Sarcopenia and Appendicular Muscle Mass as Predictors of Impaired Fasting Glucose/Type 2 Diabetes in Elderly Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Diabetes, Impaired Fasting Glucose, and Cardiovascular Risk Factor Assessment
2.2. Anthropometric and Dietary Intake Assessments
2.3. Biochemical Evaluation
2.4. Statistical Analysis
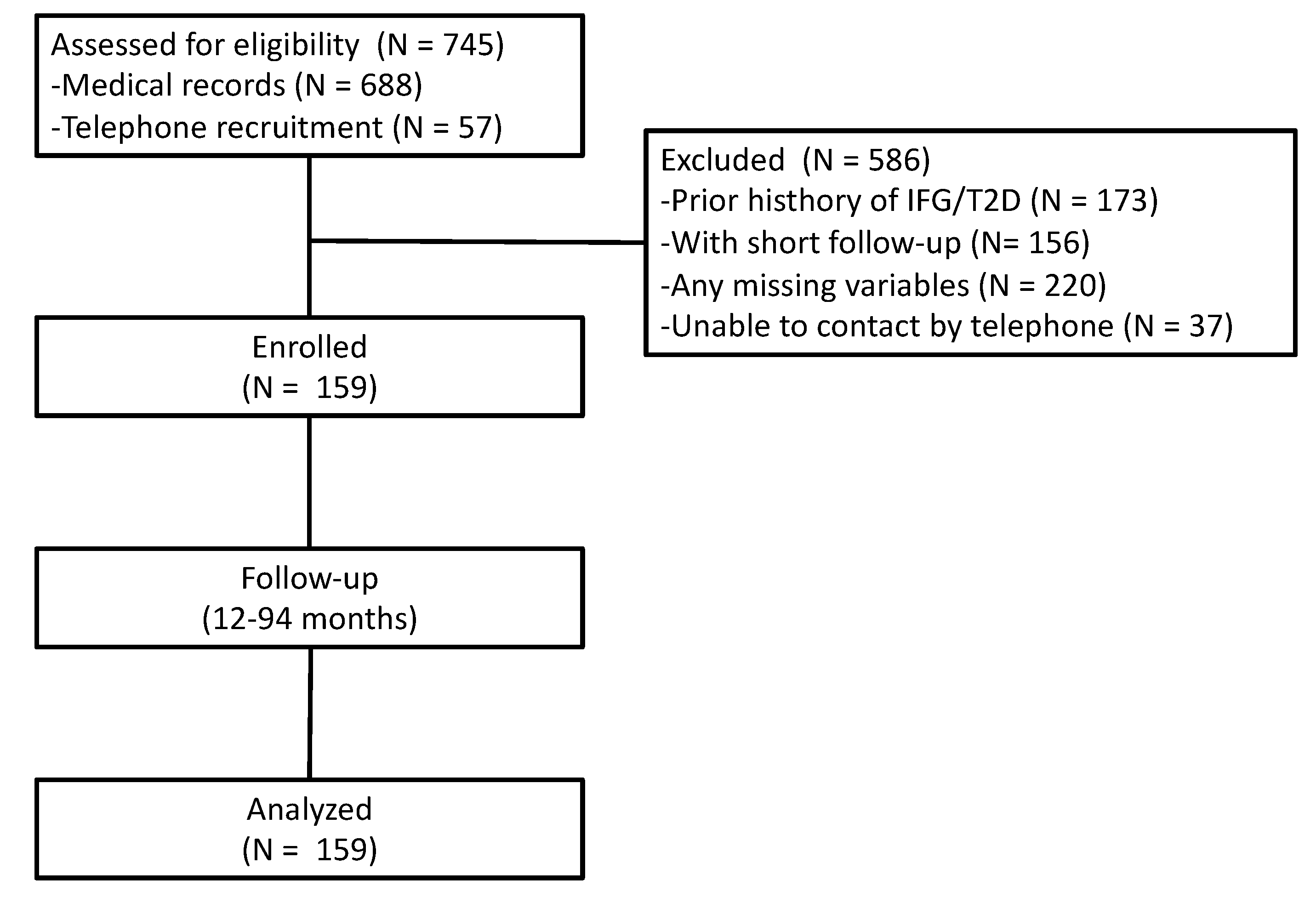
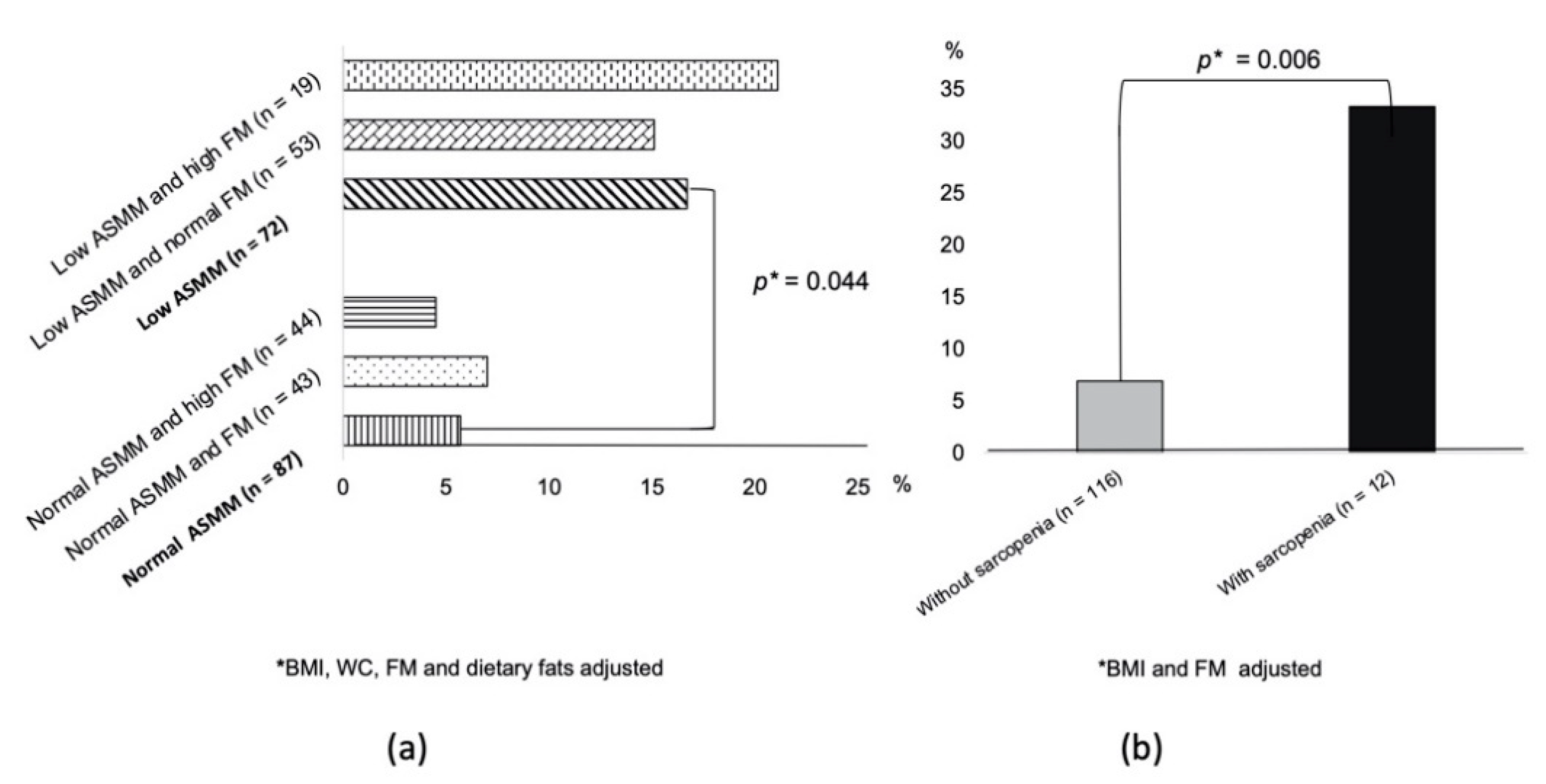
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannel, W.B.; McGee, D.L. Diabetes and Cardiovascular Disease. The Framingham Study. J. Am. Med. Assoc. 1979, 241, 2035–2038. [Google Scholar] [CrossRef]
- Barr, E.L.M.; Zimmet, P.Z.; Welborn, T.A.; Jolley, D.; Magliano, D.J.; Dunstan, D.W.; Cameron, A.J.; Dwyer, T.; Taylor, H.R.; Tonkin, A.M.; et al. Risk of Cardiovascular and All-Cause Mortality in Individuals with Diabetes Mellitus, Impaired Fasting Glucose, and Impaired Glucose Tolerance: The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007, 116, 151–157. [Google Scholar] [CrossRef]
- Aronson, D.; Edelman, E.R. Coronary Artery Disease and Diabetes Mellitus. Cardiol. Clin. 2014, 32, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Cowie, C.C.; Rust, K.F.; Ford, E.S.; Eberhardt, M.S.; Byrd-Holt, D.D.; Li, C.; Williams, D.E.; Gregg, E.W.; Bainbridge, K.E.; Saydah, S.H.; et al. Full Accounting of Diabetes and Pre-Diabetes in the U.S. Population in 1988–1994 and 2005–2006. Diabetes Care 2009, 32, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Burrows, N.R.; Gregg, E.W.; Albright, A.; Geiss, L.S. Declining Rates of Hospitalization for Nontraumatic Lower-Extremity Amputation in the Diabetic Population Aged 40 Years or Older: U.S., 1988–2008. Diabetes Care 2012, 35, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; Florez, H.; Haas, L.B.; Halter, J.B.; Huang, E.S.; Korytkowski, M.T.; Munshi, M.N.; Odegard, P.S.; et al. Diabetes in Older Adults. Diabetes Care 2012, 35, 2650–2664. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; St George, R.; Fitzpatrick, R.C. Walking Stability and Sensorimotor Function in Older People with Diabetic Peripheral Neuropathy. Arch. Phys. Med. Rehabil. 2004, 85, 245–252. [Google Scholar] [CrossRef]
- Volpato, S.; Blaum, C.; Resnick, H.; Ferrucci, L.; Fried, L.P.; Guralnik, J.M. Women’s Health and Aging Study Comorbidities and Impairments Explaining the Association between Diabetes and Lower Extremity Disability: The Women’s Health and Aging Study. Diabetes Care 2002, 25, 678–683. [Google Scholar] [CrossRef]
- Huxley, R.; Barzi, F.; Woodward, M. Excess Risk of Fatal Coronary Heart Disease Associated with Diabetes in Men and Women: Meta-Analysis of 37 Prospective Cohort Studies. Br. Med. J. 2006, 332, 73–78. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Haffner, S.M.; Miettinen, H.; Stern, M.P. Relatively More Atherogenic Coronary Heart Disease Risk Factors in Prediabetic Women than in Prediabetic Men. Diabetologia 1997, 40, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Juutilainen, A.; Kortelainen, S.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Gender Difference in the Impact of Type 2 Diabetes on Coronary Heart Disease Risk. Diabetes Care 2004, 27, 2898–2904. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.; Iannuzzo, G.; Mattiello, A.; Rubba, F.; Panico, S.; Rubba, P. Association between Body Shape Index and Small Dense LDL Particles in a Cohort of Mediterranean Women: Findings from Progetto ATENA. J. Clin. Biochem. Nutr. 2017, 61, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.; Iannuzzo, G.; Mattiello, A.; Marotta, G.; Iannuzzi, A.; Panico, S.; Rubba, P. Association between Lp (a) and Atherosclerosis in Menopausal Women without Metabolic Syndrome. Biomark. Med. 2016, 10, 397–402. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Larsen, B.A.; Wassel, C.L.; Kritchevsky, S.B.; Strotmeyer, E.S.; Criqui, M.H.; Kanaya, A.M.; Fried, L.F.; Schwartz, A.V.; Harris, T.B.; Ix, J.H.; et al. Association of Muscle Mass, Area, and Strength with Incident Diabetes in Older Adults: The Health ABC Study. J. Clin. Endocrinol. Metab. 2016, 101, 1847–1855. [Google Scholar] [CrossRef]
- Baker, C.F.; Overvad, K.; Dahm, C.C. Lean Body Mass and Risk of Type 2 Diabetes—A Danish Cohort Study. J. Diabetes Metab. Disord. 2019, 18, 445–451. [Google Scholar] [CrossRef]
- Succurro, E.; Fiorentino, T.V.; Miceli, S.; Perticone, M.; Sciacqua, A.; Andreozzi, F.; Sesti, G. Relative Risk of Cardiovascular Disease Is Higher in Women with Type 2 Diabetes, but Not in Those with Prediabetes, as Compared with Men. Diabetes Care 2020, 43, 3070–3078. [Google Scholar] [CrossRef]
- Mankowska, A.; Nowak, L.; Sypniewska, G. Adiponectin and Metabolic Syndrome in Women at Menopause. EJIFCC 2009, 19, 173–184. [Google Scholar]
- Bhardwaj, P.; Au, C.C.; Benito-Martin, A.; Ladumor, H.; Oshchepkova, S.; Moges, R.; Brown, K.A. Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J. Steroid Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Is Estradiol a Biomarker of Type 2 Diabetes Risk in Postmenopausal Women? Diabetes 2017, 66, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Yoon, J.S. Relative Skeletal Muscle Mass Is Associated with Development of Metabolic Syndrome. Diabetes Metab. J. 2013, 37, 458–464. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Metter, E.J.; Xue, Q.-L.; Egan, J.M.; Chia, C.W.; Studenski, S.; Shaffer, N.C.; Golden, S.; Al-Sofiani, M.; Florez, H.; et al. The Relationship of Lean Body Mass with Aging to the Development of Diabetes. J. Endocr. Soc. 2020, 4, bvaa043. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Chang, Y.; Jung, H.-S.; Yun, K.E.; Shin, H.; Ryu, S. Relative Muscle Mass and the Risk of Incident Type 2 Diabetes: A Cohort Study. PLoS ONE 2017, 12, e0188650. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, K.; Adamska-Patruno, E.; Goscik, J.; Lipinska, D.; Citko, A.; Krahel, A.; Miniewska, K.; Fiedorczuk, J.; Moroz, M.; Gorska, M.; et al. The Role of Muscle Decline in Type 2 Diabetes Development: A 5-Year Prospective Observational Cohort Study. Nutrients 2019, 11, 834. [Google Scholar] [CrossRef]
- Heshka, S.; Ruggiero, A.; Bray, G.A.; Foreyt, J.; Kahn, S.E.; Lewis, C.E.; Saad, M.; Schwartz, A.V. Look AHEAD Research Group Altered Body Composition in Type 2 Diabetes Mellitus. Int. J. Obes. 2008, 32, 780–787. [Google Scholar] [CrossRef]
- Rehunen, S.K.J.; Kautiainen, H.; Korhonen, P.E.; Eriksson, J.G. A High Lean Body Mass Is Not Protecting from Type 2 Diabetes in the Presence of a High Body Fat Mass. Diabetes Metab. 2021, 47, 101219. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-Related and Disease-Related Muscle Loss: The Effect of Diabetes, Obesity, and Other Diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Gallagher, D.; Visser, M.; De Meersman, R.E.; Sepúlveda, D.; Baumgartner, R.N.; Pierson, R.N.; Harris, T.; Heymsfield, S.B. Appendicular Skeletal Muscle Mass: Effects of Age, Gender, and Ethnicity. J. Appl. Physiol. 1997, 83, 229–239. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36, S67–S74. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC) Use of Cessation Methods among Smokers Aged 16–24 Years—United States, 2003. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 1351–1354.
- Psaty, B.M.; Furberg, C.D.; Kuller, L.H.; Bild, D.E.; Rautaharju, P.M.; Polak, J.F.; Bovill, E.; Gottdiener, J.S. Traditional Risk Factors and Subclinical Disease Measures as Predictors of First Myocardial Infarction in Older Adults: The Cardiovascular Health Study. Arch. Intern. Med. 1999, 159, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Pujia, A.; Mazza, E.; Ferro, Y.; Gazzaruso, C.; Coppola, A.; Doldo, P.; Grembiale, R.D.; Pujia, R.; Romeo, S.; Montalcini, T. Lipid Oxidation Assessed by Indirect Calorimetry Predicts Metabolic Syndrome and Type 2 Diabetes. Front. Endocrinol. 2019, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing Appendicular Skeletal Muscle Mass with Bioelectrical Impedance Analysis in Free-Living Caucasian Older Adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452.
- Donini, L.M.; Poggiogalle, E.; Del Balzo, V.; Lubrano, C.; Faliva, M.; Opizzi, A.; Perna, S.; Pinto, A.; Rondanelli, M. How to Estimate Fat Mass in Overweight and Obese Subjects. Int. J. Endocrinol. 2013, 285680. [Google Scholar] [CrossRef]
- Montalcini, T.; De Bonis, D.; Ferro, Y.; Carè, I.; Mazza, E.; Accattato, F.; Greco, M.; Foti, D.; Romeo, S.; Gulletta, E.; et al. High Vegetable Fats Intake Is Associated with High Resting Energy Expenditure in Vegetarians. Nutrients 2015, 7, 5933–5947. [Google Scholar] [CrossRef]
- Buscemi, S.; Rosafio, G.; Vasto, S.; Massenti, F.M.; Grosso, G.; Galvano, F.; Rini, N.; Barile, A.M.; Maniaci, V.; Cosentino, L.; et al. Validation of a Food Frequency Questionnaire for Use in Italian Adults Living in Sicily. Int. J. Food Sci. Nutr. 2015, 66, 426–438. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- de Vegt, F.; Dekker, J.M.; Jager, A.; Hienkens, E.; Kostense, P.J.; Stehouwer, C.D.; Nijpels, G.; Bouter, L.M.; Heine, R.J. Relation of Impaired Fasting and Postload Glucose with Incident Type 2 Diabetes in a Dutch Population: The Hoorn Study. J. Am. Med. Assoc. 2001, 285, 2109–2113. [Google Scholar] [CrossRef]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An Overview of Sarcopenia: Facts and Numbers on Prevalence and Clinical Impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Garg, S.K.; Maurer, H.; Reed, K.; Selagamsetty, R. Diabetes and Cancer: Two Diseases with Obesity as a Common Risk Factor. Diabetes Obes. Metab. 2014, 16, 97–110. [Google Scholar] [CrossRef]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E. Risk Factors for Type 2 Diabetes Mellitus: An Exposure-Wide Umbrella Review of Meta-Analyses. PLoS ONE 2018, 13, e0194127. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Krishnaswami, S.; Resnick, H.; Kelley, D.E.; Haggerty, C.; Harris, T.B.; Schwartz, A.V.; Kritchevsky, S.; Newman, A.B. Association Between Regional Adipose Tissue Distribution and Both Type 2 Diabetes and Impaired Glucose Tolerance in Elderly Men and Women. Diabetes Care 2003, 26, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.L.; Addison, O.; Dibble, L.E.; Foreman, K.B.; Morrell, G.; Lastayo, P. Intramuscular Adipose Tissue, Sarcopenia, and Mobility Function in Older Individuals. J. Aging Res. 2012, 629637. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; de Courten, B.; Ebeling, P.R. Sarcopenia: A Potential Cause and Consequence of Type 2 Diabetes in Australia’s Ageing Population? Med. J. Aust. 2016, 205, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Reinders, I.; Garcia, M.E.; Eiriksdottir, G.; Launer, L.J.; Benediktsson, R.; Gudnason, V.; Jonsson, P.V.; Harris, T.B. Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik) Adipose Tissue, Muscle, and Function: Potential Mediators of Associations between Body Weight and Mortality in Older Adults with Type 2 Diabetes. Diabetes Care 2014, 37, 3213–3219. [Google Scholar] [CrossRef]
- Castillo, E.M.; Goodman-Gruen, D.; Kritz-Silverstein, D.; Morton, D.J.; Wingard, D.L.; Barrett-Connor, E. Sarcopenia in Elderly Men and Women: The Rancho Bernardo Study. Am. J. Prev. Med. 2003, 25, 226–231. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Pitaraki, E.; Metaxa, V.; Stefanadis, C. ATTICA Study Group 10-Year Incidence of Diabetes and Associated Risk Factors in Greece: The ATTICA Study (2002–2012). Rev. Diabet. Stud. 2014, 11, 181–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kataja-Tuomola, M.; Sundell, J.; Männistö, S.; Virtanen, M.J.; Kontto, J.; Albanes, D.; Virtamo, J. Short-Term Weight Change and Fluctuation as Risk Factors for Type 2 Diabetes in Finnish Male Smokers. Eur. J. Epidemiol. 2010, 25, 333–339. [Google Scholar] [CrossRef]
- French, S.A.; Folsom, A.R.; Jeffery, R.W.; Zheng, W.; Mink, P.J.; Baxter, J.E. Weight Variability and Incident Disease in Older Women: The Iowa Women’s Health Study. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wallner, S.J.; Luschnigg, N.; Schnedl, W.J.; Lahousen, T.; Sudi, K.; Crailsheim, K.; Möller, R.; Tafeit, E.; Horejsi, R. Body fat distribution of overweight females with a history of weight cycling. Int. J. Obes. 2004, 28, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Frontera, W.R.; Roubenoff, R.; Evans, W.J.; Singh, M.A.F. Longitudinal Changes in Body Composition in Older Men and Women: Role of Body Weight Change and Physical Activity. Am. J. Clin. Nutr. 2002, 76, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal Muscle Mass and Distribution in 468 Men and Women Aged 18–88 Yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S. Gender Differences in Physical Activity and Walking among Older Adults. J. Women Aging 2005, 17, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Newman, A.B.; Brach, J.S. Gender Gap in Longevity and Disability in Older Persons. Epidemiol. Rev. 2001, 23, 343–350. [Google Scholar] [CrossRef]
- Maltais, M.L.; Desroches, J.; Dionne, I.J. Changes in muscle mass and strength after menopause. J. Musculoskelet Neuronal Interact. 2009, 9, 186–197. [Google Scholar]
- Montalcini, T.; Pujia, A.; Donini, L.M.; Frittitta, L.; Galvano, F.; Natali, A.; Pironi, L.; Porrini, M.; Riso, P.; Rivellese, A.A.; et al. A Call to Action: Now Is the Time to Screen Elderly and Treat Osteosarcopenia, a Position Paper of the Italian College of Academic Nutritionists MED/49 (ICAN-49). Nutrients 2020, 12, 2662. [Google Scholar] [CrossRef]
- Rimbert, V.; Boirie, Y.; Bedu, M.; Hocquette, J.-F.; Ritz, P.; Morio, B. Muscle Fat Oxidative Capacity Is Not Impaired by Age but by Physical Inactivity: Association with Insulin Sensitivity. FASEB J. 2004, 18, 737–739. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of Skeletal Muscle Mass in Mice by a New TGF-Beta Superfamily Member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Corrao, S.; Santalucia, P.; Argano, C.; Djade, C.D.; Barone, E.; Tettamanti, M.; Pasina, L.; Franchi, C.; Eldin, T.K.; Marengoni, A.; et al. Gender-differences in disease distribution and outcome in hospitalized elderly: Data from the REPOSI study. Eur. J. Intern. Med. 2014, 25, 617–623. [Google Scholar] [CrossRef]
- Kurose, S.; Onishi, K.; Takao, N.; Miyauchi, T.; Takahashi, K.; Kimura, Y. Association of serum adiponectin and myostatin levels with skeletal muscle in patients with obesity: A cross-sectional study. PLoS ONE 2021, 16, e0245678. [Google Scholar] [CrossRef] [PubMed]
- Léger, B.; Derave, W.; De Bock, K.; Hespel, P.; Russell, A.P. Human Sarcopenia Reveals an Increase in SOCS-3 and Myostatin and a Reduced Efficiency of Akt Phosphorylation. Rejuvenation Res. 2008, 11, 163B–175B. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.L.; Cleary, A.S.; Speaker, K.J.; Lindsay, S.F.; Uyenishi, J.; Reed, J.M.; Madden, M.C.; Mehan, R.S. Myostatin, Activin Receptor IIb, and Follistatin-like-3 Gene Expression Are Altered in Adipose Tissue and Skeletal Muscle of Obese Mice. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E918–E927. [Google Scholar] [CrossRef]
- Hittel, D.S.; Berggren, J.R.; Shearer, J.; Boyle, K.; Houmard, J.A. Increased Secretion and Expression of Myostatin in Skeletal Muscle from Extremely Obese Women. Diabetes 2009, 58, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tomata, Y.; Kakizaki, M.; Sugawara, Y.; Hozawa, A.; Momma, H.; Tsuji, I.; Nagatomi, R. High circulating adiponectin levels predict decreased muscle strength among older adults aged 70 years and over: A prospective cohort study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 594–601. [Google Scholar] [CrossRef]
- Rajala, M.W.; Scherer, P.E. Minireview: The adipocyte—At the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 2003, 144, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- van Popele, N.M.; Elizabeth Hak, A.; Mattace-Raso, F.U.S.; Bots, M.L.; van der Kuip, D.A.M.; Reneman, R.S.; Hoeks, A.P.G.; Hofman, A.; Grobbee, D.E.; Witteman, J.C.M. Impaired Fasting Glucose Is Associated with Increased Arterial Stiffness in Elderly People without Diabetes Mellitus: The Rotterdam Study. J. Am. Geriatr. Soc. 2006, 54, 397–404. [Google Scholar] [CrossRef]
- Coutinho, M.; Gerstein, H.C.; Wang, Y.; Yusuf, S. The Relationship between Glucose and Incident Cardiovascular Events. A Metaregression Analysis of Published Data from 20 Studies of 95,783 Individuals Followed for 12.4 Years. Diabetes Care 1999, 22, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Levitan, E.B.; Song, Y.; Ford, E.S.; Liu, S. Is Nondiabetic Hyperglycemia a Risk Factor for Cardiovascular Disease? A Meta-Analysis of Prospective Studies. Arch. Intern. Med. 2004, 164, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.K.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Me-ta-Analysis of 102 Prospective Studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Lee, G.; Kim, S.M.; Choi, S.; Kim, K.; Jeong, S.-M.; Son, J.S.; Yun, J.-M.; Park, S.M. The Effect of Change in Fasting Glucose on the Risk of Myocardial Infarction, Stroke, and All-Cause Mortality: A Nationwide Cohort Study. Cardiovasc. Diabetol. 2018, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Chen, S.; Vaidya, A.; Wu, Y.; Wu, Z.; Hu, F.B.; Kris-Etherton, P.; Wu, S.; Gao, X. Longitudinal Change in Fasting Blood Glucose and Myocardial Infarction Risk in a Population without Diabetes. Diabetes Care 2017, 40, 1565–1572. [Google Scholar] [CrossRef]
- Piconi, L.; Quagliaro, L.; Da Ros, R.; Assaloni, R.; Giugliano, D.; Esposito, K.; Szabó, C.; Ceriello, A. Intermittent High Glucose Enhances ICAM-1, VCAM-1, E-Selectin and Interleukin-6 Expression in Human Umbilical Endothelial Cells in Culture: The Role of Poly(ADP-Ribose) Polymerase. J. Thromb. Haemost. 2004, 2, 1453–1459. [Google Scholar] [CrossRef]
- Minutolo, R.; Gabbai, F.B.; Provenzano, M.; Chiodini, P.; Borrelli, S.; Garofalo, C.; Sasso, F.C.; Santoro, D.; Bellizzi, V.; Conte, G.; et al. Cardiorenal prognosis by residual proteinuria level in diabetic chronic kidney disease: Pooled analysis of four cohort studies. Nephrol. Dial. Transplant. 2018, 33, 1942–1949. [Google Scholar] [CrossRef]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyère, O.; et al. Nutrition and Physical Activity in the Prevention and Treatment of Sarcopenia: Systematic Review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef]
- Bosaeus, I.; Rothenberg, E. Nutrition and Physical Activity for the Prevention and Treatment of Age-Related Sarcopenia. Proc. Nutr. Soc. 2016, 75, 174–180. [Google Scholar] [CrossRef]
- Silva, T.R.; Spritzer, P.M. Skeletal Muscle Mass Is Associated with Higher Dietary Protein Intake and Lower Body Fat in Postmenopausal Women: A Cross-Sectional Study. Menopause 2017, 24, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Ten Haaf, D.S.M.; Eijsvogels, T.M.H.; Bongers, C.C.W.G.; Horstman, A.M.H.; Timmers, S.; de Groot, L.C.P.G.M.; Hopman, M.T.E. Protein Supplementation Improves Lean Body Mass in Physically Active Older Adults: A Randomized Placebo-Controlled Trial. J. Cachexia Sarcopenia Muscle 2019, 10, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.V.; Jung, D.H.; Yadav, D.; Kim, J.Y.; Koh, S.B. Relative contribution of obesity and menopause to the association between serum adiponectin and incident metabolic syndrome. Menopause 2018, 25, 154–159. [Google Scholar] [CrossRef] [PubMed]

| Participants (n = 159) | |
|---|---|
| Age (years) | 71 ± 5 |
| Body weight (kg) | 65.3 ± 11 |
| BMI (kg/m2) | 28 ± 4 |
| Smokers (%) | 8 |
| Physical activity (%) | 52 |
| Hyperlipidemia (%) | 42 |
| Lipid-lowering agents (%) | 24 |
| Hypertension (%) | 65 |
| Antihypertensive agents (%) | 57 |
| Calcium/vitamin D supplementation (%) | 31 |
| BMI-defined obesity (%) | 26 |
| FM-defined obesity (%) | 38 |
| Low ASMM (%) | 45 |
| Sarcopenia * (%) | 9.4 |
| WC (cm) | 92.8 ± 11 |
| HC (cm) | 103.7 ± 10 |
| HGS * (kg) | 19.6 ± 4 |
| SBP (mmHg) | 130 ± 16 |
| DBP (mmHg) | 78 ± 9 |
| Bioimpedance analysis | |
| Rz (Ω) | 554.8 ± 69 |
| Xc (Ω) | 54.0 ± 9 |
| pA (°) | 5.6 ± 0.8 |
| FFM (kg) | 22.1 ± 8.0 |
| FM (%) | 32.9 ± 6.2 |
| ASMM (kg) | 15.4 ± 2.1 |
| Blood concentrations of (mg/dL) | |
| Glucose | 89 ± 8 |
| Creatinine | 0.76 ± 0.2 |
| Total cholesterol | 215 ± 38 |
| HDL cholesterol | 64 ± 16 |
| LDL cholesterol | 131 ± 36 |
| Triglycerides | 106 ± 46 |
| ASMM | p | ||
|---|---|---|---|
| Normal (n = 87) | Low (n = 72) | ||
| ASMM (range, kg) | 15–22.5 | 10.6–14.9 | |
| Age (years) | 70 ± 4 | 71 ± 6 | 0.36 |
| Body weight (kg) | 71.9 ± 10 | 57.3 ± 6 | <0.001 |
| BMI (kg/m2) | 30.0 ± 4 | 26.0 ± 3 | <0.001 |
| Smokers (%) | 7 | 9 | 0.77 |
| Physical activity (%) | 53 | 51 | 0.86 |
| Hyperlipidemia (%) | 36 | 49 | 0.10 |
| Lipid-lowering agents (%) | 20 | 29 | 0.19 |
| Hypertension (%) | 67 | 64 | 0.74 |
| Antihypertensive agents (%) | 60 | 54 | 0.52 |
| Calcium and vitamin D supplementation (%) | 26 | 36 | 0.30 |
| BMI-defined obesity (%) | 43 | 6 | <0.001 |
| FM-defined obesity (%) | 51 | 22 | <0.001 |
| Sarcopenia * (%) | 0 | 19 | <0.001 |
| WC (cm) | 97.0 ± 12 | 87.6 ± 8 | <0.001 |
| HC (cm) | 107.9 ± 9 | 98.7 ± 8 | <0.001 |
| HGS * (kg) | 20.3 ± 3.9 | 18.8 ± 3.8 | 0.038 |
| SBP (mmHg) | 130 ± 17 | 129 ± 16 | 0.78 |
| DBP (mmHg) | 79 ± 8 | 77 ± 9 | 0.31 |
| Bioimpedance analysis | |||
| Rz (Ω) | 519 ± 58 | 598 ± 57 | <0.001 |
| Xc (Ω) | 53 ± 10 | 56 ± 9 | 0.06 |
| pA (°) | 5.8 ± 0.8 | 5.3 ± 0.7 | <0.001 |
| FM (kg) | 25.6 ± 8.2 | 17.9 ± 4.0 | <0.001 |
| FM (%) | 34.8 ± 6.3 | 30.7 ± 5.1 | <0.001 |
| Blood concentrations of (mg/dL) | |||
| Glucose | 88 ± 8 | 89 ± 7 | 0.54 |
| Creatinine | 0.78 ± 0.2 | 0.73 ± 0.1 | 0.06 |
| Total cholesterol | 217 ± 40 | 212 ± 37 | 0.50 |
| HDL cholesterol | 63 ± 16 | 65 ± 17 | 0.43 |
| LDL cholesterol | 133 ± 38 | 128 ± 35 | 0.38 |
| Triglycerides | 111 ± 52 | 100 ± 38 | 0.11 |
| Panel A (n = 159) | Multivariate Analysis | |
|---|---|---|
| HR (95% CI) | p | |
| WC | 0.98 (0.91–1.05) | 0.58 |
| BMI | 1.08 (0.84–1.38) | 0.53 |
| High fat mass | 0.94 (0.14–6.43) | 0.95 |
| Low ASMM | 5.30 (1.34–21.04) | 0.017 |
| Fat intake (%) | 1.00 (0.89–1.12) | 0.92 |
| Panel B (n = 128) | Multivariate Analysis | |
| HR (95% CI) | p | |
| BMI | 0.95 (0.80–1.12) | 0.58 |
| High fat mass | 1.70 (0.28–10.07) | 0.55 |
| Sarcopenia | 4.72 (1.38–16.18) | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buscemi, C.; Ferro, Y.; Pujia, R.; Mazza, E.; Boragina, G.; Sciacqua, A.; Piro, S.; Pujia, A.; Sesti, G.; Buscemi, S.; et al. Sarcopenia and Appendicular Muscle Mass as Predictors of Impaired Fasting Glucose/Type 2 Diabetes in Elderly Women. Nutrients 2021, 13, 1909. https://doi.org/10.3390/nu13061909
Buscemi C, Ferro Y, Pujia R, Mazza E, Boragina G, Sciacqua A, Piro S, Pujia A, Sesti G, Buscemi S, et al. Sarcopenia and Appendicular Muscle Mass as Predictors of Impaired Fasting Glucose/Type 2 Diabetes in Elderly Women. Nutrients. 2021; 13(6):1909. https://doi.org/10.3390/nu13061909
Chicago/Turabian StyleBuscemi, Carola, Yvelise Ferro, Roberta Pujia, Elisa Mazza, Giada Boragina, Angela Sciacqua, Salvatore Piro, Arturo Pujia, Giorgio Sesti, Silvio Buscemi, and et al. 2021. "Sarcopenia and Appendicular Muscle Mass as Predictors of Impaired Fasting Glucose/Type 2 Diabetes in Elderly Women" Nutrients 13, no. 6: 1909. https://doi.org/10.3390/nu13061909
APA StyleBuscemi, C., Ferro, Y., Pujia, R., Mazza, E., Boragina, G., Sciacqua, A., Piro, S., Pujia, A., Sesti, G., Buscemi, S., & Montalcini, T. (2021). Sarcopenia and Appendicular Muscle Mass as Predictors of Impaired Fasting Glucose/Type 2 Diabetes in Elderly Women. Nutrients, 13(6), 1909. https://doi.org/10.3390/nu13061909










