Meta-Analysis Examining the Importance of Creatine Ingestion Strategies on Lean Tissue Mass and Strength in Older Adults
Abstract
1. Introduction
2. Materials and Methods
Sub-Analyses
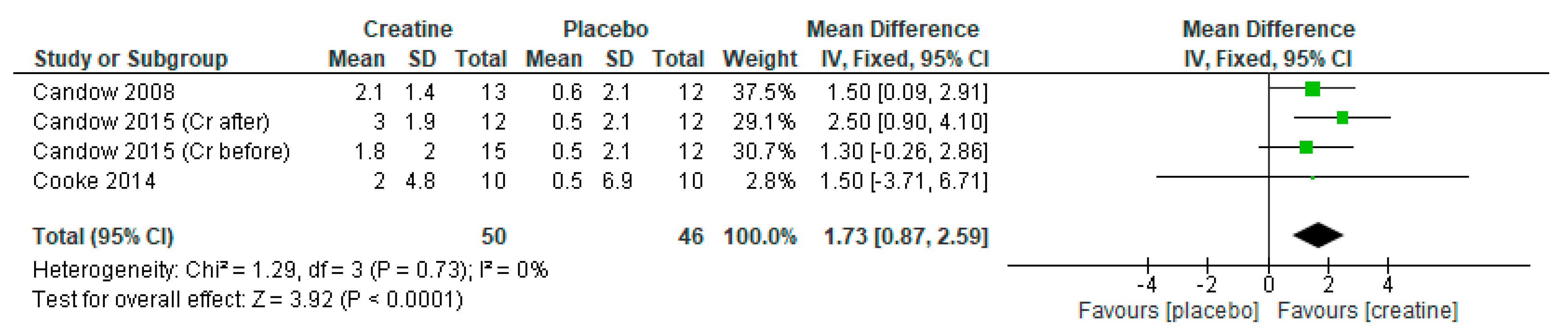
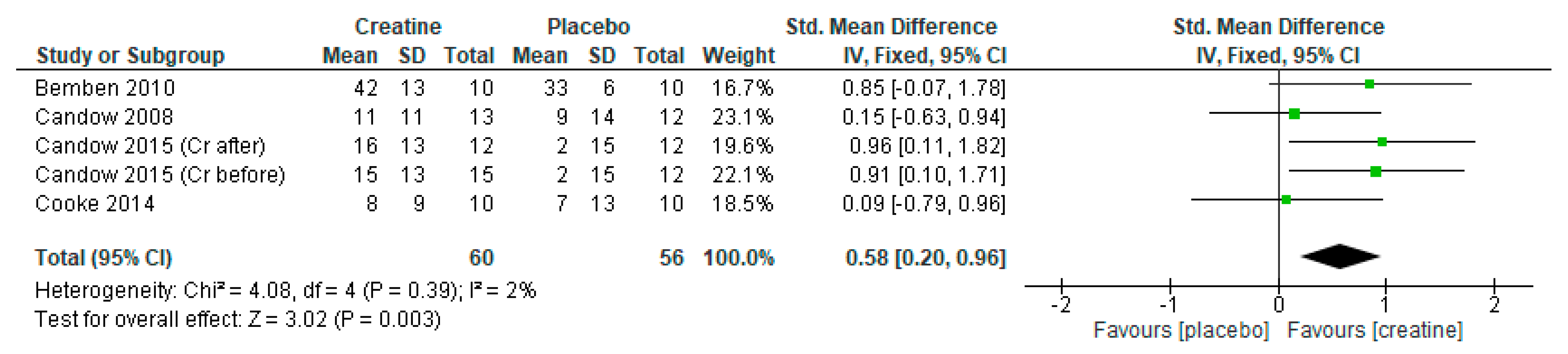
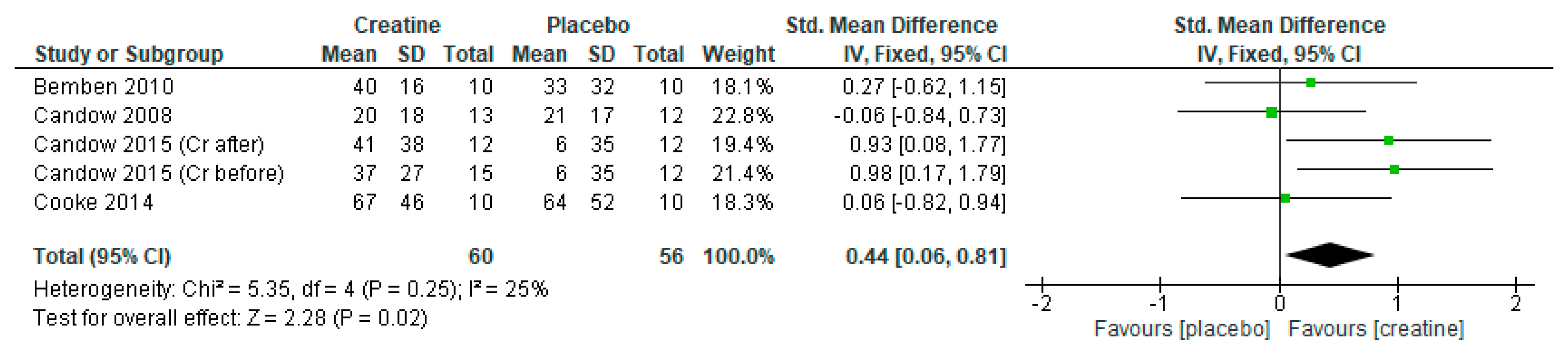
3. Results
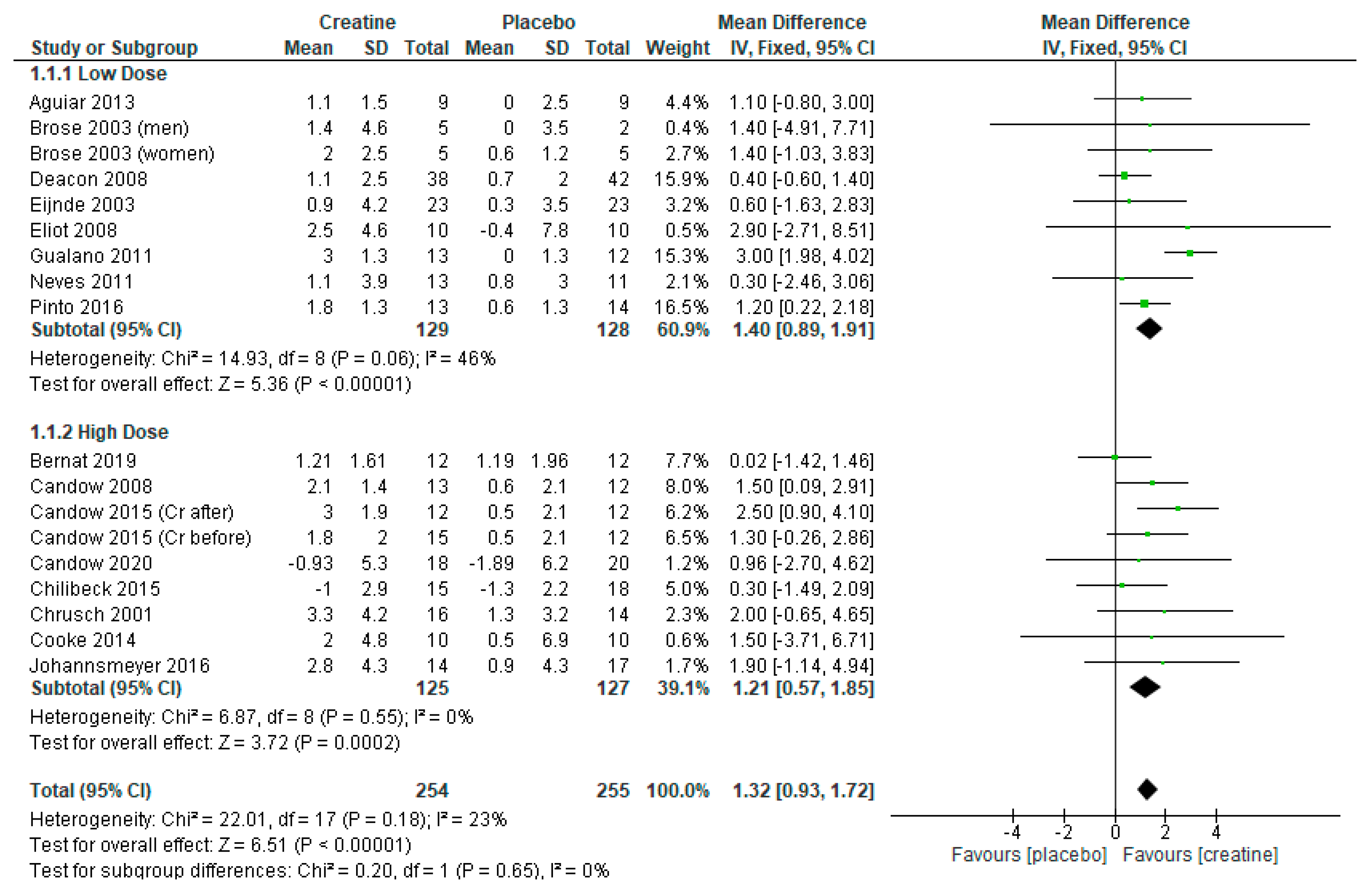
3.1. Lean Tissue Mass
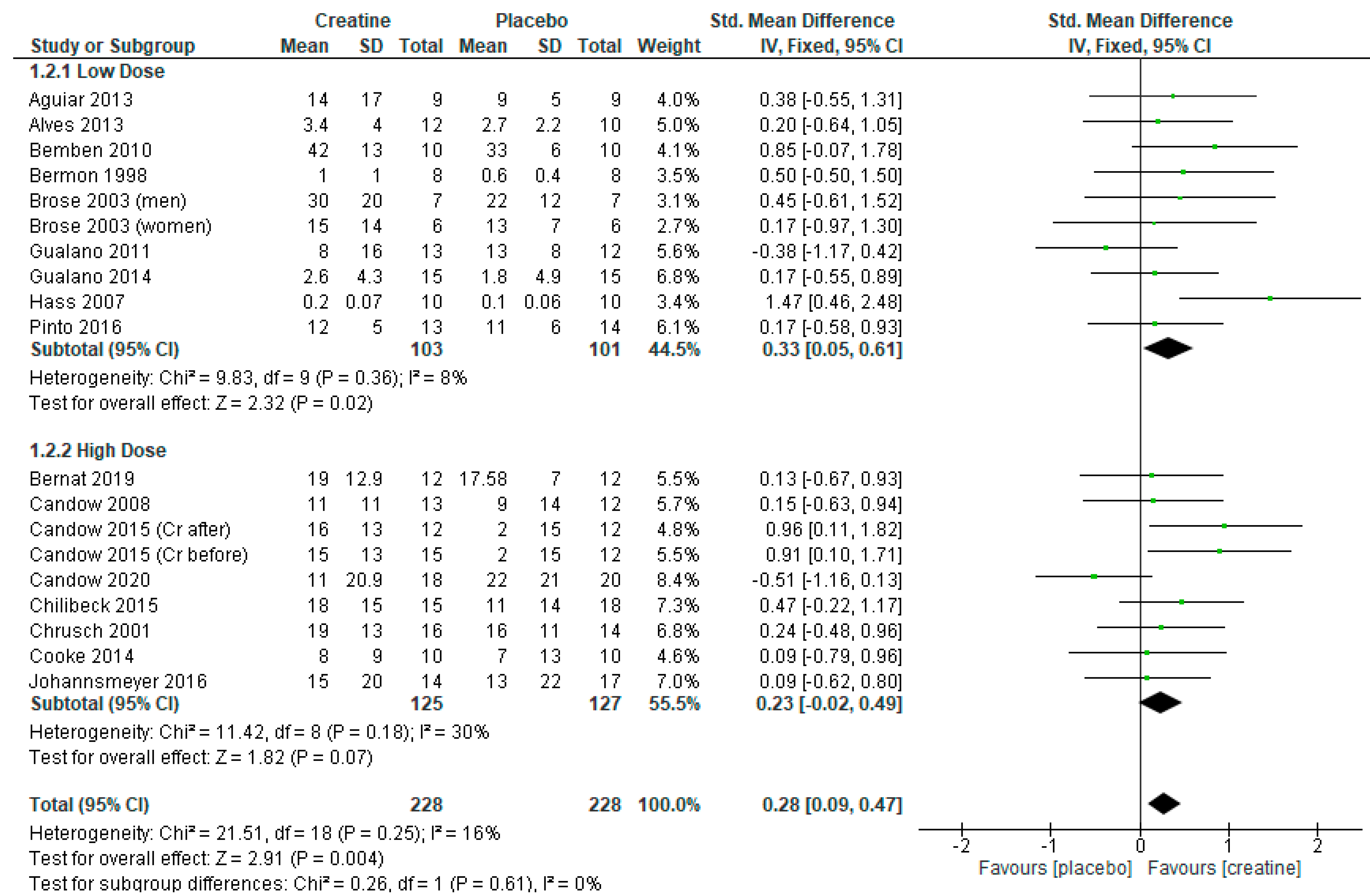
3.2. Chest Press Strength
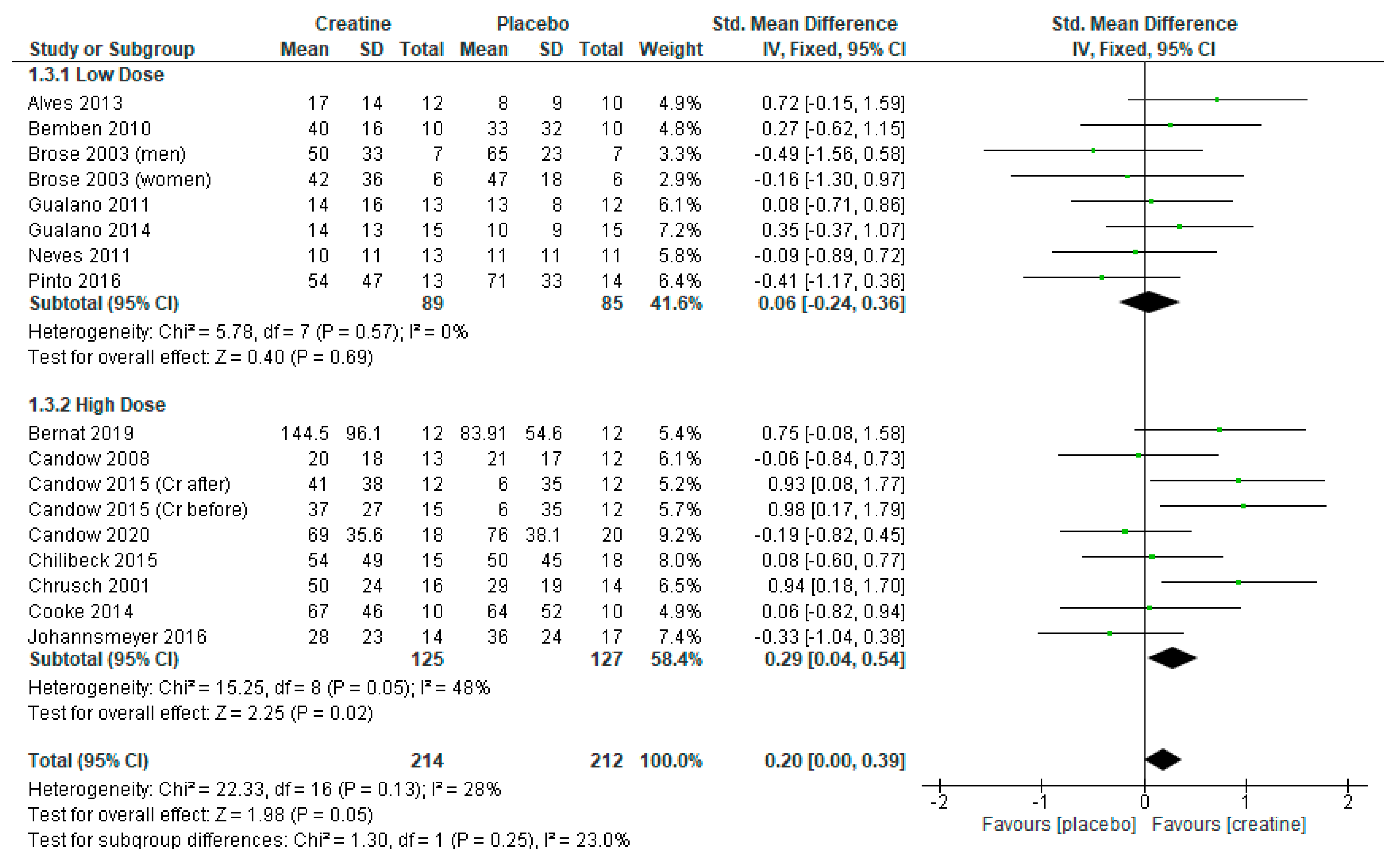
3.3. Leg Press Strength
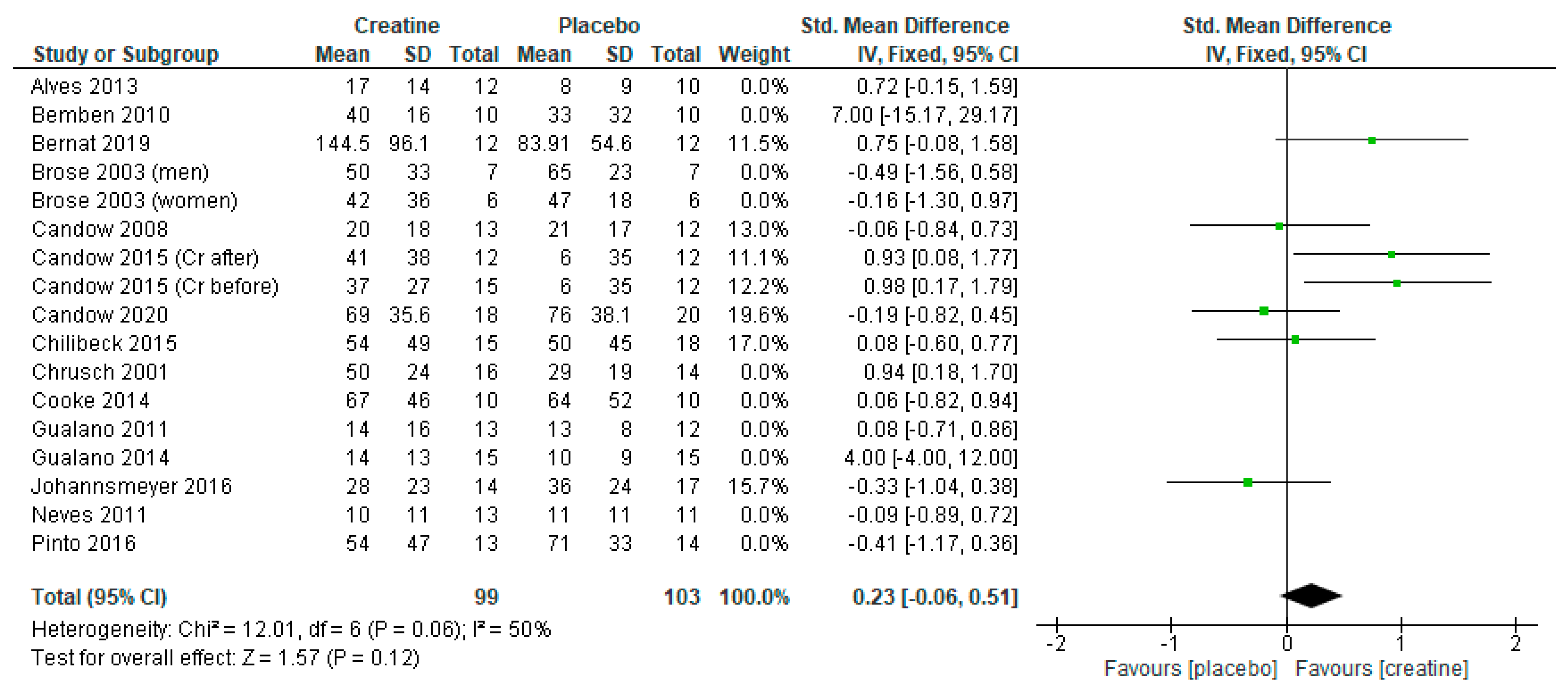
3.4. Creatine Only on Training Days
3.5. Publication Bias
3.6. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Kirk, B.; Duque, G. Current Evidence and Possible Future Applications of Creatine Supplementation for Older Adults. Nutrients 2021, 13, 745. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J. Clin. Med. 2019, 8, 488. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Prokopidis, K.; Duque, G. Nutrients to mitigate osteosarcopenia: The role of protein, vitamin D and calcium. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 25–32. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Creatine: Endogenous metabolite, dietary, and therapeutic supplement. Annu. Rev. Nutr. 2007, 27, 241–261. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Chilibeck, P.D.; Forbes, S.C. Creatine supplementation and aging musculoskeletal health. Endocrine 2014, 45, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Variables Influencing the Effectiveness of Creatine Supplementation as a Therapeutic Intervention for Sarcopenia. Front. Nutr. 2019, 6, 124. [Google Scholar] [CrossRef]
- Alves, C.R.; Merege Filho, C.A.; Benatti, F.B.; Brucki, S.; Pereira, R.M.; de Sa Pinto, A.L.; Lima, F.R.; Roschel, H.; Gualano, B. Creatine supplementation associated or not with strength training upon emotional and cognitive measures in older women: A randomized double-blind study. PLoS ONE 2013, 8, e76301. [Google Scholar] [CrossRef]
- Aguiar, A.F.; Januario, R.S.; Junior, R.P.; Gerage, A.M.; Pina, F.L.; do Nascimento, M.A.; Padovani, C.R.; Cyrino, E.S. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2013, 113, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Bemben, M.G.; Witten, M.S.; Carter, J.M.; Eliot, K.A.; Knehans, A.W.; Bemben, D.A. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J. Nutr. Health Aging 2010, 14, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Eliot, K.A.; Knehans, A.W.; Bemben, D.A.; Witten, M.S.; Carter, J.; Bemben, M.G. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J. Nutr. Health Aging 2008, 12, 208–212. [Google Scholar] [CrossRef]
- Bermon, S.; Venembre, P.; Sachet, C.; Valour, S.; Dolisi, C. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol. Scand. 1998, 164, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Brose, A.; Parise, G.; Tarnopolsky, M.A. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Deacon, S.J.; Vincent, E.E.; Greenhaff, P.L.; Fox, J.; Steiner, M.C.; Singh, S.J.; Morgan, M.D. Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 178, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Eijnde, B.O.; Van Leemputte, M.; Goris, M.; Labarque, V.; Taes, Y.; Verbessem, P.; Vanhees, L.; Ramaekers, M.; Vanden Eynde, B.; Van Schuylenbergh, R.; et al. Effects of creatine supplementation and exercise training on fitness in men 55–75 yr old. J. Appl. Physiol. 2003, 95, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; DE Salles Painneli, V.; Roschel, H.; Artioli, G.G.; Neves, M.; De Sa Pinto, A.L.; Da Silva, M.E.; Cunha, M.R.; Otaduy, M.C.; Leite Cda, C.; et al. Creatine in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Med. Sci. Sports Exerc. 2011, 43, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Macedo, A.R.; Alves, C.R.; Roschel, H.; Benatti, F.B.; Takayama, L.; de Sa Pinto, A.L.; Lima, F.R.; Pereira, R.M. Creatine supplementation and resistance training in vulnerable older women: A randomized double-blind placebo-controlled clinical trial. Exp. Gerontol. 2014, 53, 7–15. [Google Scholar] [CrossRef]
- Hass, C.J.; Collins, M.A.; Juncos, J.L. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: A randomized trial. Neurorehabil. Neural Repair 2007, 21, 107–115. [Google Scholar] [CrossRef]
- Neves, M.; Gualano, B.; Roschel, H.; Fuller, R.; Benatti, F.B.; Pinto, A.L.; Lima, F.R.; Pereira, R.M.; Lancha, A.H.; Bonfa, E. Beneficial effect of creatine supplementation in knee osteoarthritis. Med. Sci. Sports Exerc. 2011, 43, 1538–1543. [Google Scholar] [CrossRef]
- Pinto, C.L.; Botelho, P.B.; Carneiro, J.A.; Mota, J.F. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J. Cachexia Sarcopenia Muscle 2016, 7, 413–421. [Google Scholar] [CrossRef]
- Bernat, P.; Candow, D.G.; Gryzb, K.; Butchart, S.; Schoenfeld, B.J.; Bruno, P. Effects of high-velocity resistance training and creatine supplementation in untrained healthy aging males. Appl. Physiol. Nutr. Metab. 2019, 44, 1246–1253. [Google Scholar] [CrossRef]
- Candow, D.G.; Little, J.P.; Chilibeck, P.D.; Abeysekara, S.; Zello, G.A.; Kazachkov, M.; Cornish, S.M.; Yu, P.H. Low-dose creatine combined with protein during resistance training in older men. Med. Sci. Sports Exerc. 2008, 40, 1645–1652. [Google Scholar] [CrossRef]
- Candow, D.G.; Vogt, E.; Johannsmeyer, S.; Forbes, S.C.; Farthing, J.P. Strategic creatine supplementation and resistance training in healthy older adults. Appl. Physiol. Nutr. Metab. 2015, 40, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Chilibeck, P.D.; Gordon, J.; Vogt, E.; Landeryou, T.; Kaviani, M.; Paus-Jensen, L. Effect of 12 months of creatine supplementation and whole-body resistance training on measures of bone, muscle and strength in older males. Nutr. Health 2020. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Candow, D.G.; Landeryou, T.; Kaviani, M.; Paus-Jenssen, L. Effects of Creatine and Resistance Training on Bone Health in Postmenopausal Women. Med. Sci. Sports Exerc. 2015, 47, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Chrusch, M.J.; Chilibeck, P.D.; Chad, K.E.; Davison, K.S.; Burke, D.G. Creatine supplementation combined with resistance training in older men. Med. Sci. Sports Exerc. 2001, 33, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.B.; Brabham, B.; Buford, T.W.; Shelmadine, B.D.; McPheeters, M.; Hudson, G.M.; Stathis, C.; Greenwood, M.; Kreider, R.; Willoughby, D.S. Creatine supplementation post-exercise does not enhance training-induced adaptations in middle to older aged males. Eur. J. Appl. Physiol. 2014, 114, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Johannsmeyer, S.; Candow, D.G.; Brahms, C.M.; Michel, D.; Zello, G.A. Effect of creatine supplementation and drop-set resistance training in untrained aging adults. Exp. Gerontol. 2016, 83, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Chilibeck, P.D.; Kaviani, M.; Candow, D.G.; Zello, G.A. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: A meta-analysis. Open Access J. Sports Med. 2017, 8, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Creatine supplementation during resistance training in older adults-a meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.H.; Deng, Y. Potential cytotoxic effect of chronic administration of creatine, a nutrition supplement to augment athletic performance. Med. Hypotheses 2000, 54, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef]
- Dalbo, V.J.; Roberts, M.D.; Stout, J.R.; Kerksick, C.M. Putting to rest the myth of creatine supplementation leading to muscle cramps and dehydration. Br. J. Sports Med. 2008, 42, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Ju, J.S.; Smith, J.L.; Oppelt, P.J.; Fisher, J.S. Creatine feeding increases GLUT4 expression in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 347. [Google Scholar] [CrossRef][Green Version]
- Roberts, P.A.; Fox, J.; Peirce, N.; Jones, S.W.; Casey, A.; Greenhaff, P.L. Creatine ingestion augments dietary carbohydrate mediated muscle glycogen supercompensation during the initial 24 h of recovery following prolonged exhaustive exercise in humans. Amino Acids 2016, 48, 1831–1842. [Google Scholar] [CrossRef]
- Burke, D.G.; Candow, D.G.; Chilibeck, P.D.; MacNeil, L.G.; Roy, B.D.; Tarnopolsky, M.A.; Ziegenfuss, T. Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 389–398. [Google Scholar] [CrossRef]
- Safdar, A.; Yardley, N.J.; Snow, R.; Melov, S.; Tarnopolsky, M.A. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol. Genom. 2008, 32, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Bassit, R.A.; Curi, R.; Costa Rosa, L.F. Creatine supplementation reduces plasma levels of pro-inflammatory cytokines and PGE2 after a half-ironman competition. Amino Acids 2008, 35, 425–431. [Google Scholar] [CrossRef]
- Santos, R.V.; Bassit, R.A.; Caperuto, E.C.; Costa Rosa, L.F. The effect of creatine supplementation upon inflammatory and muscle soreness markers after a 30km race. Life Sci. 2004, 75, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.L.; Ferreira, A.P.; Silva, L.F.; Hoffmann, M.S.; Dutra, F.D.; Furian, A.F.; Oliveira, M.S.; Fighera, M.R.; Royes, L.F. Creatine reduces oxidative stress markers but does not protect against seizure susceptibility after severe traumatic brain injury. Brain Res. Bull. 2012, 87, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R. Creatine supplementation decreases oxidative DNA damage and lipid peroxidation induced by a single bout of resistance exercise. J. Strength Cond. Res. 2011, 25, 3448–3455. [Google Scholar] [CrossRef] [PubMed]
- Deminice, R.; Rosa, F.T.; Franco, G.S.; Jordao, A.A.; de Freitas, E.C. Effects of creatine supplementation on oxidative stress and inflammatory markers after repeated-sprint exercise in humans. Nutrition 2013, 29, 1127–1132. [Google Scholar] [CrossRef]
- Harris, R.C.; Soderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Syrotuik, D.G.; Bell, G.J. Acute creatine monohydrate supplementation: A descriptive physiological profile of responders vs. nonresponders. J. Strength Cond. Res. 2004, 18, 610–617. [Google Scholar] [PubMed]
- Candow, D.G.; Chilibeck, P.D. Differences in size, strength, and power of upper and lower body muscle groups in young and older men. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 148–156. [Google Scholar] [CrossRef]
- Robinson, T.M.; Sewell, D.A.; Hultman, E.; Greenhaff, P.L. Role of submaximal exercise in promoting creatine and glycogen accumulation in human skeletal muscle. J. Appl. Physiol. 1999, 87, 598–604. [Google Scholar] [CrossRef]
- Persky, A.M.; Brazeau, G.A.; Hochhaus, G. Pharmacokinetics of the dietary supplement creatine. Clin. Pharmacokinet. 2003, 42, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Candow, D.G. Timing of creatine supplementation and resistance training: A brief review. J. Exerc. Nutr. 2018, 1, 1. [Google Scholar]
- Ostojic, S.M.; Korovljev, D.; Stajer, V. Dietary creatine and cognitive function in U.S. adults aged 60 years and over. Aging Clin. Exp. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Candow, D.G.; Ferreira, L.H.B.; Souza-Junior, T.P. Effects of Creatine Supplementation on Properties of Muscle, Bone, and Brain Function in Older Adults: A Narrative Review. J. Diet. Suppl. 2021, 1–18. [Google Scholar] [CrossRef]
- Moon, A.; Heywood, L.; Rutherford, S.; Cobbold, C. Creatine supplementation: Can it improve quality of life in the elderly without associated resistance training? Curr. Aging Sci. 2013, 6, 251–257. [Google Scholar] [CrossRef]

| First Author, Year | Population | Supplement Protocol | Resistance Training | Duration | Outcomes | |
|---|---|---|---|---|---|---|
| Loading Protocol | Maintenance Dose | |||||
| Lower-Dose/Absolute Studies (≤5 g/day) | ||||||
| Alves et al. [11] | N = 47; healthy women, Mean age = 66.8 years (range: 60–80 years) | CR 20 g/day for 5 days | CR (5 g/day) or PLA | RT = 2 days/wk | 24 wks | ↔ 1RM strength compared to RT + PLA |
| Aguiar et al. [12] | N = 18; healthy women; Mean age = 65 years | None | CR (5 g/day) or PLA | RT = 3 days/wk | 12 wks | CR ↑ gains in fat-free mass (+3.2%), muscle mass (+2.8%), 1RM bench press, knee extension, and biceps curl compared to PLA |
| Bemben et al. and Eliot et al. [13,14] | N = 42; healthy men; age = 48–72 years | None | CR (5 g/day) | RT = 3 days/wk | 14 wks | ↔ lean tissue mass, 1RM strength |
| Bermon et al. [15] | N = 32 (16 men, 16 women); healthy; age = 67–80 years | CR 20 g/day for 5 days | CR (3 g/day) or PLA | RT = 3 days/wk | 7.4 wks (52 days) | ↔ lower limb muscular volume, 1-, 12-repetitions maxima, and the isometric intermittent endurance |
| Brose et al. [16] | N = 28 (15 men, 13 women); healthy; age: men = 68.7, women = 70.8 years | None | CR (5 g/day) or PLA | RT = 3 days/wk | 14 wks | CR ↑ gains in lean tissue mass and isometric knee extension strength; ↔ type 1, 2a, 2x muscle fiber area |
| Deacon et al. [17] | N = 80 (50 men, 30 women); COPD; age = 68.2 years | CR 22 g/day for 5 days | CR (3.76 g/day) or PLA | RT = 3 days/wk | 7 wks | ↔ lean tissue mass or muscle strength |
| Eijnde et al. [18] | N = 46; healthy men; age = 55–75 years | None | CR (5 g/day) or PLA | Cardiorespiratory + RT = 2–3 days/wk | 26 wks | ↔ lean tissue mass or isometric maximal strength |
| Gualano et al. [19] | N = 25 (9 men, 16 women); type 2 diabetes; age = 57 years | None | CR (5 g/day) or PLA | RT = 3 days/wk | 12 wks | ↔ lean tissue mass |
| Gualano et al. [20] | N = 30; “vulnerable” women; Mean age = 65.4 years | CR 20 g/day for 5 days | CR (5 g/day) or PLA | RT = 2 days/wk | 24 wks | CR + RT ↑ gains in 1RM bench press and appendicular lean mass compared to PLA + RT |
| Hass et al. [21] | N = 20 (17 men, 3 women with idiopathetic Parkinson’s disease); Mean age = 62 years | CR 20 g/day for 5 days | CR (5 g/day) or PLA | RT = 2 days/wk | 12 wks | CR ↑ chest press strength, chair rise performance; ↔ Leg extension 1RM, muscular endurance |
| Neves et al. [22] | N = 24 (postmenopausal women with knee osteoarthritis); Age = 55–65 years | CR 20 g/day for 1 week | CR 5 (g/day) or PLA | RT=3 days/wk | 12 wks | CR ↑ gains in limb lean mass. ↔ 1RM leg press |
| Pinto et al. [23] | N = 27 (men and women); healthy; age = 60–80 years | None | CR (5 g/day) or PLA | RT = 3 days/wk | 12 wks | CR ↑ gains in lean tissue mass. ↔ 10 RM bench press or leg press strength |
| Higher-Dose/Relative Studies (>5 g/day) | ||||||
| Bernat et al. [24] | N = 24 healthy men; age = 59 ± 6 years | None | CR (0.1 g/kg/day; ~9.5 g/day) or PLA | High-velocity RT = 2 days/wk | 8 wks | ↔ muscle thickness, physical performance, upper body muscle strength. CR ↑ leg press strength, total lower body strength |
| Candow et al. [25] | N = 35; healthy men; age = 59–77 years | None | CR (0.1 g/kg/day; ~8.6 g/day) or PLA | RT = 3 days/wk | 10 wks | CR ↑ muscle thickness compared to PLA. CR ↑ 1RM bench press ↔ 1RM leg press |
| Candow et al. [26] | N = 39 (17 men, 22 women); healthy; age = 50–71 years | None | CR (0.1 g/kg; ~7.7 g/day) before RT, CR (0.1 g/kg; ~8.8 g/day) after RT, or PLA | RT = 3 days/wk | 32 wks | CR after RT ↑ lean tissue mass, 1RM leg press, 1RM chest press compared to PLA |
| Candow et al. [27] | N = 38; healthy men; age = 49–67 years | None | CR (On training days: 0.05 g/kg before and 0.05 g/kg after exercise; total ~9.3 g/day) + 0.1 g/kg/day on non-training days (2 equal doses) | RT = 3 days/wk | 12 months | ↔ lean tissue mass, muscle thickness, or muscle strength |
| Chilibeck et al. [28] | N = 33; healthy women; Mean age = 57 years | None | CR (0.1 g/kg/day; ~6.9 g/day) or PLA | RT = 3 days/wk | 52 wks | ↔ lean tissue mass and muscle thickness gains between groups. ↑ relative bench press strength compared to PLA. |
| Chrusch et al. [29] | N = 30; healthy men; age = 60–84 years | CR 0.3 g/kg/d for 5 days | CR 0.07 g/kg/day; ~6.2 g/day or PLA | RT = 3 days/wk | 12 wks | CR ↑ gains in lean tissue mass. CR ↑ 1RM leg press, 1RM knee extension, leg press endurance, and knee extension endurance. ↔ 1RM bench press or bench press endurance. |
| Cooke et al. [30] | N = 20; healthy men; age = 55–70 years | CR 20 g/day for 7 days | CR 0.1 g/kg/day or ~8.8 g/day on training days | RT = 3 days/wk | 12 wks | ↔ lean tissue mass, 1RM bench press, 1RM leg press |
| Johannsmeyer et al. [31] | N = 31 (17 men, 14 women); healthy; age = 58 years | None | CR 0.1 g/kg/day; ~7.8 g/day or PLA | RT = 3 days/wk | 12 wks | CR ↑ gains in lean tissue mass and 1RM strength in men only |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forbes, S.C.; Candow, D.G.; Ostojic, S.M.; Roberts, M.D.; Chilibeck, P.D. Meta-Analysis Examining the Importance of Creatine Ingestion Strategies on Lean Tissue Mass and Strength in Older Adults. Nutrients 2021, 13, 1912. https://doi.org/10.3390/nu13061912
Forbes SC, Candow DG, Ostojic SM, Roberts MD, Chilibeck PD. Meta-Analysis Examining the Importance of Creatine Ingestion Strategies on Lean Tissue Mass and Strength in Older Adults. Nutrients. 2021; 13(6):1912. https://doi.org/10.3390/nu13061912
Chicago/Turabian StyleForbes, Scott C., Darren G. Candow, Sergej M. Ostojic, Michael D. Roberts, and Philip D. Chilibeck. 2021. "Meta-Analysis Examining the Importance of Creatine Ingestion Strategies on Lean Tissue Mass and Strength in Older Adults" Nutrients 13, no. 6: 1912. https://doi.org/10.3390/nu13061912
APA StyleForbes, S. C., Candow, D. G., Ostojic, S. M., Roberts, M. D., & Chilibeck, P. D. (2021). Meta-Analysis Examining the Importance of Creatine Ingestion Strategies on Lean Tissue Mass and Strength in Older Adults. Nutrients, 13(6), 1912. https://doi.org/10.3390/nu13061912










