Low- and No-Calorie Sweetener (LNCS) Consumption Patterns Amongst the Spanish Adult Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Sample
2.3. Data Collection
2.4. Assessment of LNCS Intake
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA Panel on Nutrition Novel Foods and Food Allergens. Scientific Opinion on the substantiation of health claims related to intense sweeteners and contribution to the maintenance or achievement of a normal body weight (ID 1136, 1444, 4299), reduction of post-prandial glycaemic responses (ID 4298), maintenance of normal blood glucose concentrations (ID 1221, 4298), and maintenance of tooth mineralisation by decreasing tooth demineralisation (ID 1134, 1167, 1283) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2229. [Google Scholar]
- World Health Organization (WHO). Controlling the Global Obesity Epidemic. Available online: https://www.who.int/nutrition/topics/obesity/en/ (accessed on 20 April 2020).
- World Health Organization (WHO). Diet, Nutrition and the Prevention of Chronic Diseases. In Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Ministerio de Sanidad, Seguridad Sociale Igualdad; Agencia Española de Consumo, Seguridad Alimentaria y Nutrición. Plan de Colaboración Para la Mejora de la Composición de Alimentos y Bebidas y Otras Medidas 2017–2020; Ministerio de Sanidad, Seguridad Sociale Igualdad: Madrid, Spain, 2015. [Google Scholar]
- Dunford, E.K.; Taillie, L.S.; Miles, D.R.; Eyles, H.; Tolentino-Mayo, L.; Ng, S.W. Non-Nutritive Sweeteners in the Packaged Food Supply-An Assessment across 4 Countries. Nutrients 2018, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Vaesken, M.L.; Ruiz, E.; Partearroyo, T.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G. Added Sugars and Low- and No-Calorie Sweeteners in a Representative Sample of Food Products Consumed by the Spanish ANIBES Study Population. Nutrients 2018, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- European Union (EU). Commisssion Regulation (EU) No 1129/2011 of 11 November 2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives; OJ L 295, 12.11.2011; European Union: Brussels, Belgium, 2011; pp. 1–177. [Google Scholar]
- European Union (EU). Commisssion Regulation (EU) No 1333/2008 of 16 December 2008 of the European Parlamient and of the Council on food additives. Off. J. Eur. Union 2008, 354, 16–33. [Google Scholar]
- Mattes, R.D.; Popkin, B.M. Nonnutritive sweetener consumption in humans: Effects on appetite and food intake and their putative mechanisms. Am. J. Clin. Nutr. 2009, 89, 1–14. [Google Scholar] [CrossRef]
- Renwick, A.G. Incidence and Severity in Relation to Magnitude of Intake above the ADI or TDI: Use of Critical Effect Data. Regul. Toxicol. Pharmacol. 1999, 30, S79–S86. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Rother, K.I. Trends in the consumption of low-calorie sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef]
- Martyn, D.; Darch, M.; Roberts, A.; Lee, H.Y.; Yaqiong Tian, T.; Kaburagi, N.; Belmar, P. Low-/No-Calorie Sweeteners: A Review of Global Intakes. Nutrients 2018, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Toews, I.; Lohner, S.; Küllenberg de Gaudry, D.; Sommer, H.; Meerpohl, J.J. Association between intake of non-sugar sweeteners and health outcomes: Systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ 2019, 364, k4718. [Google Scholar] [CrossRef]
- Ruiz, E.; Rodriguez, P.; Valero, T.; Ávila, J.M.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G. Dietary Intake of Individual (Free and Intrinsic) Sugars and Food Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 275. [Google Scholar] [CrossRef]
- Latasa, P.; Louzada, M.; Martinez Steele, E.; Monteiro, C.A. Added sugars and ultra-processed foods in Spanish households (1990-2010). Eur. J. Clin. Nutr. 2018, 72, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Azaïs-Braesco, V.; Sluik, D.; Maillot, M.; Kok, F.; Moreno, L.A. A review of total & added sugar intakes and dietary sources in Europe. Nutr. J. 2017, 16, 6. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Ribas, L.; Inglès, C.; Fuentes, M.; Lloveras, G.; Salleras, L. Cyclamate consumption in Catalonia, Spain (1992): Relationship with the body mass index. Food Addit. Contam. 1996, 13, 695–703. [Google Scholar] [CrossRef] [PubMed]
- European Union (EU). Commisssion Regulation (EU) No 1169/2011 of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Off. J. Eur. Union 2011, 304, 18–63. [Google Scholar]
- Piernas, C.; Ng, S.W.; Popkin, B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr. Obes. 2013, 8, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Vaesken, M.L.; Partearroyo, T.; Cano, A.; Urrialde, R.; Varela-Moreiras, G. Novel database of declared low- and no-calorie sweeteners from foods and beverages available in Spain. J. Food Compost. Anal. 2019, 82, 103234. [Google Scholar] [CrossRef]
- Ministerio de Agricultura Pesca y Alimentación. Infome del Consumo Alimentario en España 2018; Ministerio de Agricultura, Pesca y Alimentación Secretaría General Técnica: Madrid, Spain, 2019. [Google Scholar]
- Popkin, B.M.; Hawkes, C. Sweetening of the global diet, particularly beverages: Patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016, 4, 174–186. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y. Metabolic effects of non-nutritive sweeteners. Physiol. Behav. 2015, 152, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S. Non-sugar sweeteners and health. BMJ 2019, 364, k5005. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Raposo, A.; Aranceta-Bartrina, J.; Varela-Moreiras, G.; Logue, C.; Laviada, H.; Socolovsky, S.; Pérez-Rodrigo, C.; Aldrete-Velasco, J.A.; Meneses Sierra, E.; et al. Ibero⁻American Consensus on Low- and No-Calorie Sweeteners: Safety, Nutritional Aspects and Benefits in Food and Beverages. Nutrients 2018, 10, 818. [Google Scholar] [CrossRef]
- Rodríguez, I.T.; Ballart, J.F.; Pastor, G.C.; Jordà, E.B.; Val, V.A. Validation of a short questionnaire on frequency of dietary intake: Reproducibility and validity. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar] [PubMed]
- Laja, A.; Samaniego-Vaesken, M.L.; Partearroyo, T.; Varela-Moreiras, G. Informe de Expertos en el campo de los edulcorantes bajos o sin calorías (científicos, prescriptores, administraciones, industria, asociaciones) basado en metodología DAFO (Debilidades, Amenazas, Fortalezas, Oportunidades) Farmacia; Departamento de Ciencias Farmacéuticas y de la Salud, Facultad de Farmacia: Madrid, Spain, 2020. [Google Scholar]
- Spanish Society of Community Nutrition (SENC). Guide to Healthy Eating for Primary Healthcare and Citizen Groups. Recommendations for a Healthy, Responsible and Sustainable Individual, Family or Collective Diet; Editorial Planeta: Madrid, Spain, 2018. [Google Scholar]
- Russell, C.; Grimes, C.; Baker, P.; Sievert, K.; Lawrence, M.A. The drivers, trends and dietary impacts of non-nutritive sweeteners in the food supply: A narrative review. Nutr. Res. Rev. 2020, 10, 1–24. [Google Scholar] [CrossRef]
- Sambra, V.; López-Arana, S.; Cáceres, P.; Abrigo, K.; Collinao, J.; Espinoza, A.; Valenzuela, S.; Carvajal, B.; Prado, G.; Peralta, R.; et al. Overuse of Non-caloric Sweeteners in Foods and Beverages in Chile: A Threat to Consumers’ Free Choice? Front. Nutr. 2020, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Martínez, X.; Zapata, Y.; Pinto, V.; Cornejo, C.; Elbers, M.; Graaf, M.V.; Villarroel, L.; Hodgson, M.I.; Rigotti, A.; Echeverría, G. Intake of Non-Nutritive Sweeteners in Chilean Children after Enforcement of a New Food Labeling Law that Regulates Added Sugar Content in Processed Foods. Nutrients 2020, 12, 1594. [Google Scholar] [CrossRef] [PubMed]
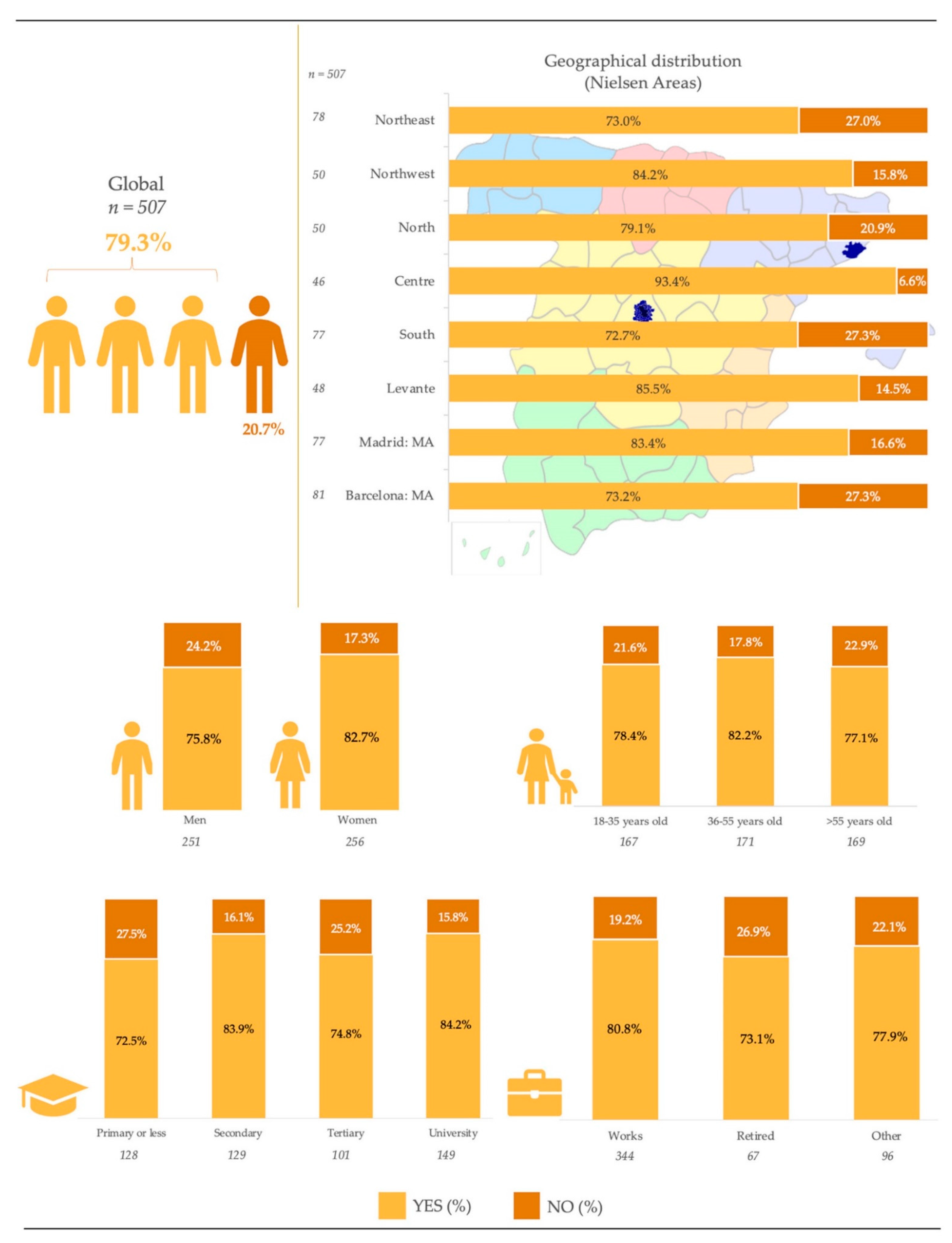

 |  |  | |||||
| n | % | n | % | n | % | ||
| Global | 507 | 100 | 251 | 49.5 | 256 | 50.5 | |
 | 18–35 years | 167 | 33.0 | 86 | 51.5 | 81 | 48.5 |
| 36–55 years | 171 | 33.7 | 80 | 46.8 | 91 | 53.2 | |
| >55 years | 169 | 33.3 | 85 | 50.3 | 84 | 49.7 | |
 | Northeast | 78 | 15.4 | 39 | 50.0 | 39 | 50.0 |
| Northwest | 50 | 9.9 | 25 | 50.0 | 25 | 50.0 | |
| North | 50 | 9.9 | 23 | 46.0 | 27 | 54.0 | |
| Centre | 46 | 9.1 | 24 | 52.2 | 22 | 47.8 | |
| South | 77 | 15.1 | 40 | 51.9 | 37 | 48.1 | |
| Levante | 48 | 9.5 | 23 | 47.9 | 25 | 52.1 | |
| Madrid: MA (1) | 77 | 15.2 | 38 | 49.4 | 39 | 50.6 | |
| Barcelona: MA (1) | 81 | 16.0 | 39 | 48.1 | 42 | 51.9 | |
 | Primary or less | 128 | 25.2 | 67 | 52.3 | 61 | 47.7 |
| Secondary | 129 | 25.4 | 58 | 45.0 | 71 | 55.0 | |
| Tertiary | 101 | 19.9 | 51 | 50.5 | 50 | 49.5 | |
| University | 149 | 29.4 | 75 | 50.3 | 74 | 49.7 | |
 | Works | 344 | 67.9 | 177 | 51.7 | 167 | 48.3 |
| Retired | 67 | 13.2 | 44 | 65.7 * | 23 | 34.3 | |
| Other: unemployed, students, housewives | 96 | 18.9 | 29 | 30.2 * | 67 | 69.8 | |
| Presence of LNCS | ||
|---|---|---|
| Food Group | Yes (%) | No (%) |
| Appetizers | 1.7 | 98.3 |
| Beverages: alcoholic | 0.0 | 100.0 |
| Beverages: non-alcoholic | 36.1 | 63.9 |
| Canned fruit | 0.0 | 100.0 |
| Cereals and derivatives | 4.3 | 95.7 |
| Eggs | 0.0 | 100.0 |
| Fish and shellfish | 0.0 | 100.0 |
| Fruits | 0.0 | 100.0 |
| Meat: red and/or processed | 5.1 | 94.9 |
| Milk and dairy products | 7.0 | 93.0 |
| Nuts and seeds | 0.0 | 100.0 |
| Pulses | 0.0 | 100.0 |
| Ready-to-eat meals | 0.0 | 100.0 |
| Sauces and condiments | 1.0 | 99.0 |
| Sugar and sweets | 14.2 | 85.9 |
| Vegetables | 0.0 | 100.0 |
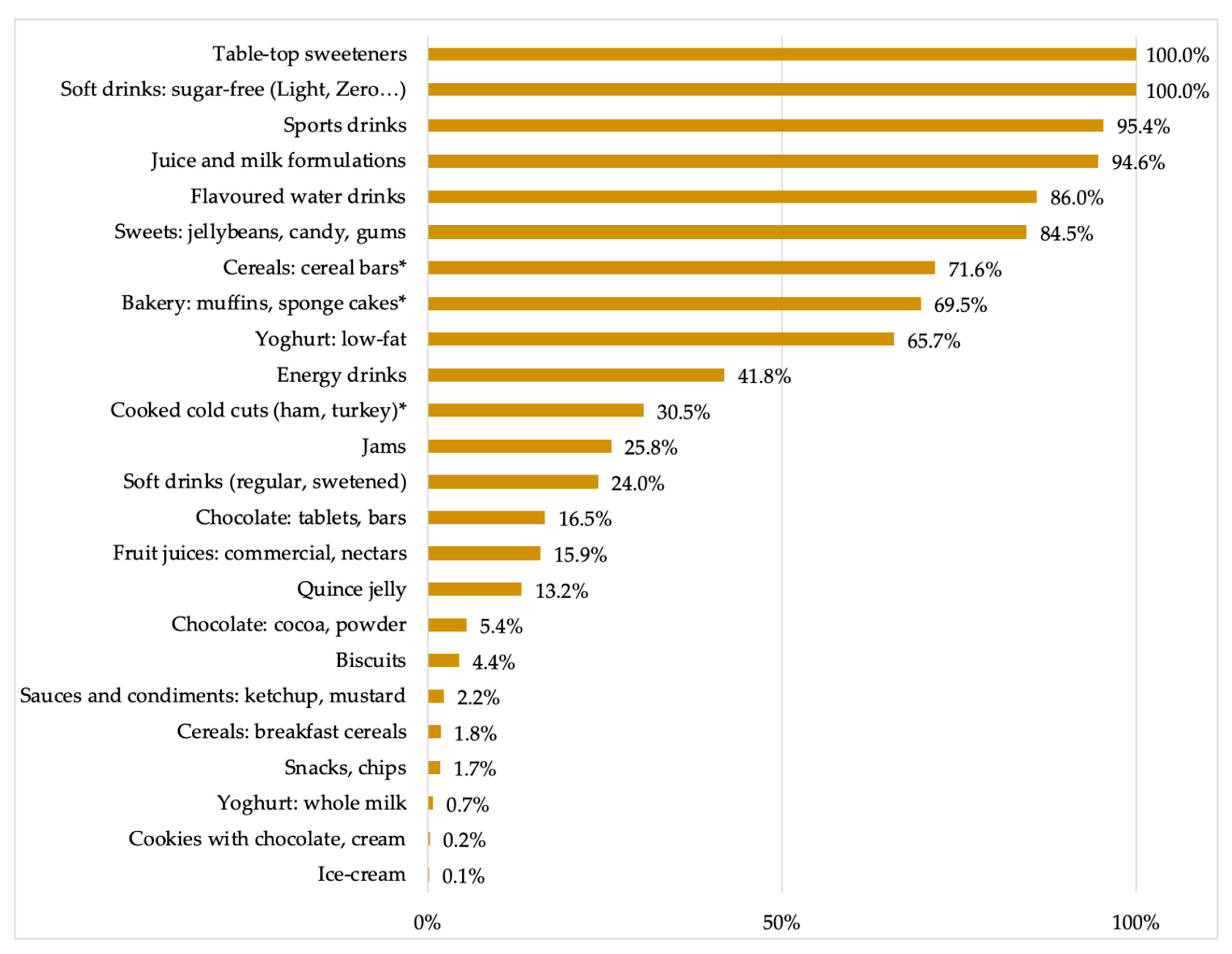
| Food Product | % | Nº | LNCS | Food Product | % | Nº | LNCS |
|---|---|---|---|---|---|---|---|
| Table top sweeteners | 100.0 | 6 | Acesulfame K (E-950) Aspartame (E-951) Cyclamate (E-952) Saccharin and its salts (E-954) Steviol glycosides (E-960) Thaumatin (E-957) | Soft drinks: Regular (sweetened) | 24.0 | 7 | Acesulfame K (E-950) Aspartame (E-951) Cyclamate (E-952) Steviol glycosides (E-960) Neohesperidine-DC (E-959) Saccharin and its salts (E-954) Sucralose (E-955) |
| Soft drinks: Low or sugar-free (Light, Diet, Zero…) | 100.0 | 6 | Acesulfame K (E-950) Aspartame (E-951) Cyclamate (E-952) Saccharin and its salts (E-954) Steviol glycosides (E-960) Sucralose (E-955) | Chocolate: tablets, bars | 16.5 | 2 | Steviol glycosides (E-960) Maltitol (E-965) |
| Sports drinks | 95.4 | 3 | Acesulfame K (E-950) Aspartame (E-951) Sucralose (E-955) | Fruit juices: commercial, nectars | 15.9 | 6 | Acesulfame K (E-950) Cyclamate (E-952) Neohesperidine-DC (E-959) Saccharin and its salts (E-954) Steviol glycosides (E-960) Sucralose (E-955) |
| Juice and milk formulations | 94.6 | 2 | Acesulfame K (E-950) Sucralose (E-955) | Quince jelly | 13.2 | 3 | Maltitol (E-965) Steviol glycosides (E-960) Sucralose (E-955) |
| Flavoured water drinks | 86.0 | 4 | Acesulfame K (E-950) Isomalt (E-953) Steviol glycosides (E-960) Sucralose (E-955) | Chocolate: cocoa, powder | 5.4 | 2 | Acesulfame K (E-950) Salt of aspartame-acesulfame K (E-962) |
| Sweets: jelly beans, candy, chewing gum | 84.5 | 12 | Acesulfame K (E-950) Aspartame (E-951) Isomalt (E-953) Maltitol (E-965) Mannitol (E-421) Neohesperidine-DC (E-959) Saccharin and its salts (E-954) Salt of aspartame-acesulfame K (E-962) Sorbitol (E-420) Steviol glycosides (E-960) Sucralose (E-955) Xylitol (E-967) | Biscuits | 4.4 | 3 | Isomalt (E-953) Maltitol (E-965) Sucralose (E-955) |
| Cereals: cereal bars | 71.6 | 1 | Sorbitol (E-420) * | Sauces and condiments: ketchup, mustard | 2.2 | 3 | Cyclamate (E-952) Saccharin and its salts (E-954) Sucralose (E-955) |
| Bakery: muffins, sponge cakes | 69.5 | 1 | Sorbitol (E-420) * | Cereals: breakfast cereals | 1.8 | 2 | Acesulfame K (E-950) Maltitol (E-965) |
| Yoghurt: low-fat | 65.7 | 6 | Acesulfame K (E-950) Aspartame (E-951) Neotame (E-961) Salt of aspartame-acesulfame K (E-962) Steviol glycosides (E-960) Sucralose (E-955) | Appetizers: snacks, chips | 1.7 | 1 | Aspartame (E-951) |
| Energy drinks | 41.8 | 3 | Acesulfame K (E-950) Aspartame (E-951) Sucralose (E-955) | Yoghurt: whole milk | 0.7 | 3 | Acesulfame K (E-950) Neotame (E-961) Sucralose (E-955) |
| Cooked cold cuts (ham, turkey) | 30.5 | 1 | Sorbitol (E-420) * | Cookies: with chocolate, cream | 0.2 | 1 | Maltitol (E-965) |
| Jams | 25.8 | 6 | Acesulfame K (E-950) Aspartame (E-951) Steviol glycosides (E-960) Maltitol (E-965) Sorbitol (E-420) Sucralose (E-955) | Ice-cream | 0.1 | 2 | Acesulfame K (E-950) Maltitol (E-965) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redruello-Requejo, M.; González-Rodríguez, M.; Samaniego-Vaesken, M.d.L.; Montero-Bravo, A.; Partearroyo, T.; Varela-Moreiras, G. Low- and No-Calorie Sweetener (LNCS) Consumption Patterns Amongst the Spanish Adult Population. Nutrients 2021, 13, 1845. https://doi.org/10.3390/nu13061845
Redruello-Requejo M, González-Rodríguez M, Samaniego-Vaesken MdL, Montero-Bravo A, Partearroyo T, Varela-Moreiras G. Low- and No-Calorie Sweetener (LNCS) Consumption Patterns Amongst the Spanish Adult Population. Nutrients. 2021; 13(6):1845. https://doi.org/10.3390/nu13061845
Chicago/Turabian StyleRedruello-Requejo, Marina, María González-Rodríguez, Mª de Lourdes Samaniego-Vaesken, Ana Montero-Bravo, Teresa Partearroyo, and Gregorio Varela-Moreiras. 2021. "Low- and No-Calorie Sweetener (LNCS) Consumption Patterns Amongst the Spanish Adult Population" Nutrients 13, no. 6: 1845. https://doi.org/10.3390/nu13061845
APA StyleRedruello-Requejo, M., González-Rodríguez, M., Samaniego-Vaesken, M. d. L., Montero-Bravo, A., Partearroyo, T., & Varela-Moreiras, G. (2021). Low- and No-Calorie Sweetener (LNCS) Consumption Patterns Amongst the Spanish Adult Population. Nutrients, 13(6), 1845. https://doi.org/10.3390/nu13061845









