The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
1. Introduction
1.1. Description of the Intervention
1.2. How the Intervention Might Work
1.3. Why It Is Important to do This Review
1.4. Aim
2. Methods
2.1. Types of Studies
2.2. Types of Participants
2.3. Types of Interventions
2.4. Types of Outcome Measures
- The relative abundance of gut microbiota (genera only) (Bifidobacterium);
- Lipopolysaccharides and lipopolysaccharides binding protein;
- Lipid profile, i.e., high density lipoprotein (HDL) cholesterol, total cholesterol, low density lipoprotein (LDL) cholesterol, triglycerides;
- Inflammatory markers, i.e., high sensitivity C-reactive protein (hsCRP), interleukin 6 (IL-6), tumour necrosis factor α (TNF-α), adeponectin, and leptin;
- Body mass index (BMI).
2.5. Search Methods for Identification of Studies
2.6. Data Collection and Analysis
2.6.1. Selection of Studies
2.6.2. Data Extraction and Management
2.6.3. Assessment of Risk of Bias in Included Studies
2.6.4. Data Analysis
2.6.5. Effect Size
3. Results
Risk of Bias of Included Studies
- (a)
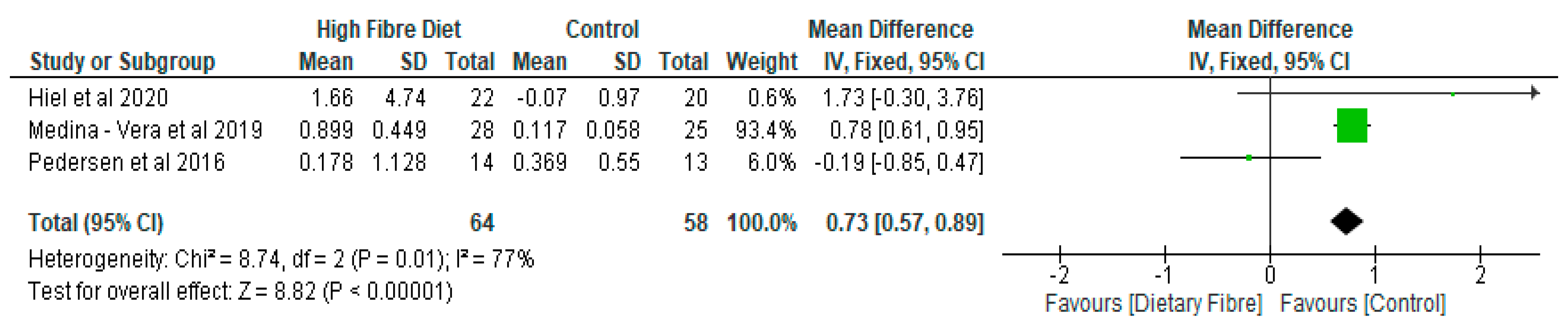
- Gut Microbiota
- (b)
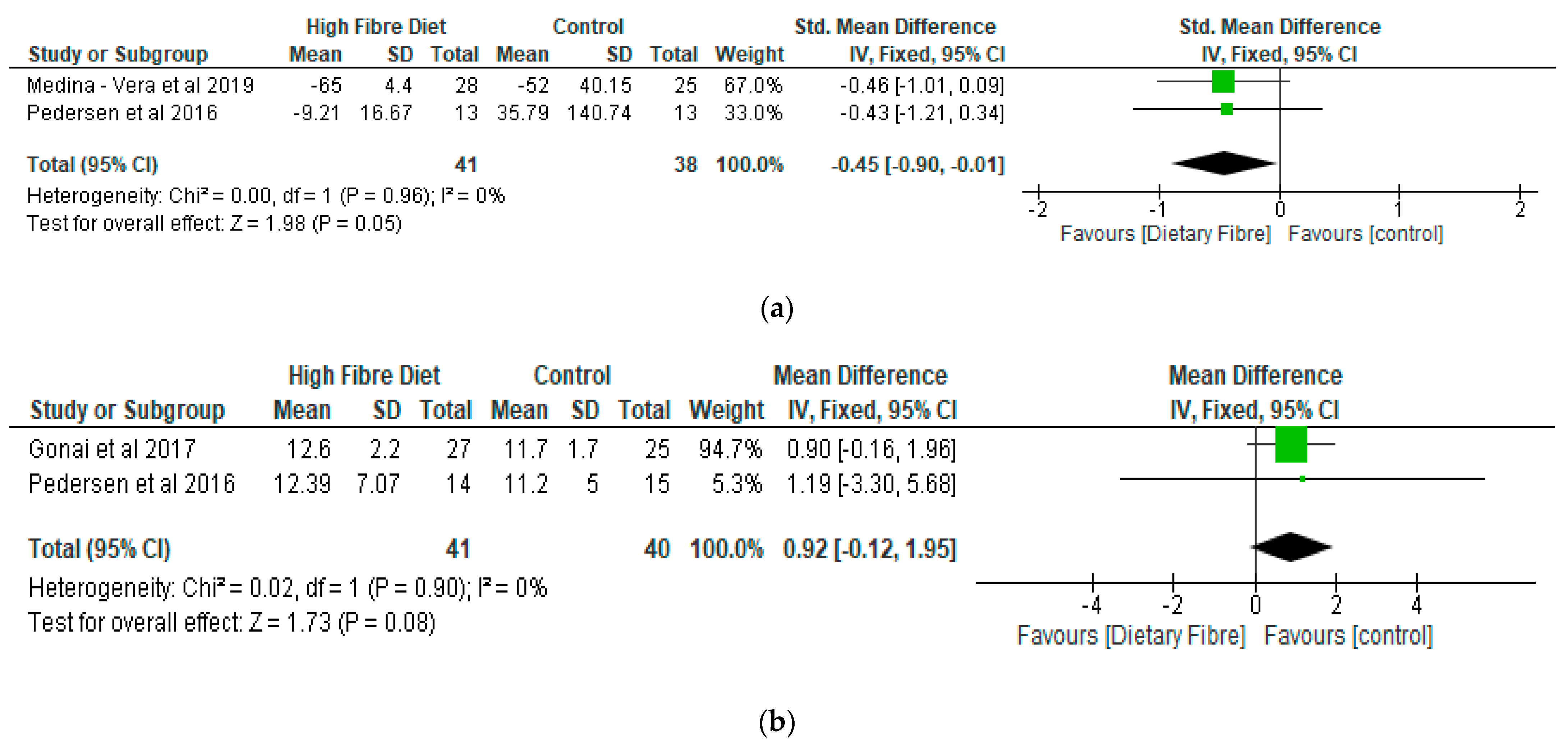
- Lipopolysaccharide (LPS) and Lipopolysaccharide Binding Protein (LBP)
- (c)
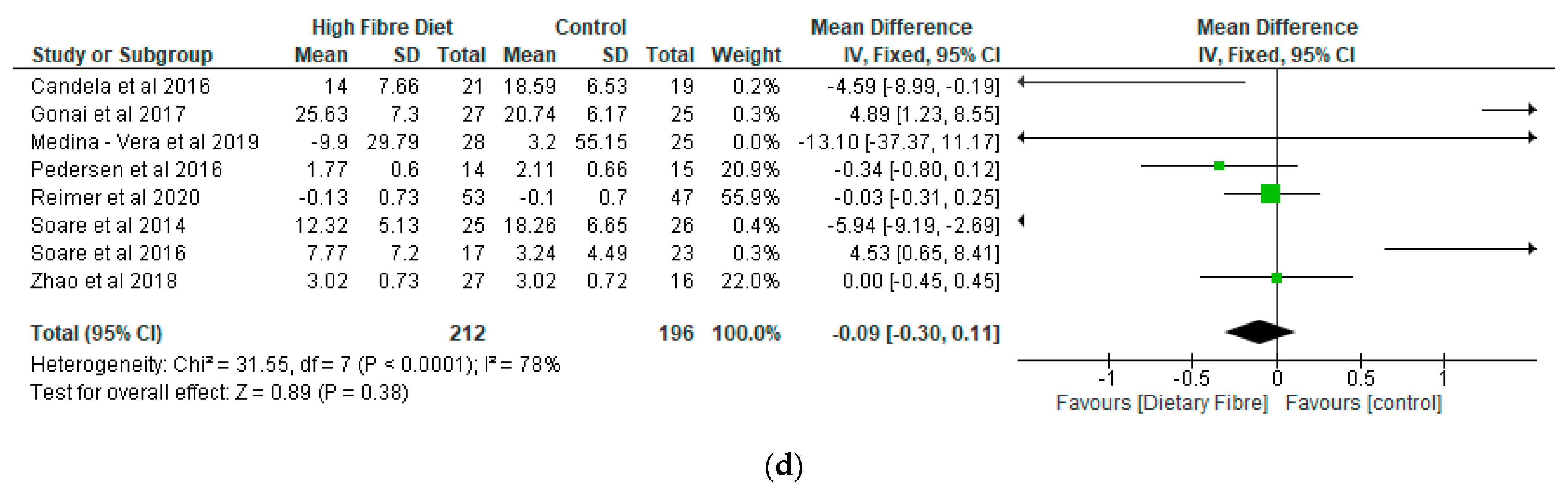
- Lipid Profile
- (d)
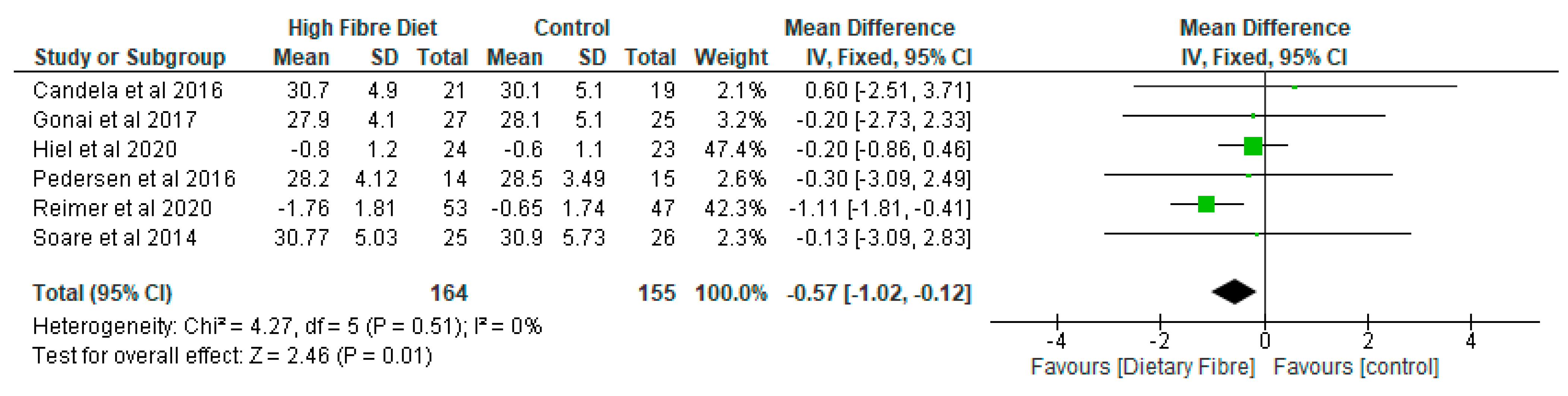
- Inflammatory Markers
- (e)
- Body Mass Index (BMI)
4. Discussion
Limitation of the Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; López Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals that influence gut microbiota as prophylactics and for the treatment of obesity and inflammatory diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Woldeamlak, B.; Yirdaw, K.; Biadgo, B. Role of gut microbiota in type 2 diabetes mellitus and its complications: Novel insights and potential intervention strategies. Korean J. Gastroenterol. 2019, 74, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Aliasgharzadeh, A.; Aliloo, A.; Ghotaslou, R.; Arbabi, S. Comparison of bifidobacterium spp. and lactobacillus spp. count in faeces of patients with type 2 diabetes mellitus and healthy people. Middle East J. Fam. Med. 2018, 16, 102–106. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Leylabadlo, H.E.; Sanaie, S.; Sadeghpour Heravi, F.; Ahmadian, Z.; Ghotaslou, R. From role of gut microbiota to microbial-based therapies in type 2-diabetes. Infect. Genet. Evol. 2020, 81, 104268. [Google Scholar] [CrossRef]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef]
- Davison, K.M.; Temple, N.J. Cereal fiber, fruit fiber, and type 2 diabetes: Explaining the paradox. J. Diabetes Complicat. 2018, 32, 240–245. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Davy, B.M.; Halliday, T.M.; Hulver, M.W.; Neilson, A.P.; Ponder, M.A.; Davy, K.P. The effect of prebiotic supplementation with inulin on cardiometabolic health: Rationale, design, and methods of a controlled feeding efficacy trial in adults at risk of type 2 diabetes. Contemp. Clin. Trials 2015, 45, 328–337. [Google Scholar] [CrossRef]
- Fallucca, F.; Porrata, C.; Fallucca, S.; Pianesi, M. Influence of diet on gut microbiota, inflammation and type 2 diabetes mellitus. First experience with macrobiotic Ma-Pi 2 diet. Diabetes Metab. Res. Rev. 2014, 30, 48–54. [Google Scholar] [CrossRef]
- Scientific Advisory Committee on Nutrition. Carbohydrate and Health. 2015. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf (accessed on 16 March 2021).
- Scientific Advisory Committee on Nutrition. Statement on Dietary Fibre. 2008. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/339367/SACN_Draft_position_statement_on_dietary_fibre_and_health_and_dietary_fibre_definition_2008.pdf (accessed on 16 March 2021).
- Fang, Q.; Hu, J.; Nie, Q.; Nie, S. Effects of polysaccharides on glycometabolism based on gut microbiota alteration. Trends Food Sci. Technol. 2019, 92, 65–70. [Google Scholar] [CrossRef]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Nie, Q.; Chen, H.; Hu, J.; Fan, S.; Nie, S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 848–863. [Google Scholar] [CrossRef]
- Jackson, M.A.; Verdi, S.; Maxan, M.-E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.; Williams, F.M.K.; Menni, C.; Bell, J.T.; et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Feng, Q.-Q.; Ojo, O.O.; Wang, X.-H. The role of dietary fibre in modulating gut microbiota dysbiosis in patients with type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2020, 12, 3239. [Google Scholar]
- Gong, J.; Yang, C. Advances in the methods for studying gut microbiota and their relevance to the research of dietary fiber functions. Food Res. Int. 2012, 48, 916–929. [Google Scholar] [CrossRef]
- Diamant, M.; Blaak, E.E.; de Vos, W.M. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes. Rev. 2011, 12, 272–281. [Google Scholar] [CrossRef]
- Logan, A.C.; Jacka, F.N.; Prescott, S.L. Immune-microbiota interactions: Dysbiosis as a global health issue. Curr. Allergy Asthma Rep. 2016, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Bomba, A.; Strojný, L.; Chmelárová, A.; Hijová, E.; Bertková, I.; Mojžišová, G.; Žofcáková, J.; Sáláj, R.; Supuková, A.; Šoltéšoviá, A. The role of gut microflora in the pathogenesis of chronic diseases and possibilities for its modulation for their prevention. Microb. Ecol. Health Dis. 2013, 24, 4. [Google Scholar] [CrossRef]
- Houghton, D.; Hardy, T.; Stewart, C.; Errington, L.; Day, C.P.; Trenell, M.I.; Avery, L. Systematic review assessing the effectiveness of dietary intervention on gut microbiota in adults with type 2 diabetes. Diabetologia 2018, 61, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Web Plot Digitizer. 2021. Available online: https://apps.automeris.io/wpd/ (accessed on 5 March 2021).
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.; Wu, H.; Jaiyeola, E.; Diribe, O.; La Ragione, R.; Robertson, M.D.; Wright, J.; Gallagher, E.; Horton, F.; Hinton, P.; et al. Host–microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br. J. Nutr. 2016, 116, 1869–1877. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- The Nordic Cochrane Centre. Review Manager, Version 5.3; The Nordic Cochrane Centre; The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Critical Appraisal Skills Programme (CASP). CASP Randomised Controlled Trial Checklist. Available online: https://casp-uk.net/wp-content/uploads/2018/03/CASP-Randomised-Controlled-Trial-Checklist-2018_fillable_form.pdf (accessed on 3 March 2021).
- Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheault, D.; Leyrolle, Q.; Bindels, L.B.; Gomes da Silveira Cauduro, C.; Mulders, M.D.G.H.; Zamariola, G.; Azzi, A.-S.; et al. Link between gut microbiota and health outcomes in inulin -treated obese patients: Lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin. Nutr. 2020, 39, 3618–3628. [Google Scholar] [CrossRef]
- Reimer, R.A.; Wharton, S.; Green, T.J.; Manjoo, P.; Ramay, H.R.; Lyon, M.R.; Gahler, R.J.; Wood, S. Effect of a functional fibre supplement on glycemic control when added to a year-long medically supervised weight management program in adults with type 2 diabetes. Eur. J. Nutr. 2021, 60, 1237–1251. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Gonai, M.; Shigehisa, A.; Kigawa, I.; Kurasaki, K.; Chonan, O.; Matsuki, T.; Yoshida, Y.; Aida, M.; Hamano, K.; Terauchi, Y. Galacto-oligosaccharides ameliorate dysbiotic Bifidobacteriaceae decline in Japanese patients with type 2 diabetes. Benef. Microbes 2017, 8, 705–716. [Google Scholar] [CrossRef]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.-M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef]
- Candela, M.; Biagi, E.; Soverini, M.; Consolandi, C.; Quercia, S.; Severgnini, M.; Peano, C.; Turroni, S.; Rampelli, S.; Pozzilli, P.; et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br. J. Nutr. 2016, 116, 80–93. [Google Scholar] [CrossRef]
- Soare, A.; Khazrai, Y.M.; Del Toro, R.; Roncella, E.; Fontana, L.; Fallucca, S.; Angeletti, S.; Formisano, V.; Capata, F.; Ruiz, V.; et al. The effect of the macrobiotic Ma-Pi 2 diet vs. The recommended diet in the management of type 2 diabetes: The randomized controlled MADIAB trial. Nutr. Metab. 2014, 11, 39. [Google Scholar] [CrossRef]
- Soare, A.; Del Toro, R.; Roncella, E.; Khazrai, Y.M.; Angeletti, S.; Dugo, L.; Fallucca, S.; Fontana, L.; Altomare, M.; Formisano, V.; et al. The effect of macrobiotic Ma-Pi 2 diet on systemic inflammation in patients with type 2 diabetes: A post hoc analysis of the MADIAB trial. BMJ Open Diabetes Res. Care 2015, 3, e000079. [Google Scholar] [CrossRef]
- Soare, A.; Del Toro, R.; Khazrai, Y.M.; Di Mauro, A.; Fallucca, S.; Angeletti, S.; Skrami, E.; Gesuita, R.; Tuccinardi, D.; Manfrini, S.; et al. A 6-month follow-up study of the randomized controlled Ma-Pi macrobiotic dietary intervention (MADIAB trial) in type 2 diabetes. Nutr. Diabetes 2016, 6, e222. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Gholami, A.; Hariri, M. Effect of resistant starch type 2 on inflammatory mediators: A systematic review and meta-analysis of randomized controlled trials. Complementary Ther. Med. 2021, 56, 102597. [Google Scholar] [CrossRef] [PubMed]
- Tilves, C.M.; Zmuda, J.M.; Kuipers, A.L.; Nestlerode, C.S.; Evans, R.W.; Bunker, C.H.; Patrick, A.L.; Miljkovic, I. Association of lipopolysaccharide-binding protein with aging-related adiposity change and prediabetes among african ancestry men. Diabetes Care 2016, 39, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Kupsch, S.; Schromm, A.B. Lipopolysaccharide-binding protein is bound and internalized by host cells and colocalizes with LPS in the cytoplasm: Implications for a role of LBP in intracellular LPS-signaling. Biochim. Biophys. Acta 2016, 1863, 660–672. [Google Scholar] [CrossRef]
- Lim, P.S.; Chang, Y.-K.; Wu, T.-K. Serum Lipopolysaccharide-Binding Protein is Associated with Chronic Inflammation and Metabolic Syndrome in Hemodialysis Patients. Blood Purif. 2019, 47, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M.. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Durrer Schutz, D.; Busetto, L.; Dicker, D.; Farpour-Lambert, N.; Pryke, R.; Toplak, H.; Widmer, D.; Yumuk, V.; Schutz, Y. European practical and patient-centred guidelines for adult obesity management in primary care. Obes. Facts 2019, 12, 40–66. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Saford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefts of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]





| Population | Interventions | Outcome | Design of Study | Search Terms Combined |
|---|---|---|---|---|
| Patients with diabetes | Dietary fibre | Gut microbiota | Randomised controlled trial | |
| Type 2 diabetes OR Patients with diabetes OR Diabetes OR diabetes mellitus, type 2 OR Diabetes complications OR diabetes mellitus | Fibre OR Dietary fibre OR Supplement OR Prebiotics OR Dietary supplements OR Dietary fibre OR Dietary carbohydrate OR Polysaccharide OR Wheat bran | Microbiome OR Microbiota OR Gastrointestinal microbiota OR Gut microbiota | #1 Controlled clinical trial OR Randomised controlled trial OR randomly OR trial randomised OR placebo OR groups OR drug therapy #2 “Animals” NOT “Humans” #3 #1 NOT #2 | Column 1 AND Column 2 AND Column 3 AND Column 4 |
| Authors/Country of Study | Type of Study | Details of Sample | Mean Age/Range (Years) | Aim | Type of Interventions | Findings |
|---|---|---|---|---|---|---|
| Birkeland et al. [37], Norway | RCT | n = 25 | 63.1: 41–73 | To examine the effect of inulin-type fructans on faecal microbiota and short chain fatty acids in patients with type 2 diabetes. | Inulin-type fructans (a mixture of oligofructose and inulin) versus placebo (maltodextrin) A 4 week washout separated 6 weeks of treatment | The results found a moderate potential of inulin-type fructans to promote the composition of gut microbiota and to increase microbial fermentation in T2D. |
| Candela et al. [38], Italy | RCT | Ma-Pi 2 diet (n = 21), control diet (n = 19) | 66 | Two different energy-restricted dietary approaches were explored, i.e., the fibre-rich macrobiotic Ma-Pi 2 diet or a control diet | Macrobiotic Ma-Pi 2 diet rich in fibre versus control diet. A 21-day treatment | The Ma-Pi 2 diet was effective in alleviating the increase of possible proinflammatory groups, in the gut ecosystem, but not the control diet. It demonstrated the possibility of reversing proinflammatory dysbiosis in patients with T2D and its effectiveness in improving metabolic control. |
| Gonai et al. [36], Japan | RCT | GOS (n = 27), placebo (n = 25) | GOS (55 ± 11) Placebo (54 ± 12) | To evaluate the role of GOS on glycaemic control, gut microbiota, and metabolites in patients with type 2 diabetes. | GOS versus placebo (maltodextrin) A 4-week treatment | Bifidobacteriaceae was significantly restored in patients with diabetes after consuming GOS. On the other hand, there was no improvement in LBP and glucose tolerance during this short period of trial. It was shown that GOS could mitigate dysbiosis in patients with diabetes, and continuous intake of GOS may be useful in managing type 2 diabetes. |
| Hiel et al. [33], Belgium | RCT | 47 Metformin-treated participants (all diabetic, prebiotic n = 24, placebo n = 23) | Age ranged from 18 to 65 years. | To explore the effect of inulin supplementation with metformin in obese patients with T2D and their beneficial effects through modulation of gut microbiota. | Subjects were randomly assigned to the prebiotic or placebo arm A 3-month treatment | A large increase in Bifidobacterium may be due to inulin intake rather than a driver of prebiotic-linked biological outcomes. |
| Medina-Vera et al. [28], Mexico | RCT | T2D (n = 81) Final Group numbers analysed: DF (n = 28), placebo (n = 25) | DP (50.4 ± 8.7) Placebo (49.8 ± 10.6) | To examine the role of dietary intervention (functional food-based) on faecal microbiota and biochemical parameters in patients with type 2 diabetes. | A dietary portfolio (DP) versus placebo A 3-month treatment | The long term use of diets that are high in fibre, rich in polyphenol and vegetable-protein-based provide advantages in enhancing the faecal microbiota composition and may be used as therapies for managing dyslipidaemia and inflammation. |
| Pedersen et al. [29], UK | RCT | GOS (n = 14), placebo (n = 15) | GOS (56.7 ± 1.6) Placebo (58.1 ± 1.7) | To compare the effects of prebiotic supplementation with placebo treatment in patients with type 2 diabetes. | GOS versus placebo (maltodextrin) A 12-week treatment | As compared with the placebo, supplementation with prebiotic fibre did not appear to show any significant impact on clinical outcomes or bacterial abundances. |
| Reimer et al. [34], Canada | RCT | PGX® (n = 147), placebo (n = 143) | PGX® (56.2 ± 8.6) Placebo (53.4 ± 9.9) | To evaluate the adjunct effect of the soluble viscous fibre PGX® on glycemic control in patients with T2D. | PGX® versus placebo A 52-week treatment. | PGX® may be a useful adjunct to weight loss programs in patients with type 2 diabetes based on improvements in other metabolic parameters. |
| Soare et al. [39], Italy | RCT | Ma-Pi 2 diet (n = 25), control diet (n = 26) | Ma-Pi 2 diet (67 ± 8.163) Control diet (65 ± 7.284) | The effect of various dietary methods (the macrobiotic Ma-Pi 2 diet) were compared with standard diets recommended for patients with type 2 diabetes. | Fibre-rich macrobiotic Ma-Pi 2 diet versus control diet A 21-day treatment | There was significantly better improvements in metabolic control in patients with type 2 diabetes following the intervention with a short-term Ma-Pi 2 diet. |
| Soare et al. [40], Italy | RCT | Ma-Pi 2 diet (n = 25), control diet (n = 26) | Age ranged from 40 to 75 years | To investigate the effects of macrobiotic Ma-Pi 2 diet versus a standard recommended diet (control diet) on inflammatory markers in patients with T2D. | This was a post hoc analysis of the MADIAB trial A 21-day RCT. | As compared with the baseline data, it was found that Ma-Pi 2 diet was a safe dietary method of reducing levels of inflammatory markers, in the short term. |
| Soare et al. [41], Italy | RCT | Ma-Pi 2 diet (n = 17), control diet (n = 23) | Ma-Pi 2 diet (65 ± 8.89) Control diet (64 ± 8.15) | Evaluation of the advantages of the original 21-day intensive dietary interventions beyond the original MADIAB trial duration and into everyday life. | Fibre-rich macrobiotic Ma-Pi 2 diet versus control diet A 6-month follow-up study | There was higher percentage reduction in body weight and a higher percentage increase in LDL cholesterol in the Ma-Pi diet. Furthermore, all the participants’ total and LDL cholesterol levels were within recommended levels. |
| Zhao et al. [35], China | RCT | High dietary fibre (n = 27), control (n =16) | High dietary fibre (58.4 ± 6.2) Control (59.7 ± 6.0) | To assess the effect of gut microbiota and its role in glucose homeostasis in patients with type 2 diabetes. | High dietary fibre versus usual care A 84 days study | Dietary fibre was effective in promoting a group of SCFA-producing strains, while most of the other potential producers were either reduced or unchanged in patients with type 2 diabetes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojo, O.; Ojo, O.O.; Zand, N.; Wang, X. The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 1805. https://doi.org/10.3390/nu13061805
Ojo O, Ojo OO, Zand N, Wang X. The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients. 2021; 13(6):1805. https://doi.org/10.3390/nu13061805
Chicago/Turabian StyleOjo, Omorogieva, Osarhumwese Osaretin Ojo, Nazanin Zand, and Xiaohua Wang. 2021. "The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials" Nutrients 13, no. 6: 1805. https://doi.org/10.3390/nu13061805
APA StyleOjo, O., Ojo, O. O., Zand, N., & Wang, X. (2021). The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients, 13(6), 1805. https://doi.org/10.3390/nu13061805







