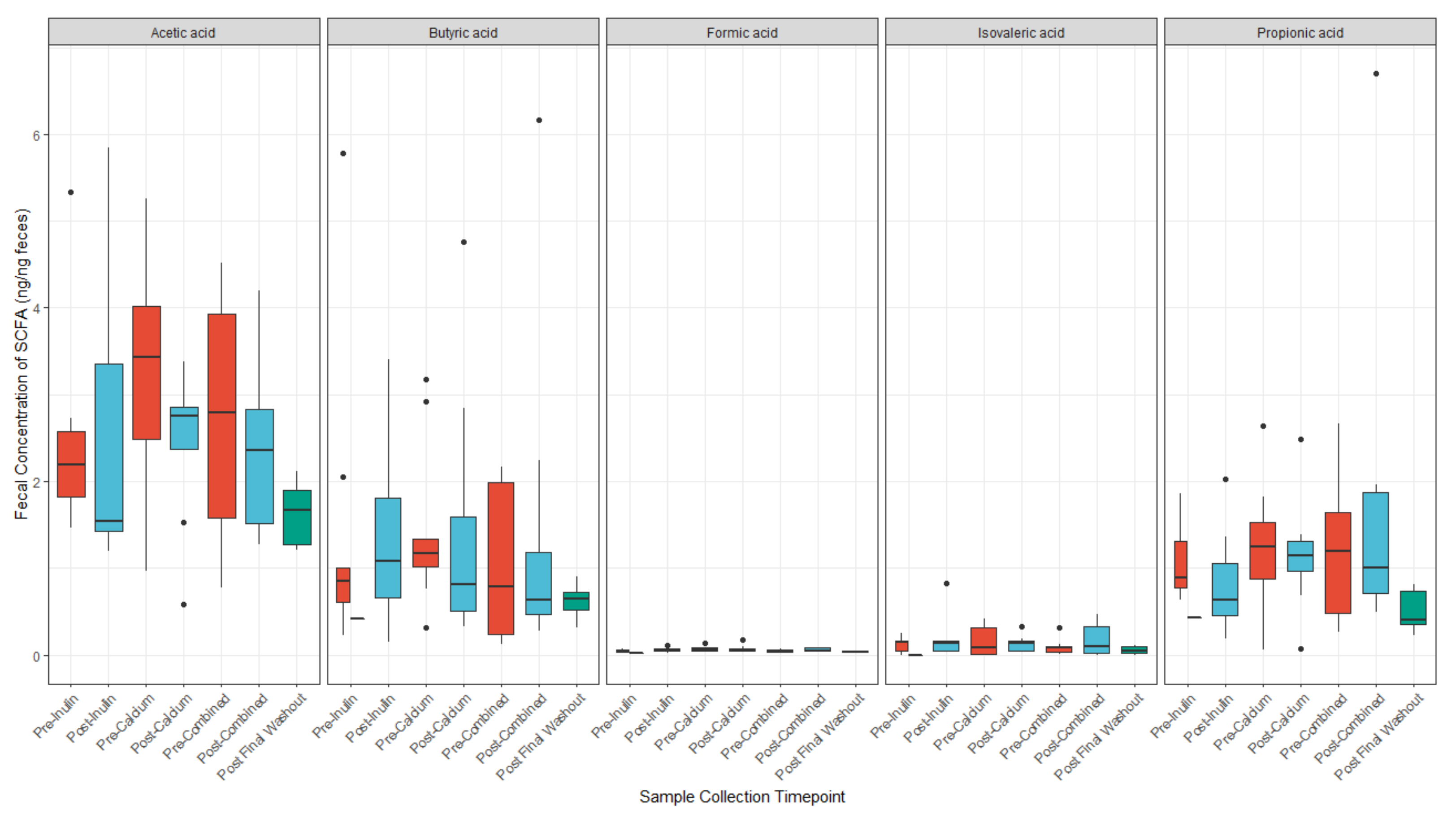
3.4. Calcium and Inulin Consumption Are Not Associated with Shifts in Fecal SCFA Concentrations
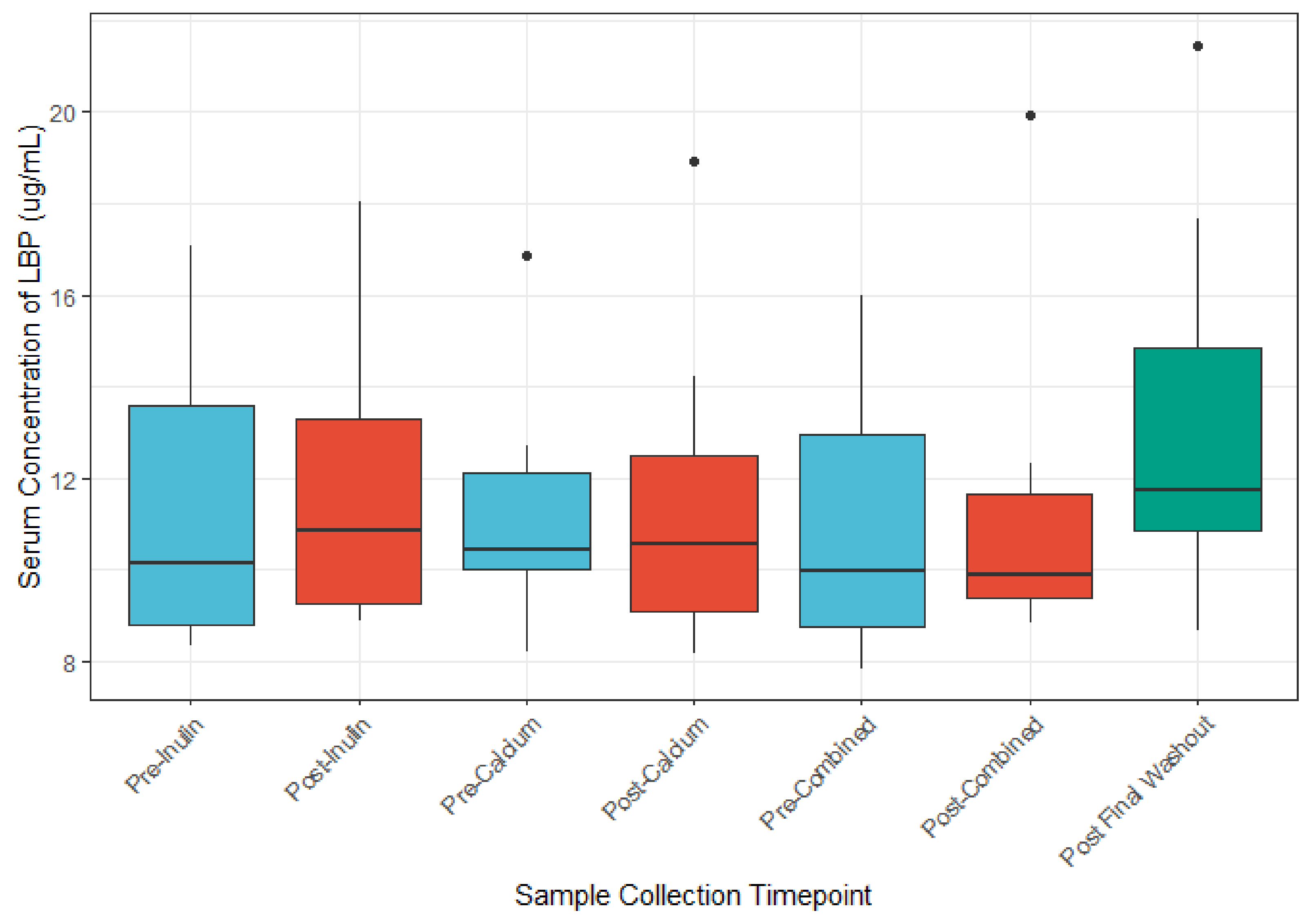
3.5. The Role of Calcium and Inulin in Modulating Systemic LBP and Zonulin Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, L.K.; O’Grady, G.; Du, P.; Egbuji, J.U.; Windsor, J.A.; Pullan, A.J. Gastrointestinal System. WIREs Syst. Biol. Med. 2010, 2, 65–79. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Michels, K.B. The Role of the Microbiome in the Developmental Origins of Health and Disease. Pediatrics 2018, 141, e20172437. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. Chapter Three-The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 91–119. [Google Scholar]
- Van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-Chain Fatty Acids: Microbial Metabolites That Alleviate Stress-Induced Brain-Gut Axis Alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Parra, P.; Laraichi, S.; Serra, F.; Palou, A. Calcium Supplementation Modulates Gut Microbiota in a Prebiotic Manner in Dietary Obese Mice. Mol. Nutr. Food Res. 2016, 60, 468–480. [Google Scholar] [CrossRef]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a Diet Based on Inulin-Rich Vegetables on Gut Health and Nutritional Behavior in Healthy Humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Xu, X.; Jia, X.; Mo, L.; Liu, C.; Zheng, L.; Yuan, Q.; Zhou, X. Intestinal Microbiota: A Potential Target for the Treatment of Postmenopausal Osteoporosis. Bone Res. 2017, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2018, 23, 41–53.e4. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.G.; Costa, J.A.; Alfenas, R.C. Could the Beneficial Effects of Dietary Calcium on Obesity and Diabetes Control Be Mediated by Changes in Intestinal Microbiota and Integrity? Br. J. Nutr. 2015, 114, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Raschka, L.; Daniel, H. Mechanisms Underlying the Effects of Inulin-Type Fructans on Calcium Absorption in the Large Intestine of Rats. Bone 2005, 37, 728–735. [Google Scholar] [CrossRef]
- Abrams, S.A.; Hawthorne, K.M.; Aliu, O.; Hicks, P.D.; Chen, Z.; Griffin, I.J. An Inulin-Type Fructan Enhances Calcium Absorption Primarily via an Effect on Colonic Absorption in Humans. J. Nutr. 2007, 137, 2208–2212. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Hofstaedter, C.E.; Zhao, C.; Mattei, L.; Tanes, C.; Clarke, E.; Lauder, A.; Sherrill-Mix, S.; Chehoud, C.; Kelsen, J.; et al. Optimizing Methods and Dodging Pitfalls in Microbiome Research. Microbiome 2017, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.R.; Rajilic-Stojanovic, M.; Salonen, A.; Sakwinska, O.; Gill, C.; Meynier, A.; Fança-Berthon, P.; Schelkle, B.; Segata, N.; Shortt, C.; et al. Considerations for the Design and Conduct of Human Gut Microbiota Intervention Studies Relating to Foods. Eur. J. Nutr. 2020, 59, 3347–3368. [Google Scholar] [CrossRef] [PubMed]
- Blatch-Jones, A.J.; Pek, W.; Kirkpatrick, E.; Ashton-Key, M. Role of Feasibility and Pilot Studies in Randomised Controlled Trials: A Cross-Sectional Study. BMJ Open 2018, 8, e022233. [Google Scholar] [CrossRef] [PubMed]
- Bernard, R. Fundamentals of Biostatistics, 8th ed.; Cengage Learning: Boston, MA, USA, 2016. [Google Scholar]
- Tong, M.; Jacobs, J.P.; McHardy, I.H.; Braun, J. Sampling of Intestinal Microbiota and Targeted Amplification of Bacterial 16S RRNA Genes for Microbial Ecologic Analysis. Curr. Protoc. Immunol. 2014, 107, 7.41.1–7.41.11. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Lin, L.; Goudarzi, M.; Ruegger, P.; McGovern, D.P.B.; Jr, A.J.F.; Borneman, J.; Xia, L.; Braun, J. Microbial, Metabolomic, and Immunologic Dynamics in a Relapsing Genetic Mouse Model of Colitis Induced by T-Synthase Deficiency. Gut Microbes 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor Workflow for Microbiome Data Analysis: From Raw Reads to Community Analyses. F1000Res 2016, 5, 1492. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by Gas Chromatography–Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H. The Vegan Package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-Scale Meta-Omics Studies. bioRxiv 2021. [Google Scholar] [CrossRef]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole Grain-Rich Diet Reduces Body Weight and Systemic Low-Grade Inflammation without Inducing Major Changes of the Gut Microbiome: A Randomised Cross-over Trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Korem, T.; Zeevi, D.; Zmora, N.; Weissbrod, O.; Bar, N.; Lotan-Pompan, M.; Avnit-Sagi, T.; Kosower, N.; Malka, G.; Rein, M.; et al. Bread Affects Clinical Parameters and Induces Gut Microbiome-Associated Personal Glycemic Responses. Cell Metab. 2017, 25, 1243–1253.e5. [Google Scholar] [CrossRef]
- Schmedes, M.; Brejnrod, A.D.; Aadland, E.K.; Kiilerich, P.; Kristiansen, K.; Jacques, H.; Lavigne, C.; Graff, I.E.; Eng, Ø.; Holthe, A.; et al. The Effect of Lean-Seafood and Non-Seafood Diets on Fecal Metabolites and Gut Microbiome: Results from a Randomized Crossover Intervention Study. Mol. Nutr. Food Res. 2019, 63, 1700976. [Google Scholar] [CrossRef] [PubMed]
- Karusheva, Y.; Koessler, T.; Strassburger, K.; Markgraf, D.; Mastrototaro, L.; Jelenik, T.; Simon, M.-C.; Pesta, D.; Zaharia, O.-P.; Bódis, K.; et al. Short-Term Dietary Reduction of Branched-Chain Amino Acids Reduces Meal-Induced Insulin Secretion and Modifies Microbiome Composition in Type 2 Diabetes: A Randomized Controlled Crossover Trial. Am. J. Clin. Nutr. 2019, 110, 1098–1107. [Google Scholar] [CrossRef]
- Hansen, T.H.; Thomassen, M.T.; Madsen, M.L.; Kern, T.; Bak, E.G.; Kashani, A.; Allin, K.H.; Hansen, T.; Pedersen, O. The Effect of Drinking Water PH on the Human Gut Microbiota and Glucose Regulation: Results of a Randomized Controlled Cross-over Intervention. Sci. Rep. 2018, 8, 16626. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.M.; Mohammad, M.; Ma, J.; Chu, D.; Haymond, M.; Aagaard, K. 66: Maternal Diet Alters the Breast Milk Microbiome and Microbial Gene Content. Am. J. Obstet. Gynecol. 2016, 214, S47–S48. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Introducing Inulin-Type Fructans. Br. J. Nutr. 2005, 93, S13–S25. [Google Scholar] [CrossRef]
- Crichton, G.E.; Howe, P.R.; Buckley, J.D.; Coates, A.M.; Murphy, K.J.; Bryan, J. Long-Term Dietary Intervention Trials: Critical Issues and Challenges. Trials 2012, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiong, Q.; Zhao, J.; Lin, X.; He, S.; Wu, N.; Yao, Y.; Liang, W.; Zuo, X.; Ying, C. Inulin-Type Fructan Intervention Restricts the Increase in Gut Microbiome–Generated Indole in Patients with Peritoneal Dialysis: A Randomized Crossover Study. Am. J. Clin. Nutr. 2020, 111, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Brueton, V.C.; Tierney, J.F.; Stenning, S.; Meredith, S.; Harding, S.; Nazareth, I.; Rait, G. Strategies to Improve Retention in Randomised Trials: A Cochrane Systematic Review and Meta-Analysis. BMJ Open 2014, 4, e003821. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.; Hutchesson, M.; Patterson, A.; Chalmers, K.; Collins, C. Recruitment and Retention of Young Women into Nutrition Research Studies: Practical Considerations. Trials 2014, 15, 23. [Google Scholar] [CrossRef]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual Dietary Fibre Intake Influences Gut Microbiota Response to an Inulin-Type Fructan Prebiotic: A Randomised, Double-Blind, Placebo-Controlled, Cross-over, Human Intervention Study. Br. J. Nutr. 2018, 119, 176–189. [Google Scholar] [CrossRef]
- Micka, A.; Siepelmeyer, A.; Holz, A.; Theis, S.; Schön, C. Effect of Consumption of Chicory Inulin on Bowel Function in Healthy Subjects with Constipation: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Food Sci. Nutr. 2017, 68, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The Effects of Inulin on Gut Microbial Composition: A Systematic Review of Evidence from Human Studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Li, Z.; Summanen, P.H.; Downes, J.; Thames, G.; Corbett, K.; Dowd, S.; Krak, M.; Heber, D. Xylooligosaccharide Increases Bifidobacteria but Not Lactobacilli in Human Gut Microbiota. Food Funct. 2014, 5, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Summanen, P.H.; Henning, S.M.; Hsu, M.; Lam, H.M.; Huang, J.; Tseng, C.-H.; Dowd, S.E.; Finegold, S.M.; Heber, D.; et al. Xylooligosaccharide Supplementation Alters Gut Bacteria in Both Healthy and Prediabetic Adults: A Pilot Study. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Swanson, K.S.; de Vos, W.M.; Martens, E.C.; Gilbert, J.A.; Menon, R.S.; Soto-Vaca, A.; Hautvast, J.; Meyer, P.D.; Borewicz, K.; Vaughan, E.E.; et al. Effect of Fructans, Prebiotics and Fibres on the Human Gut Microbiome Assessed by 16S RRNA-Based Approaches: A Review. Benef. Microbes 2020, 11, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Schoterman, M.H.C.; Nakatsu, C.H.; McCabe, L.D.; McCabe, G.P.; Wastney, M.E.; van den Heuvel, E.G.H.M.; Weaver, C.M. Galacto-Oligosaccharides Increase Calcium Absorption and Gut Bifidobacteria in Young Girls: A Double-Blind Cross-over Trial. Br. J. Nutr. 2013, 110, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; Story, J.A.; MacDonald-Clarke, C.J.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M. Soluble Corn Fiber Increases Calcium Absorption Associated with Shifts in the Gut Microbiome: A Randomized Dose-Response Trial in Free-Living Pubertal Females. J. Nutr. 2016, 146, 1298–1306. [Google Scholar] [CrossRef]
- Martinez-Puig, D.; Castillo, M.; Nofrarias, M.; Creus, E.; Perez, J.F. Long-Term Effects on the Digestive Tract of Feeding Large Amounts of Resistant Starch: A Study in Pigs. J. Sci. Food Agric. 2007, 87, 1991–1999. [Google Scholar] [CrossRef]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Microbial. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Alonso, M.; Campos, J.; Vizcaino, L.; Loidi, L.; Gude, F. Determinants of Serum Concentrations of Lipopolysaccharide-Binding Protein (LBP) in the Adult Population: The Role of Obesity. PLoS ONE 2013, 8, e54600. [Google Scholar]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary Supplementation with Inulin-Propionate Ester or Inulin Improves Insulin Sensitivity in Adults with Overweight and Obesity with Distinct Effects on the Gut Microbiota, Plasma Metabolome and Systemic Inflammatory Responses: A Randomised Cross-over Trial. Gut 2019, 68, 1430–1438. [Google Scholar] [PubMed]
- Rojo, Ó.P.; Román, A.L.S.; Arbizu, E.A.; de la Hera Martínez, A.; Sevillano, E.R.; Martínez, A.A. Serum Lipopolysaccharide-Binding Protein in Endotoxemic Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2007, 13, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Citronberg, J.S.; Curtis, K.R.; White, E.; Newcomb, P.A.; Newton, K.; Atkinson, C.; Song, X.; Lampe, J.W.; Hullar, M.A. Association of Gut Microbial Communities with Plasma Lipopolysaccharide-Binding Protein (LBP) in Premenopausal Women. ISME J. 2018, 12, 1631. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hullar, M.A.J.; Schwarz, Y.; Lampe, J.W. Human Gut Bacterial Communities Are Altered by Addition of Cruciferous Vegetables to a Controlled Fruit- and Vegetable-Free Diet. J. Nutr. 2009, 139, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n | % |
|---|---|---|
| Female | 7 | 77.8 |
| Age, years (mean, SD) | 26.1 | 3.0 |
| BMI, kg/m2 (mean, SD) | 21.9 | 1.9 |
| Weight, lbs (mean, SD) | 131.4 | 20.5 |
| Race | ||
| Non-hispanic white | 5 | 55.6 |
| Hispanic/Latino | 1 | 11.1 |
| Asian | 3 | 33.3 |
| Diet | ||
| Self-rated health of diet | ||
| Excellent | 3 | 33.3 |
| Good | 6 | 66.7 |
| Fair | 0 | 0 |
| Poor | 0 | 0 |
| Self-reported weekly food consumption, days per week (mean, SD) | ||
| Calcium | 4.7 | 1.3 |
| Fiber | 3.9 | 0.9 |
| Sugar | 2.4 | 1.7 |
| Red meat | 2.4 | 1.9 |
| Self-reported health | ||
| Excellent | 2 | 22.2 |
| Good | 7 | 77.8 |
| Fair | 0 | 0 |
| Poor | 0 | 0 |
| Physical activity, days per week (mean, SD) | 3.2 | 2.0 |
| Bowel movements per week (mean, SD) | 8.7 | 3.3 |
| Inulin Intervention | Calcium Intervention | Combined Intervention | |
|---|---|---|---|
| n 1 (%) | n (%) | n (%) | |
| Experienced abdominal pain or gastrointestinal comfort | 1 (11.1) | 1 (11.1) | 5 (55.6) |
| Days/week experiencing GI discomfort | |||
| 0 | 6 (66.7) | 7 (77.8) | 4 (44.4) |
| ≥1 | 2 (22.2) | 2 (22.2) | 5 (55.6) |
| Experienced changes in bowel movements | 3 (33.3) | 0 (0) | 4 (44.4) |
| Bowel movements per week during the intervention period (mean [SD]) | 9.3 (3.6) | 9.3 (3.9) | 9.4 (5.6) |
| Component | Mean 1 (SD) |
|---|---|
| Interest in topic | 4.2 (0.9) |
| Ease of following dietary guidelines | 4.4 (0.9) |
| Ease of stool sample collection | 3.9 (1.3) |
| Ease of blood collection | 4.6 (0.5) |
| Ease of powder consumption | 2.9 (1.3) |
| Discomfort following powder consumption | 3.1 (1) |
| Clarity of study instructions | 5 (0) |
| Organization of study | 5 (0) |
| OTU | Phylum | Genus; Species | Coef 1 | SE | p-Value 2 | q-Value 3 |
|---|---|---|---|---|---|---|
| Comparison: Calcium vs. Inulin (reference) | ||||||
| OTU125 | Firmicutes | Butyricicoccus; | 0.366 | 0.161 | 0.038 | 0.858 |
| OTU20 | Firmicutes | Streptococcus; | −0.582 | 0.260 | 0.040 | 0.858 |
| OTU170 | Firmicutes | Christensenellaceae_R-7_group; | 0.375 | 0.171 | 0.044 | 0.858 |
| OTU21 | Firmicutes | Anaerostipes; hadrus | −0.360 | 0.167 | 0.048 | 0.858 |
| Comparison: Combined vs. Inulin (reference) | ||||||
| OTU89 | Bacteroidetes | Bacteroides; | 0.721 | 0.228 | 0.006 | 0.858 |
| OTU300 | Bacteroidetes | Odoribacter; splanchnicus | 0.508 | 0.181 | 0.013 | 0.858 |
| OTU6 | Actinobacteria | Bifidobacterium; | −0.669 | 0.250 | 0.017 | 0.858 |
| OTU158 | Bacteroidetes | Alistipes; putredinis | 0.609 | 0.232 | 0.019 | 0.858 |
| OTU26 | Firmicutes | 0.611 | 0.239 | 0.022 | 0.858 | |
| OTU3 | Firmicutes | Anaerostipes; hadrus | −0.307 | 0.129 | 0.031 | 0.858 |
| OTU21 | Firmicutes | Anaerostipes; hadrus | −0.397 | 0.167 | 0.031 | 0.858 |
| OTU47 | Firmicutes | Roseburia; intestinalis | −0.473 | 0.207 | 0.037 | 0.858 |
| OTU189 | Firmicutes | Veillonella; | 0.812 | 0.356 | 0.037 | 0.858 |
| OTU170 | Firmicutes | Christensenellaceae_R-7_group; | 0.371 | 0.171 | 0.047 | 0.858 |
| OTU65 | Firmicutes | CAG-352; | 0.443 | 0.206 | 0.048 | 0.858 |
| OTU39 | Firmicutes | −0.202 | 0.095 | 0.050 | 0.858 | |
| Feature | Phylum | Genus; Species | Coef 1 | SE | p-Value 2 | q-Value 3 |
|---|---|---|---|---|---|---|
| OTU105 | Actinobacteria | Eggerthella; lenta | 0.38 | 0.096 | 0.000 | 0.042 |
| OTU8 | Actinobacteria | Bifidobacterium | 0.258 | 0.076 | 0.001 | 0.122 |
| OTU258 | Firmicutes | Lachnoclostridium | −0.293 | 0.095 | 0.003 | 0.200 |
| OTU45 | Actinobacteria | Bifidobacterium; animalis | 0.341 | 0.117 | 0.005 | 0.200 |
| OTU255 | Actinobacteria | Gordonibacter | 0.261 | 0.088 | 0.005 | 0.200 |
| OTU273 | Firmicutes | Lachnospiraceae_UCG-004 | −0.199 | 0.076 | 0.017 | 0.551 |
| OTU225 | Firmicutes | Ruminiclostridium_5 | −0.193 | 0.082 | 0.022 | 0.591 |
| OTU108 | Firmicutes | Blautia | 0.169 | 0.076 | 0.029 | 0.696 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, L.S.; Michels, K.B. Characterizing the Effects of Calcium and Prebiotic Fiber on Human Gut Microbiota Composition and Function Using a Randomized Crossover Design—A Feasibility Study. Nutrients 2021, 13, 1937. https://doi.org/10.3390/nu13061937
Yoon LS, Michels KB. Characterizing the Effects of Calcium and Prebiotic Fiber on Human Gut Microbiota Composition and Function Using a Randomized Crossover Design—A Feasibility Study. Nutrients. 2021; 13(6):1937. https://doi.org/10.3390/nu13061937
Chicago/Turabian StyleYoon, Lara S., and Karin B. Michels. 2021. "Characterizing the Effects of Calcium and Prebiotic Fiber on Human Gut Microbiota Composition and Function Using a Randomized Crossover Design—A Feasibility Study" Nutrients 13, no. 6: 1937. https://doi.org/10.3390/nu13061937
APA StyleYoon, L. S., & Michels, K. B. (2021). Characterizing the Effects of Calcium and Prebiotic Fiber on Human Gut Microbiota Composition and Function Using a Randomized Crossover Design—A Feasibility Study. Nutrients, 13(6), 1937. https://doi.org/10.3390/nu13061937






