Vitamin A Status Improvement in Obesity: Findings and Perspectives Using Encapsulation Techniques
Abstract
:1. Introduction
2. Vitamin A
3. Vitamin A Metabolism
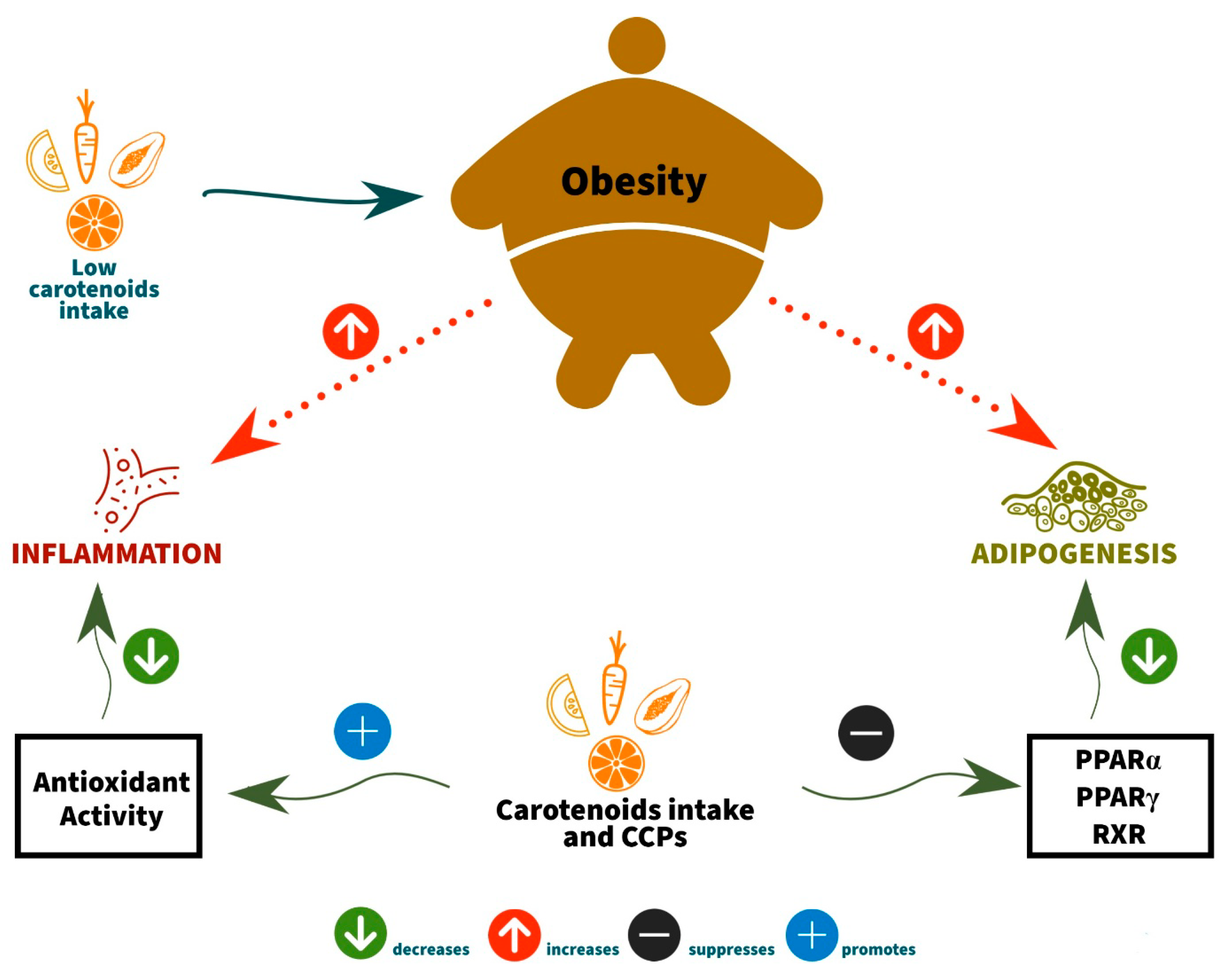
4. Association between Obesity, Vitamin A, and Carotenoids (Provitamin A Activity)
4.1. The Anti-Obesity Activities of Carotenoids and Carotenoid Converting Products (CCPs) by Inhibition of Adipocyte Differentiation
4.2. The Anti-Obesity Activities of Carotenoids and Carotenoid Converting Products (CCPs) by Antioxidant Capacity
5. Encapsulation Technology and Vitamin A
6. Prospects for Vitamin A Encapsulated in Food
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xiao, S.; Li, Q.; Hu, K.; He, Y.; Ai, Q.; Hu, L.; Yu, J. Vitamin A and Retinoic Acid Exhibit Protective Effects on Necrotizing Enterocolitis by Regulating Intestinal Flora and Enhancing the Intestinal Epithelial Barrier. Arch. Med. Res. 2018, 49, 1–9. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther. 2019, 197, 153–178. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Galmés, S.; Serra, F.; Palou, A. Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: Pre-clinical and human studies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158676. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, A.P.; Carvalho, R.; Jesus, P.; Bull, A.; Pereira, S.; Saboya, C.; Ramalho, A. Serum Antioxidant Associations with Metabolic Characteristics in Metabolically Healthy and Unhealthy Adolescents with Severe Obesity: An Observational Study. Nutrients 2018, 10, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, A.; Hoogerland, J.A.; Wessel, H.; Heegsma, J.; Derks, T.G.J.; Van Der Veer, E.; Mithieux, G.; Rajas, F.; Oosterveer, M.H.; Faber, K.N. Glycogen storage disease type 1a is associated with disturbed vitamin A metabolism and elevated serum retinol levels. Hum. Mol. Genet. 2020, 29, 264–273. [Google Scholar] [CrossRef]
- Bento, C.; Matos, A.C.; Cordeiro, A.; Ramalho, A. Vitamin A deficiency is associated with body mass index and body adiposity in women with recommended intake of vitamin A. Nutr. Hosp. 2018, 35, 1072–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Peng, R.; Cao, J.; Kang, Y.; Qu, P.; Liu, Y.; Xiao, X.; Li, T. Serum vitamin A status is associated with obesity and the metabolic syndrome among school-age children in Chongqing, China. Asia Pac. J. Clin. Nutr. 2016, 25, 563–570. [Google Scholar] [PubMed]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A. Chemistry, encapsulation, and health benefits of β-carotene—A review. Cogent Food Agric. 2015, 1, 1018696. [Google Scholar] [CrossRef]
- Banasaz, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Encapsulation of Lipid-Soluble Bioactives by Nanoemulsions. Molecules 2020, 25, 3966. [Google Scholar] [CrossRef]
- Zinder, R.; Cooley, R.; Vlad, L.G.; Molnar, J.A. Vitamin A and Wound. Nutr. Clin. Pract. 2019, 34, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.P.; Cabezuelo, M.T.; Torres, L.; Viña, J.R.; Barber, T. Vitamin A deficiency and the lung. Nutrients 2018, 10, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resende, D.; Lima, S.A.C.; Reis, S. Nanoencapsulation approaches for oral delivery of vitamin A. Colloids Surf. B Biointerfaces 2020, 193, 111121. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Amaya, D.B.R. Carotenoids and Food Preparation: The Retention of Provitamin A Carotenoids in Prepared, Processed, and Stored Foods; State University of Campinas, Department of Food Sciences: Campinas, Brazil, 1997; pp. 1–93. [Google Scholar]
- Corrêa-Filho, L.C.; Lourenço, M.M.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of β-Carotene by Spray Drying: Effect of Wall Material Concentration and Drying Inlet Temperature. Int. J. Food Sci. 2019, 2019, 8914852. [Google Scholar] [CrossRef] [Green Version]
- Battistoni, M.; Bacchetta, R.; Di Renzo, F.; Metruccio, F.; Menegola, E. Effect of nano-encapsulation of β-carotene on Xenopus laevis embryos development (FETAX). Toxicol. Rep. 2020, 7, 510–519. [Google Scholar] [CrossRef]
- Lundquist, P.; Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurya, V.K.; Aggarwal, M.; Ranjan, V.; Gothandam, K.M. Improving Bioavailability of Vitamin A in Food by Encapsulation: An Update. Nanosci. Med. 2020, 1, 117–145. [Google Scholar] [CrossRef]
- Sauvant, P.; Cansell, M.; Sassi, A.H.; Atgié, C. Vitamin A enrichment: Caution with encapsulation strategies used for food applications. Food Res. Int. 2012, 46, 469–479. [Google Scholar] [CrossRef]
- von Lintig, J.; Moon, J.; Lee, J.; Ramkumar, S. Carotenoid metabolism at the intestinal barrier. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158580. [Google Scholar] [CrossRef]
- Blaner, W.S.; Li, Y.; Brun, P.J.; Yuen, J.J.; Lee, S.A.; Clugston, R.D. Vitamin A Absorption, Storage and Mobilization; Department of Medicine, College of Physicians and Surgeons, Columbia University: New York, NY, USA, 2016; pp. 95–125. [Google Scholar] [CrossRef]
- Nishimoto, K.; Toya, Y.; Davis, C.R.; Tanumihardjo, S.A.; Welham, N.V. Dynamics of vitamin A uptake, storage, and utilization in vocal fold mucosa. Mol. Metab. 2020, 40, 101025. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Dullaart, R.P.F.; Schreuder, T.C.M.A.; Blokzijl, H.; Faber, K.N. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, D.C.; Noy, N. Signalling by vitamin A and retinol-binding protein in regulation of insulin responses and lipid homeostasis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Netto, M.P.; Priore, S.E.; Franceschini, S.C.C. Indicators of the nutritional state of vitamin A. J. Braz. Soc. Food Nutr. 2006, 31, 127–150. [Google Scholar]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Trasino, S.E.; Tang, X.-H.; Jessurun, J.; Gudas, L.J. Obesity Leads to Tissue, but not Serum Vitamin A Deficiency. Sci. Rep. 2015, 5, 15893. [Google Scholar] [CrossRef]
- Coronel, J.; Pinos, I.; Amengual, J. β-carotene in Obesity Research: Technical Considerations and Current Status of the Field. Nutrients 2019, 11, 842. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.P.; Coronel, J.; Amengual, J. The role of β-carotene and vitamin A in atherogenesis: Evidences from preclinical and clinical studies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158635. [Google Scholar] [CrossRef] [PubMed]
- Mounien, L.; Tourniaire, F.; Landrier, J.-F. Anti-Obesity Effect of Carotenoids: Direct Impact on Adipose Tissue and Adipose Tissue-Driven Indirect Effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef] [Green Version]
- Harari, A.; Coster, A.C.F.; Jenkins, A.; Xu, A.; Greenfield, J.R.; Harats, D.; Shaish, A.; Bonet, D.S. Obesity and Insulin Resistance Are Inversely Associated with Serum and Adipose Tissue Carotenoid Concentrations in Adults. J. Nutr. 2020, 150, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Brun, P.J.; Grijalva, A.; Rausch, R.; Watson, E.; Yuen, J.J.; Das, B.C.; Shudo, K.; Kagechika, H.; Leibel, R.L.; Blaner, W.S. Retinoic acid receptor signaling is required to maintain glucose-stimulated insulin secretion and β-cell mass. FASEB J. 2015, 29, 671–683. [Google Scholar] [CrossRef] [Green Version]
- Roohbakhsh, A.; Karimi, G.; Iranshahi, M. Carotenoids in the treatment of diabetes mellitus and its complications: A mechanistic review. Biomed. Pharmacother. 2017, 91, 31–42. [Google Scholar] [CrossRef]
- Östh, M.; Öst, A.; Kjolhede, P.; Stralfors, P. The concentration of β-carotene in human adipocytes, but not the whole-body adipocyte stores, is reduced in obesity. PLoS ONE 2014, 9, e85610. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Chen, X.; Jha, K.; Beydoun, H.A.; Zonderman, A.B.; Canas, J.A. Carotenoids, vitamin A, and their association with the metabolic syndrome: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 32–45. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, J.; Zhang, Y.; Tang, J.; Sun, B.; Xu, W.; Wang, X.; Chen, Y.; Sun, Z. Changes in Intestinal Microbiota Are Associated with Islet Function in a Mouse Model of Dietary Vitamin A Deficiency. J. Diabetes Res. 2020, 2020, 2354108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godala, M.; Materek-Kuśmierkiewicz, I.; Moczulski, D.; Rutkowski, M.; Szatko, F.; Gaszyńska, E.; Tokarski, S.; Kowalski, J. The risk of plasma vitamin A, C, E and D deficiency in patients with metabolic syndrome: A case-control study. Adv. Clin. Exp. Med. 2017, 26, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Kuang, H.; Wei, C.H.; Wang, T.; Eastep, J.; Li, Y.; Chen, G. Vitamin A Status Affects Weight Gain and Hepatic Glucose Metabolism in Rats Fed a High-Fat Diet. Biochem. Cell Biol. 2019, 97, 545–553. [Google Scholar] [CrossRef]
- Mody, N. Alterations in vitamin A/retinoic acid homeostasis in diet-induced obesity and insulin resistance. Proc. Nutr. Soc. 2017, 76, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.P.; Andrade, L.D.A.; Flôres, S.H.; Rios, A.D.O. Nanoencapsulation of carotenoids: A focus on different delivery systems and evaluation parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef] [Green Version]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Melo, R.L.F.; Souza, I.C.C.; Carvalho, A.J.R.; Bezerra, E.M.; Costa, R.F. Nanoparticles as biological tools: An exploratory review. Res. Soc. Dev. 2020, 9, 1–18. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Zhang, Y.; Xiong, H.; Wang, F.; Wang, Y.; Lu, Z. Effects of starch and gelatin encapsulated vitamin A on growth performance, immune status and antioxidant capacity in weaned piglets. Anim. Nutr. 2020, 6, 130–133. [Google Scholar] [CrossRef]
- Rovoli, M.; Pappas, I.; Lalas, S.; Gortzi, O.; Kontopidis, G. In vitro and in vivo assessment of vitamin A encapsulation in a liposome-protein delivery system. J. Liposome Res. 2018, 29, 142–152. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, H.M.C. Encapsulação: Aplicação à tecnologia de alimentos. Alim. Nutr. 2005, 16, 89–97. [Google Scholar]
- Sachaniya, J.; Savaliya, R.; Goyal, R.; Singh, S. Liposomal formulation of vitamin A for the potential treatment of osteoporosis. Int. J. Nanomed. 2018, 13, 51–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, R.; Shoemaker, C.F.; Yang, X.; Zhong, F.; Huang, Q. Stability and Bioaccessibility of β-Carotene in Nanoemulsions Stabilized by Modified Starches. J. Agric. Food Chem. 2013, 61, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.; Sugahara, L.Y.; Leimann, F.V.; Oliveira, S.; Brum, E.D.S.; Calhelha, R.C.; Barreiro, M.F.; Ferreira, I.C.F.R.; Ineu, R.P.; Gonçalves, O.H. Nanodispersions of β-carotene: Effects on antioxidant enzymes and cytotoxic properties. Food Funct. 2018, 9, 3698–3706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, E.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of thermal and UV-light stability of β-carotene-loaded nanoemulsions by water-soluble chitosan coating. Int. J. Biol. Macromol. 2020, 165, 1156–1163. [Google Scholar] [CrossRef]
- Medeiros, A.K.D.O.C.; Gomes, C.D.C.; Amaral, M.L.Q.D.A.; De Medeiros, L.D.G.; Medeiros, I.; Porto, D.L.; Aragão, C.F.S.; Maciel, B.L.L.; Morais, A.H.D.A.; Passos, T.S. Nanoencapsulation improved water solubility and color stability of carotenoids extracted from Cantaloupe melon (Cucumis melo L.). Food Chem. 2019, 270, 562–572. [Google Scholar] [CrossRef]
- de Oliveira, G.L.R.; Medeiros, I.; Nascimento, S.S.D.C.; Viana, R.L.S.; Porto, D.L.; Rocha, H.A.O.; Aragão, C.F.S.; Maciel, B.L.L.; de Assis, C.F.; Morais, A.H.D.A.; et al. Antioxidant stability enhancement of carotenoid rich-extract from Cantaloupe melon (Cucumis melo L.) nanoencapsulated in gelatin under different storage conditions. Food Chem. 2021, 348, 129055. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, Y.; Chen, L. Intestinal uptake of barley protein-based nanoparticles for β-carotene delivery. Acta Pharm. Sin. B 2019, 9, 87–96. [Google Scholar] [CrossRef]
- Liu, X.; Wanga, P.; Zoub, Y.X.; Luoa, Z.G.; Tamer, T.M. Co-encapsulation of Vitamin C and β-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef]
- Aditya, N.P.; Ko, S. Solid lipid nanoparticles (SLNs): Delivery vehicles for food bioactives. RSC Adv. 2015, 5, 30902–30911. [Google Scholar] [CrossRef]
- Salah, E.; Abouelfetou, M.M.; Pana, Y.; Chena, D.; Xie, S. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloids Surf. B Biointerfaces 2020, 196, 111305. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Long, P.; Zhou, J.; Ho, C.-T.; Zou, X.; Chen, B.; Zhang, L. Improved absorption of β-carotene by encapsulation in an oil-in-water nanoemulsion containing tea polyphenols in the aqueous phase. Food Res. Int. 2019, 116, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhou, Y.; Bai, L.; Liu, F.; Deng, Y.; McClements, D.J. Fabrication of β-carotene nanoemulsion-based delivery systems using dual-channel microfluidization: Physical and chemical stability. J. Colloid Interface Sci. 2017, 490, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Miyazawa, T.; Harigae, T.; Onuma, R.; Kimura, F.; Fujii, T.; Miyazawa, T. Distribution of β-carotene-encapsulated polysorbate 80-coated poly(d, l-lactide-co-glycolide) nanoparticles in rodent tissues following intravenous administration. Int. J. Nanomed. 2015, 10, 7223–7230. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; McClements, D.J.; Xiao, H. Improving oral bioavailability of nutraceuticals by engineered nanoparticle-based delivery systems. Curr. Opin. Food Sci. 2015, 2, 14–19. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
| Reference | Study Type | Objectives | Findings Related to Obesity |
|---|---|---|---|
| Östh et al. (2014) [36] | Clinical | Evaluate clinical parameters such as triglycerides, total cholesterol, HDL/LDL, fasting glucose and insulin, β-carotene concentration in isolated subcutaneous abdominal adipocytes obtained from eutrophic, overweight, obese, and obese humans with type 2 diabetes. | It was observed that the concentration of β-carotene was 50% lower in the adipocytes of obese and obese diabetic groups in comparison with the eutrophic and overweight groups. Thus, the concentration of β-carotene in adipocytes appears to be inversely related to the nutritional status of obesity. |
| Trasino et al. (2015) [29] | Ex-vivo and pre-clinical | Analyze the histology of frozen human liver and mice to demonstrate that even with adequate vitamin A in the diet, obesity dramatically reduces vitamin A levels and signaling in major organs. | Obese mice (induced by eating a high-fat diet or genetic mutations) drastically reduced retinol levels in various organs. Organs from obese rats show impaired vitamin A transcriptional signaling, including reductions in retinoic acid receptor mRNAs and lower levels of intracellular retinol-binding protein Crbp1 (RBP1) in vitamin storage in stellate cells. Reductions in vitamin A signaling in the organs of obese mice correlate with increased adiposity and fatty liver. |
| Wei et al. (2016) [7] | Cross-sectional | Examine the association of vitamin A status with obesity and metabolic syndrome (MS) in school-aged children, assessing body height, weight, waist circumference, blood pressure, blood glucose, and lipids. | The serum vitamin A levels in the obese group were significantly lower than the overweight and eutrophic group (p < 0.05). BMI, waist circumference, and fasting glucose were significantly higher in the group with low vitamin A concentration (p < 0.05). As a result, vitamin A deficiency was significantly associated with metabolic syndrome (p < 0.05). |
| Godala et al. (2017) [39] | Case-control study | Assess the risk of vitamin A, C, E, and D deficiency in the plasma of patients with multiple sclerosis. | The results showed that plasma vitamin A levels were significantly lower (p < 0.05) in patients with metabolic syndrome. They also showed that hyperglycemia increased the risk of vitamin A deficiency. |
| Stenzel et al. (2018) [4] | Observational | Investigate the relationship between the state of antioxidant micronutrients and the components of metabolic syndrome in metabolically healthy (MH) and unhealthy (MU) obesity. | The results showed that obese MU adolescents have a significant inverse association between vitamin A and waist circumference. |
| Harari et al. (2020) [33] | Cohort | Evaluate the relationships between adipose tissue and serum carotenoids with body fat, abdominal fat distribution, muscle insulin resistance, adipose and liver tissue, and food intake. In addition, evaluate the relationships and distributions of carotenoids detected in adipose tissue and serum compared to serum carotenoids and retinol concentrations in individuals with and without obesity. | All serum carotenoid concentrations were significantly lower (p < 0.05) in samples collected from individuals with obesity compared with eutrophic individuals. Similar to serum carotenoids, most carotenoids in adipose tissue were inversely related to anthropometric measurements (weight, BMI, waist circumference, and total and central body fat). Total carotenoids, β-carotene, and α-carotene were inversely associated with fasting insulin and HOMA-IR. The insulin resistance of the adipose tissue correlated inversely with the total concentration of carotenoids. |
| Reference | Objective | Encapsulation Technique | Materials | PD | EE (%) | Functionalities Associated | Related Mechanisms |
|---|---|---|---|---|---|---|---|
| Liu et al. (2019) [56] | Investigate the potential of particles in improving the absorption of active compounds such as β-carotene, using Caco-2 cells in vitro and small intestine ex vivo. | Emulsification oil in water (O/W) | β-carotene, barley protein, canola oil. | 351 nm | 90.7% | Uptake is dependent on time, concentration, and temperature. The uptake and transport of encapsulated β-carotene were higher (p < 0.05) (15%) compared to the free form (2.6%). There was also a greater capacity for retention and permeation in the intestinal tissue. | The particles could more easily enter Caco-2 cells through endocytosis. Therefore, the particle size is inversely proportional to the presence of β-carotene in the cell monolayers. |
| Rocha et al. (2018) [52] | Evaluate the effects of particles containing β-carotene on the activity of the enzymes glutathione-S-transferase (GST) and acetylcholinesterase (AChE) (Drosophila melanogaster homogenate), superoxide dismutase (SOD), and catalase and the cytotoxic properties in tumor cell lines and not tumoral. | Solid dispersion | Tween-80, β-carotene and polyvinylpyrrolidone (PVP). | 337 nm | - | High dispersion in water and modulating AChE, presenting a high potential for controlling the cholinergic system. In low concentrations, particles showed mimetic activity in vitro for SOD and altered activity for GST. Particles also showed activity against four different tumor cell lines. | The performance of the carotenoid encapsulated in aqueous medium was significantly improved due to the reduction of the particle size, allowing enhancement of its biological activity. |
| Liu et al. (2020) [57] | Encapsulate vitamin C and β-carotene in liposomes (L-VC-βC) and evaluate structural characteristics, stability (4 °C and 25 °C), antioxidant activity, in vitro gastrointestinal digestion, and release kinetics. | Liposomes | Egg yolk lecithin, cholesterol, β-carotene, and vitamin C. | ~250 nm | ~98% | The storage stability of L-VC-βC was higher (p < 0.05) compared to L-βC. In gastrointestinal digestion, the carotenoid present in the two liposomes was released slowly, with more than 70% being released in the simulated gastric fluid. | In the gastric phase, the release of βC was proportional to the time of contact with the gastric fluid. In the intestinal phase, expanded, cracked, and fragmented liposomes were observed, possibly due to contact with bile salts, or due to increased membrane fluidity due to the permeation of bile salts, resulting in high adsorption of lipases. |
| Resende et al. (2020) [13] | Develop and characterize lipid particles containing vitamin A for food fortification, ensuring oral stability and bioaccessibility. | Organic solvent-free sonication method | Tween 80, vitamin A (VA), Gelucire (GEL), stearic acid (SA) and oleic acid (OA), Miglyol (MIG). | 228 nm to 612 nm | 5% to 97% | In vitro tests simulating gastrointestinal digestion showed that the particles were not altered in the stomach and that the biocompatibility of the formulations did not show toxicity in fibroblasts. With the developed particles, 80% of the added vitamin reached the intestine in the digestibility test. | After two hours, the size remained unchanged, showing stability of the particles in the stomach. In the intestine, bile salts, together with pancreatic lipases, can promote the digestion of the di- and triacylglycerols that make up the particles. Loss of structure and lipid aggregation can occur, as indicated by the increase in particle diameter. |
| Baek et al. (2020) [53] | Obtain nanoemulsions containing β-carotene (BC-NEs) and coated with water-soluble chitosan (WSC-BC-NEs) to improve the stability of β-carotene against high temperature and ultraviolet light. | Emulsification O/W | Medium chain triglyceride (MCT) oil, Tween 80, lecithin, β-carotene (BC), and chitosan. | BC-NEs = 64 nm. WSC-BC-NEs = 218 nm. | - | WSC-BC-NEs (2% chitosan) increased the thermal and ultraviolet stability of the encapsulated BC compared to BC-NEs. After 21 days of storage at 37 °C, WSC-BC-NEs preserved about 96.7% of β-carotene and 77.6% in UV light exposure (253 nm) at room temperature. | BC was dispersed in a lipid, which can be surrounded by additional protective layers. The increased stability can be explained by the limited interaction between the BC contained in the droplet nucleus and oxygen and radicals in the environment, due to the physical barrier provided by the wall material. |
| Medeiros et al. (2019) [54] | Produce nanoparticles based on carotenoids from cantaloupe melon pulp (Cucumis melo L.) rich in β-carotene (CE), and evaluate water dispersion and color stability in yogurt for 60 days of storage. | Emulsification O/W | CE, soybean oil, Tween 20, porcine gelatine (EPG), whey protein concentrate (EWPC), and isolated (EWPI). | EWPC = 123 nm EWPI = 161 nm EPG = 59.3 nm | EWPC = 77% EWPI = 58% EPG = 90% | EPG showed an increase in water solubility by about 267% compared to the crude extract. EPG added to yogurt simulated yellow fruit-flavored yogurt coloration and promoted high color stability and homogeneity compared to the CE. | The smallest particle size of EPG associated to the high chemical interaction between the CE and porcine gelatin justify the increase in water solubility and color stability. |
| Oliveira et al. (2021) [55] | Evaluate if the nanoencapsulation of the carotenoids of cantaloupe melon pulp (Cucumis melo L.) rich in β-carotene (CE) promoted the preservation or enhancement of the antioxidant potential when stored at different conditions (light and temperature). | Emulsification O/W | CE, soybean oil, Tween 20, porcine gelatin (EPG). | 90.9 nm | 94.80% | Carotenoid antioxidant activity increased after nanoencapsulation in porcine gelatin (57–59%). After 60 days, EPG preserved the β-carotene in the light (83.1%) and dark at 25 °C (99.0%) and in the dark (99.0%) at 5 °C, maintaining the antioxidant potential (68.7–48.3%) compared to CE. | The chemical interaction between carotenoid-protein may be related to the increase in water solubility. In addition, the photoprotective effects (absorption/dissipation of energy or extinction of reactive oxygen species) promote increased activity and antioxidant stability of carotenoids. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, C.d.C.; Passos, T.S.; Morais, A.H.A. Vitamin A Status Improvement in Obesity: Findings and Perspectives Using Encapsulation Techniques. Nutrients 2021, 13, 1921. https://doi.org/10.3390/nu13061921
Gomes CdC, Passos TS, Morais AHA. Vitamin A Status Improvement in Obesity: Findings and Perspectives Using Encapsulation Techniques. Nutrients. 2021; 13(6):1921. https://doi.org/10.3390/nu13061921
Chicago/Turabian StyleGomes, Camila de Carvalho, Thais Souza Passos, and Ana Heloneida Araújo Morais. 2021. "Vitamin A Status Improvement in Obesity: Findings and Perspectives Using Encapsulation Techniques" Nutrients 13, no. 6: 1921. https://doi.org/10.3390/nu13061921
APA StyleGomes, C. d. C., Passos, T. S., & Morais, A. H. A. (2021). Vitamin A Status Improvement in Obesity: Findings and Perspectives Using Encapsulation Techniques. Nutrients, 13(6), 1921. https://doi.org/10.3390/nu13061921






