Maternal Serum Albumin Redox State Is Associated with Infant Birth Weight in Japanese Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
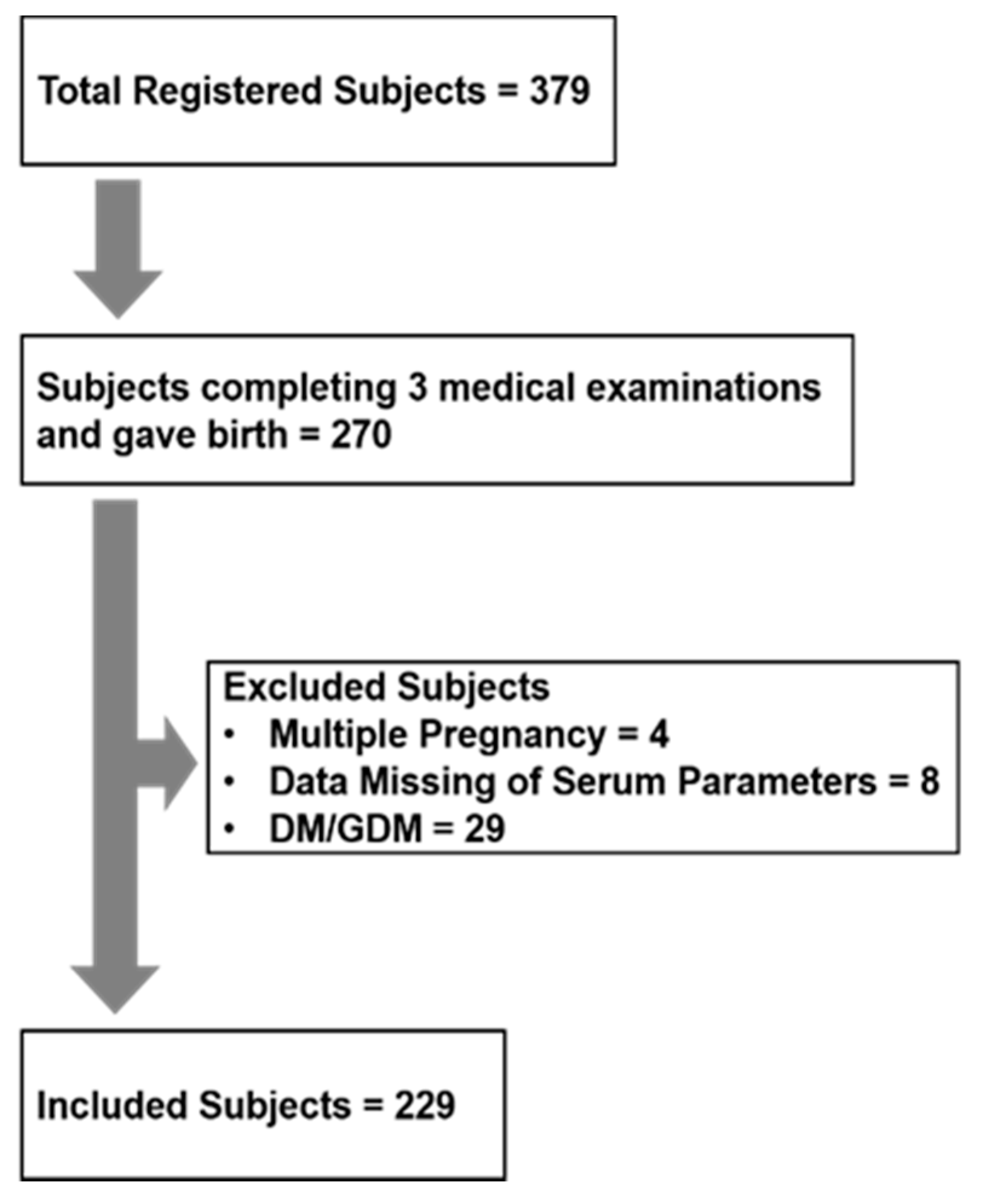
2.1. Human Observational Study
2.2. Animal Experiment Study
2.3. Blood Biochemistry
2.4. Statistical Analyses
3. Results
3.1. Human Observational Study
3.1.1. Characteristics of participants
3.1.2. Blood Biochemistry in Pregnant Women in the First, Second, and Third Trimesters
3.1.3. Relationship between Maternal Parameters and Infant Birth Weight
3.2. Animal Experiment
3.2.1. Animal Characteristics
3.2.2. Blood Biochemistry in Pregnant rats on PD9, PD16, and PD19
3.2.3. Relationship between Maternal Serum Parameters and Birth Weight in the Litter.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cutland, C.L.; Lackritz, E.M.; Mallett-Moore, T.; Bardají, A.; Chandrasekaran, R.; Lahariya, C.; Nisar, M.I.; Tapia, M.D.; Pathirana, J.; Kochhar, S.; et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017, 35, 6492–6500. [Google Scholar] [CrossRef]
- Harita, N.; Kariya, M.; Hayashi, T.; Sato, K.K.; Aoki, T.; Nakamura, K.; Endo, G.; Narimoto, K. Gestational bodyweight gain among underweight Japanese women related to small-for-gestational-age birth. J. Obstet. Gynaecol. Res. 2012, 38, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Itoh, H.; Tasaka, M.; Naito, H.; Fukuoka, Y.; Muramatsu Kato, K.; Kohmura, Y.K.; Sugihara, K.; Kanayama, N. Changes of maternal dietary intake, bodyweight and fetal growth throughout pregnancy in pregnant Japanese women. J. Obstet. Gynaecol. Res. 2013, 39, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Aoki, S.; Kurasawa, K.; Okuda, M.; Takahashi, T.; Hirahara, F. Associations of maternal pre-pregnancy underweight with small-for-gestational-age and spontaneous preterm birth, and optimal gestational weight gain in Japanese women. J. Obstet. Gynaecol. Res. 2014, 40, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, K.; Aoki, S.; Toma, R.; Fujiwara, K.; Sakamaki, K.; Hirahara, F. Pregnancy Outcomes Based on Pre-Pregnancy Body Mass Index in Japanese Women. PLoS ONE 2016, 11, e0157081. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Nagashima, K.; Suzuki, S.; Itoh, H. Application of Japanese guidelines for gestational weight gain to multiple pregnancy outcomes and its optimal range in 101,336 Japanese women. Sci. Rep. 2019, 9, 17310. [Google Scholar] [CrossRef] [PubMed]
- Shindo, R.; Aoki, M.; Yamamoto, Y.; Misumi, T.; Miyagi, E.; Aoki, S. Optimal gestational weight gain for underweight pregnant women in Japan. Sci. Rep. 2019, 9, 18129. [Google Scholar] [CrossRef]
- Gete, D.G.; Waller, M.; Mishra, G.D. Effects of maternal diets on preterm birth and low birth weight: A systematic review. Br. J. Nutr. 2020, 123, 446–461. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Reynolds, R.M.; Hardy, D.B. Developmental origins of health and disease: Current knowledge and potential mechanisms. Nutr. Rev. 2017, 75, 951–970. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. Living with the Past: Evolution, Development, and Patterns of Disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef]
- Torres, N.; Bautista, C.J.; Tovar, A.R.; Ordáz, G.; Rodríguez-Cruz, M.; Ortiz, V.; Granados, O.; Nathanielsz, P.W.; Larrea, F.; Zambrano, E. Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E270–E277. [Google Scholar] [CrossRef] [PubMed]
- Bautista, C.J.; Bautista, R.J.; Montaño, S.; Reyes-Castro, L.A.; Rodriguez-Peña, O.N.; Ibáñez, C.A.; Nathanielsz, P.W.; Zambrano, E. Effects of maternal protein restriction during pregnancy and lactation on milk composition and offspring development. Br. J. Nutr. 2019, 122, 141–151. [Google Scholar] [CrossRef]
- Armengaud, J.-B.; Dennebouy, Z.; Labes, D.; Fumey, C.; Wilson, A.; Candotti, F.; Yzydorczyk, C.; Simeoni, U. Intra-uterine growth restriction induced by maternal low-protein diet causes long-term alterations of thymic structure and function in adult male rat offspring. Br. J. Nutr. 2020, 123, 892–900. [Google Scholar] [CrossRef]
- Vickers, M.H.; Gluckman, P.D.; Coveny, A.H.; Hofman, P.L.; Cutfield, W.S.; Gertler, A.; Breier, B.H.; Harris, M. Neonatal Leptin Treatment Reverses Developmental Programming. Endocrinology 2005, 146, 4211–4216. [Google Scholar] [CrossRef] [PubMed]
- Durrant, L.M.; Khorram, O.; Buchholz, J.N.; Pearce, W.J. Maternal food restriction modulates cerebrovascular structure and contractility in adult rat offspring: Effects of metyrapone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R401–R410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pedroso, A.P.; Souza, A.P.; Dornellas, A.P.S.; Oyama, L.M.; Nascimento, C.M.O.; Santos, G.M.S.; Rosa, J.C.; Bertolla, R.P.; Klawitter, J.; Christians, U.; et al. Intrauterine Growth Restriction Programs the Hypothalamus of Adult Male Rats: Integrated Analysis of Proteomic and Metabolomic Data. J. Proteome Res. 2017, 16, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Ghosh, S.; Shin, B.C.; Devaskar, S.U. Role of microRNA-122 in hepatic lipid metabolism of the weanling female rat offspring exposed to prenatal and postnatal caloric restriction. J. Nutr. Biochem. 2019, 73, 108220. [Google Scholar] [CrossRef]
- Takemoto, Y.; Ota, E.; Yoneoka, D.; Mori, R.; Takeda, S. Japanese secular trends in birthweight and the prevalence of low birthweight infants during the last three decades: A population-based study. Sci. Rep. 2016, 6, 31396. [Google Scholar] [CrossRef]
- Hayashi, F.; Takimoto, H.; Yoshita, K.; Yoshiike, N. Perceived body size and desire for thinness of young Japanese women: A population-based survey. Br. J. Nutr. 2006, 96, 1154–1162. [Google Scholar] [CrossRef]
- Kitamura, Y.; Kogomori, C.; Hamano, H.; Maekawa, I.; Shimizu, T.; Shiga, S. Relationship between Changes in Fatty Acid Composition of the Erythrocyte Membranes and Fatty Acid Intake during Pregnancy in Pregnant Japanese Women. Ann. Nutr. Metab. 2017, 70, 268–276. [Google Scholar] [CrossRef]
- Fuhrman, M.P. The Albumin-nutrition connection: Separating myth from fact. Nutrition 2002, 18, 199–200. [Google Scholar] [CrossRef]
- Wada, Y.; Takeda, Y.; Kuwahata, M. Potential Role of Amino Acid/Protein Nutrition and Exercise in Serum Albumin Redox State. Nutrients 2018, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Sato, Y.; Miyazaki, K.; Takeda, Y.; Kuwahata, M. The reduced/oxidized state of plasma albumin is modulated by dietary protein intake partly via albumin synthesis rate in rats. Nutr. Res. 2017, 37, 46–57. [Google Scholar] [CrossRef]
- Wada, Y.; Komatsu, Y.; Izumi, H.; Shimizu, T.; Takeda, Y.; Kuwahata, M. Increased Ratio of Non-mercaptalbumin-1 Among Total Plasma Albumin Demonstrates Potential Protein Undernutrition in Adult Rats. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef]
- Wada, Y.; Izumi, H.; Shimizu, T.; Takeda, Y. A More Oxidized Plasma Albumin Redox State and Lower Plasma HDL Particle Number Reflect Low-Protein Diet Ingestion in Adult Rats. J. Nutr. 2020, 150, 256–266. [Google Scholar] [CrossRef]
- Bar-Or, D.; Heyborne, K.D.; Bar-Or, R.; Rael, L.T.; Winkler, J.V.; Navot, D. Cysteinylation of maternal plasma albumin and its association with intrauterine growth restriction. Prenat. Diagn. 2005, 25, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, S.B.; Ovesen, P.G.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. [Google Scholar] [CrossRef]
- Hayashi, T.; Era, S.; Kawai, K.; Imai, H.; Nakamura, K.; Onda, E.; Yoh, M. Observation for redox state of human serum and aqueous humor albumin from patients with senile cataract. Pathophysiology 2000, 6, 237–243. [Google Scholar] [CrossRef]
- Kubota, K.; Nakayama, A.; Takehana, K.; Kawakami, A.; Yamada, N.; Suzuki, E. A simple stabilization method of reduced albumin in blood and plasma for the reduced/oxidized albumin ratio measurement. Int. J. Biomed. Sci. 2009, 5, 293–301. [Google Scholar]
- Moore, V.M.; Davies, M.J.; Willson, K.J.; Worsley, A.; Robinson, J.S. Dietary Composition of Pregnant Women Is Related to Size of the Baby at Birth. J. Nutr. 2004, 134, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Halldorsson, T.I.; Willett, W.C.; Knudsen, V.K.; Gillman, M.W.; Mikkelsen, T.B.; Olsen, J.; The, N.C. Milk consumption during pregnancy is associated with increased infant size at birth: Prospective cohort study. Am. J. Clin. Nutr. 2007, 86, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Buettner, P.; Watt, K.; Clough, A.; Brimblecombe, J.; Judd, J. The effect of balanced protein energy supplementation in undernourished pregnant women and child physical growth in low- and middle-income countries: A systematic review and meta-analysis. Matern. Child. Nutr. 2015, 11, 415–432. [Google Scholar] [CrossRef]
- Geraghty, A.A.; O’Brien, E.C.; Alberdi, G.; Horan, M.K.; Donnelly, J.; Larkin, E.; Segurado, R.; Mehegan, J.; Molloy, E.J.; McAuliffe, F.M. Maternal protein intake during pregnancy is associated with child growth up to 5 years of age, but not through insulin-like growth factor-1: Findings from the ROLO study. Br. J. Nutr. 2018, 120, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, N.; Nagata, C.; Yasuo, S.; Morokuma, S.; Kato, K.; Sanefuji, M.; Shibata, E.; Tsuji, M.; Senju, A.; Kawamoto, T.; et al. Optimal protein intake during pregnancy for reducing the risk of fetal growth restriction: The Japan Environment and Children’s Study. Br. J. Nutr. 2018, 120, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Stephens, T.V.; Woo, H.; Innis, S.M.; Elango, R. Healthy pregnant women in Canada are consuming more dietary protein at 16- and 36-week gestation than currently recommended by the Dietary Reference Intakes, primarily from dairy food sources. Nutr. Res. 2014, 34, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.L.; Collins, C.E. High-protein diets during pregnancy: Healthful or harmful for offspring? Am. J. Clin. Nutr. 2014, 100, 993–995. [Google Scholar] [CrossRef]
- Ota, E.; Hori, H.; Mori, R.; Tobe-Gai, R.; Farrar, D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Stephens, T.V.; Payne, M.; Ball, R.O.; Pencharz, P.B.; Elango, R. Protein Requirements of Healthy Pregnant Women during Early and Late Gestation Are Higher than Current Recommendations. J. Nutr. 2014, 145, 73–78. [Google Scholar] [CrossRef]
- Payne, M.; Stephens, T.; Lim, K.; Ball, R.O.; Pencharz, P.B.; Elango, R. Lysine Requirements of Healthy Pregnant Women are Higher During Late Stages of Gestation Compared to Early Gestation. J. Nutr. 2018, 148, 94–99. [Google Scholar] [CrossRef]
- Ennis, M.A.; Rasmussen, B.F.; Lim, K.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G.; Elango, R. Dietary phenylalanine requirements during early and late gestation in healthy pregnant women. Am. J. Clin. Nutr. 2020, 111, 351–359. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | |
|---|---|
| Maternal background | |
| Participants (n) | 229 |
| Primipara (n) | 83 (36.2%) |
| Age (years)*1 | 31.6 ± 5.0 |
| Height (cm)*1 | 158.1 ± 5.4 |
| Pre-pregnancy body weight (kg)*1 & 2 | 51.7 ± 9.7 |
| Pre-pregnancy BMI*2 | 21.1 ± 3.4 |
| Pre-pregnancy BMI < 18.5 (n) *2 | 40 (17.5%) |
| Gestational outcome | |
| Gestation period (weeks)*1 | 39.2 ± 1.2 |
| Body weight at delivery (kg)*1 | 63.9 ± 9.9 |
| Weight gain (kg)*1 | 11.0 ± 4.0 |
| Vaginal delivery, Caesarean section (n, n) | 182, 47 |
| Male, Female (n, n) | 116, 113 |
| Birth weight (g)*1 | 3107 ± 388 |
| Low birth weight delivery (<2500 g; n) | 9 (3.9%) |
| Preterm delivery (<37 weeks; n) | 10 (4.4%) |
| Independent Variable | R | p |
|---|---|---|
| Serum reduced ALB ratio | ||
| 1st trimester | 0.066 | 0.322 |
| 2nd trimester | 0.108 | 0.105 |
| 3rd trimester | 0.177 | <0.01 |
| Serum ALB concentration | ||
| 1st trimester | 0.095 | 0.154 |
| 2nd trimester | 0.009 | 0.888 |
| 3rd trimester | −0.066 | 0.325 |
| Serum BUN concentration | ||
| 1st trimester | 0.069 | 0.296 |
| 2nd trimester | 0.024 | 0.718 |
| 3rd trimester | −0.011 | 0.864 |
| Independent Variable | Coefficient | SE | β | VIF | p | |
|---|---|---|---|---|---|---|
| Model 1 | (Intercept) | 1930.68 | 281.31 | − | <0.0001 | |
| R2 = 0.079 p < 0.0001 | Serum reduced ALB*2 | 12.02 | 3.94 | 0.196 | 1.01 | <0.01 |
| Pre-pregnancy body weight | 8.72 | 2.57 | 0.218 | 1.01 | <0.001 | |
| Model 2 | (Intercept) | 1978.40 | 288.32 | − | <0.0001 | |
| R2 = 0.06 p <0.001 | Serum reduced ALB*2 | 11.33 | 3.95 | 0.184 | 1.00 | <0.01 |
| Pre-pregnancy BMI | 21.54 | 7.42 | 0.187 | 1.00 | <0.01 | |
| Model 3 | (Intercept) | 1794.11 | 285.69 | − | <0.0001 | |
| R2 = 0.095 p < 0.0001 | Serum reduced ALB*2 | 11.39 | 3.89 | 0.185 | 1.00 | <0.01 |
| Body weight at delivery | 9.94 | 2.49 | 0.253 | 1.00 | <0.0001 | |
| Model 4 | (Intercept) | −2911.12 | 766.32 | − | <0.001 | |
| R2 = 0.216 p < 0.0001 | Serum reduced ALB*2 | 5.60 | 3.69 | 0.091 | 1.04 | 0.131 |
| Gestation period | 145.16 | 19.87 | 0.439 | 1.04 | <0.0001 | |
| Model 5 | (Intercept) | −3186.05 | 755.99 | - | <0.0001 | |
| R2 = 0.251 p < 0.0001 | Serum reduced ALB*2 | 6.73 | 3.63 | 0.110 | 1.05 | 0.065 |
| Pre-pregnancy body weight | 7.45 | 2.33 | 0.186 | 1.01 | <0.01 | |
| Gestation period | 140.40 | 19.54 | 0.424 | 1.05 | <0.0001 | |
| Model 6 | (Intercept) | −3186.05 | 755.99 | − | <0.0001 | |
| R2 = 0.240 p < 0.001 | Serum reduced ALB*2 | 6.10 | 3.64 | 0.099 | 1.04 | 0.096 |
| Pre-pregnancy BMI | 17.92 | 6.73 | 0.155 | 1.01 | <0.01 | |
| Gestation period | 141.25 | 19.67 | 0.427 | 1.05 | <0.0001 | |
| Model 7 | (Intercept) | −3180.65 | 750.21 | − | <0.0001 | |
| R2 = 0.260 P-p < 0.0001 | Serum reduced ALB*2 | 6.28 | 3.60 | 0.102 | 1.04 | 0.082 |
| Body weight at delivery | 8.22 | 2.27 | 0.209 | 1.01 | <0.001 | |
| Gestation period | 137.59 | 19.47 | 0.416 | 1.05 | <0.0001 |
| Independent Variable | R | p |
|---|---|---|
| Reduced ALB | ||
| PD9 | −0.102 | 0.687 |
| PD16 | 0.198 | 0.432 |
| PD19 | 0.697 | <0.01 |
| PD19inc | 0.614 | <0.01 |
| ALB | ||
| PD9 | 0.514 | <0.05 |
| PD16 | 0.381 | 0.119 |
| PD19 | 0.294 | 0.237 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, Y.; Ehara, T.; Tabata, F.; Komatsu, Y.; Izumi, H.; Kawakami, S.; Noshiro, K.; Umazume, T.; Takeda, Y. Maternal Serum Albumin Redox State Is Associated with Infant Birth Weight in Japanese Pregnant Women. Nutrients 2021, 13, 1764. https://doi.org/10.3390/nu13061764
Wada Y, Ehara T, Tabata F, Komatsu Y, Izumi H, Kawakami S, Noshiro K, Umazume T, Takeda Y. Maternal Serum Albumin Redox State Is Associated with Infant Birth Weight in Japanese Pregnant Women. Nutrients. 2021; 13(6):1764. https://doi.org/10.3390/nu13061764
Chicago/Turabian StyleWada, Yasuaki, Tatsuya Ehara, Fuka Tabata, Yosuke Komatsu, Hirohisa Izumi, Satomi Kawakami, Kiwamu Noshiro, Takeshi Umazume, and Yasuhiro Takeda. 2021. "Maternal Serum Albumin Redox State Is Associated with Infant Birth Weight in Japanese Pregnant Women" Nutrients 13, no. 6: 1764. https://doi.org/10.3390/nu13061764
APA StyleWada, Y., Ehara, T., Tabata, F., Komatsu, Y., Izumi, H., Kawakami, S., Noshiro, K., Umazume, T., & Takeda, Y. (2021). Maternal Serum Albumin Redox State Is Associated with Infant Birth Weight in Japanese Pregnant Women. Nutrients, 13(6), 1764. https://doi.org/10.3390/nu13061764






