Probiotic Supplementation and High-Intensity Interval Training Modify Anxiety-Like Behaviors and Corticosterone in High-Fat Diet-Induced Obesity Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets and Supplement
2.2. Inducing Obesity and Differentiation of Groups
2.3. Probiotic Supplement
2.4. Treadmill Training Protocol
2.5. Open Field (OF) Test
2.6. Elevated Plus Maze (EPM) Test
2.7. Blood Sampling and Corticosterone Analyses
2.8. Statistical Analysis
3. Results
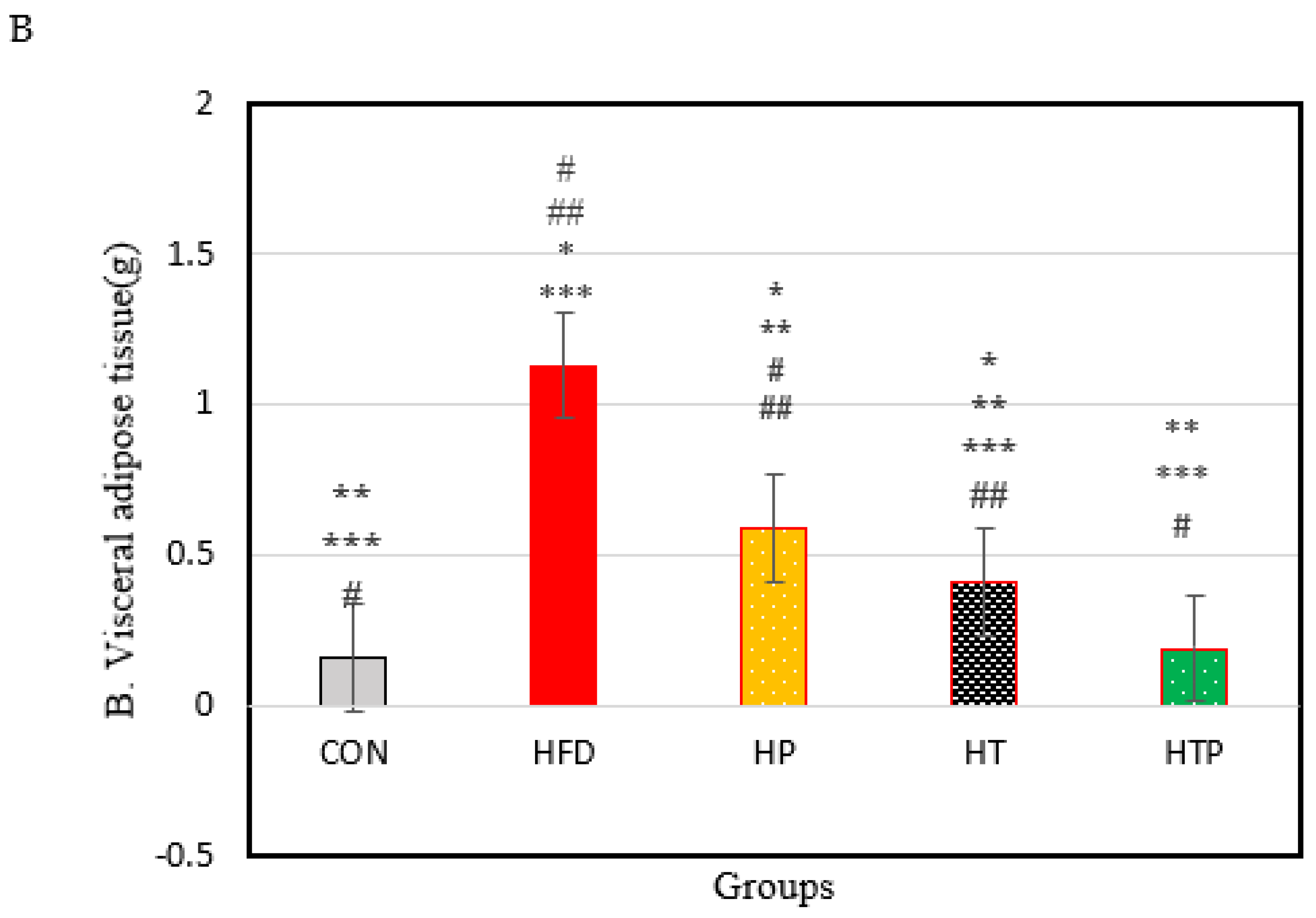
3.1. Body Weight and Visceral Fat Mass
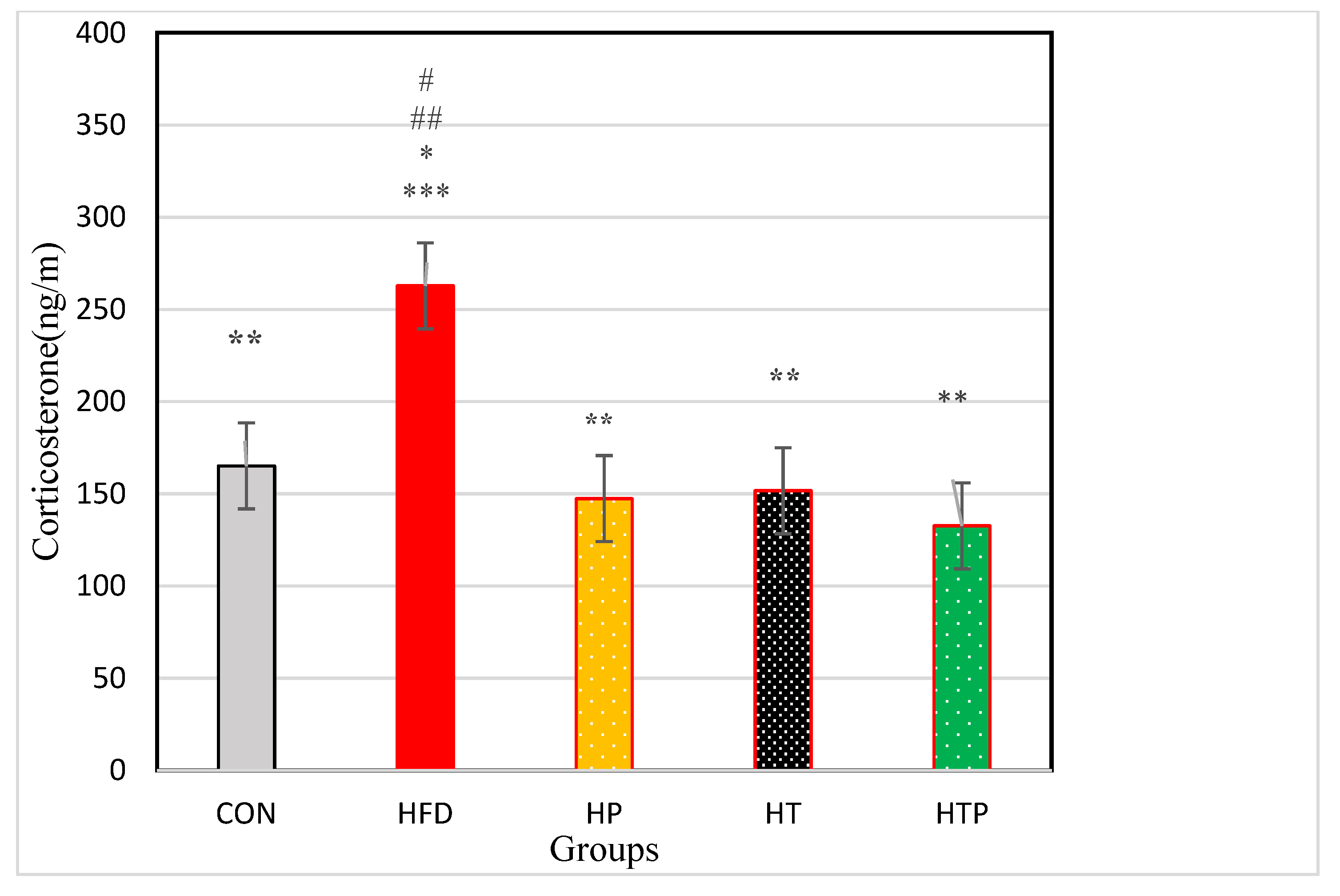
3.2. Serum Corticosterone Levels
3.3. Anxiety-Related Behavior Test
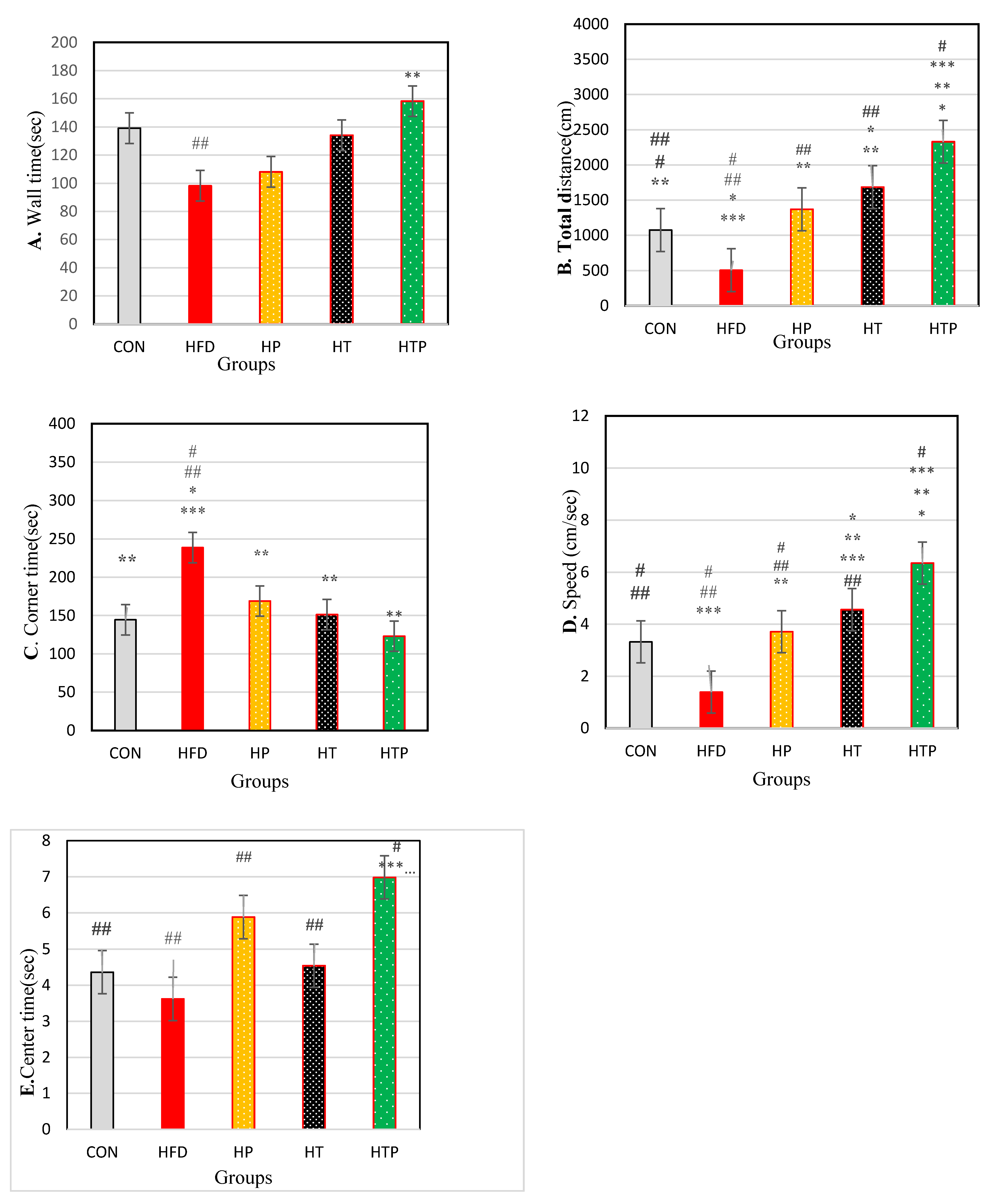
3.3.1. Open Field Measures
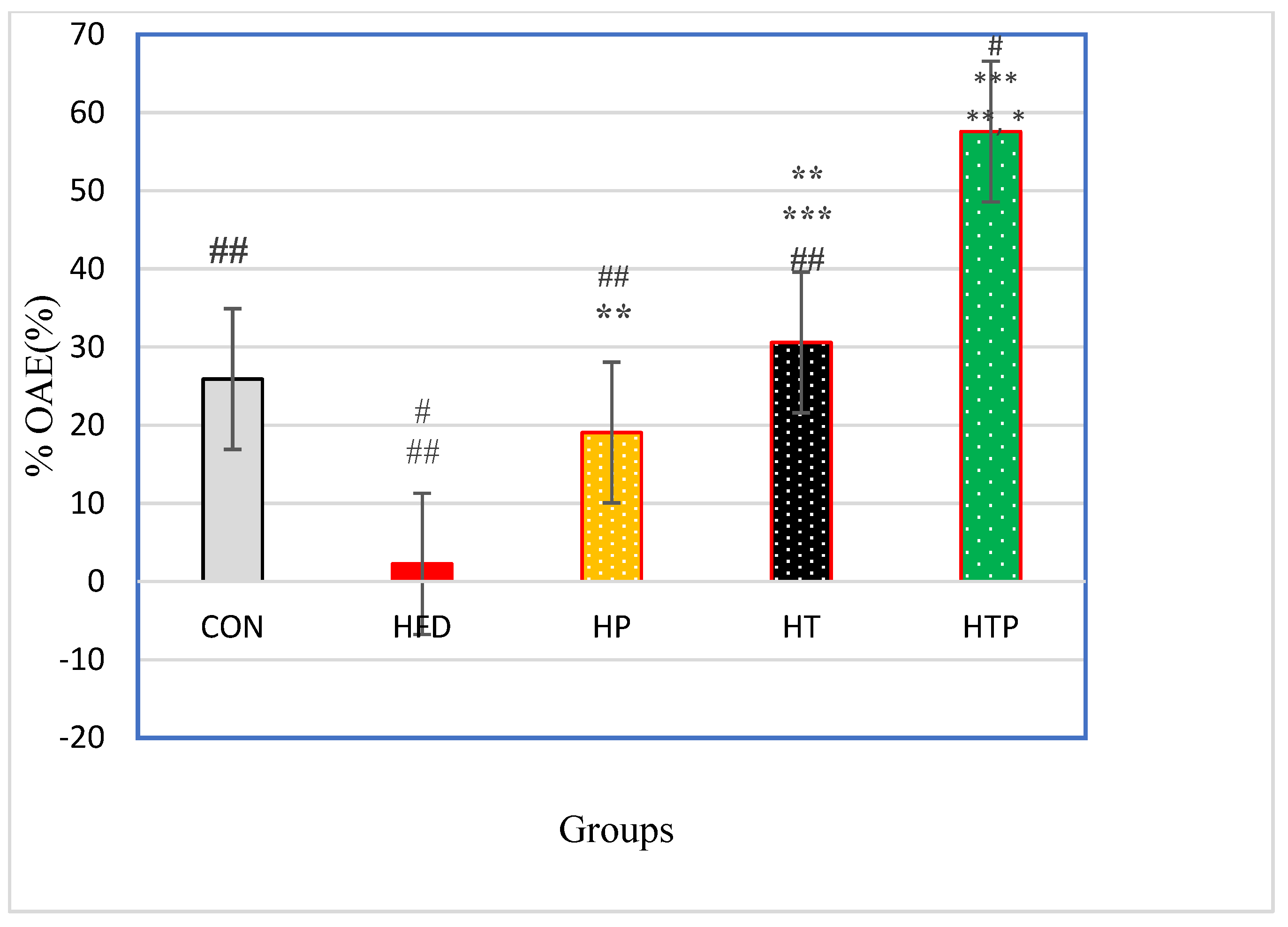
3.3.2. Elevated Plus Maze (EPM) Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almeida-Suhett, C.P.; Graham, A.; Chen, Y.; Deuster, P. Behavioral changes in male mice fed a high-fat diet are associated with IL-1β expression in specific brain regions. Physiol. Behav. 2017, 169, 130–140. [Google Scholar] [CrossRef]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R.; Grosso, G. Diet and Mental Health: Review of the Recent Updates on Molecular Mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, G.; Nitka, D.; Schmitz, N. The association between obesity and anxiety disorders in the population: A systematic review and meta-analysis. Int. J. Obes. 2010, 34, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.; O’Hara, K.; Xu, Z.; Yang, Y. HFD-induced energy states-dependent bidirectional control of anxiety levels in mice. Int. J. Obes. 2017, 41, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Prakash, R.; Chawla, D.; Du, W.; Didion, S.P.; Filosa, J.A.; Zhang, Q.; Brann, D.W.; Lima, V.V.; Tostes, R.C.; et al. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R1001. [Google Scholar] [CrossRef]
- Sivanathan, S.; Thavartnam, K.; Arif, S.; Elegino, T.; McGowan, P.O. Chronic high fat feeding increases anxiety-like behaviour and reduces transcript abundance of glucocorticoid signalling genes in the hippocampus of female rats. Behav. Brain. Res. 2015, 286, 265–270. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, G.; McCormick, B.P.; Song, T.; Xu, X. Effects of high-intensity interval versus mild-intensity endurance training on metabolic phenotype and corticosterone response in rats fed a high-fat or control diet. PLoS ONE 2017, 12, e0181684. [Google Scholar] [CrossRef]
- Ceppa, F.; Mancini, A.; Tuohy, K. Current evidence linking diet to gut microbiota and brain development and function. Int. J. Food. Sci. Nutr. 2019, 70, 1–19. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-glucose or-fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Avolio, E.; Fazzari, G.; Zizza, M.; De Lorenzo, A.; Di Renzo, L.; Alò, R.; Facciolo, R.M.; Canonaco, M. Probiotics modify body weight together with anxiety states via pro-inflammatory factors in HFD-treated Syrian golden hamster. Behav. Brain. Res. 2019, 356, 390–399. [Google Scholar] [CrossRef]
- Park, S.; Bae, J.H. Probiotics for weight loss: A systematic review and meta-analysis. Nutr. Res. 2015, 35, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Oral probiotics ameliorate the behavioral deficits induced by chronic mild stress in mice via the gut microbiota-inflammation axis. Front. Behav. Neurosci. 2018, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.F.; Kim, H.-M.; Lim, S.; Chung, M.-J.; Lim, C.-Y.; Koo, B.-S.; Kang, S.-S. Effect of probiotic administration on gut microbiota and depressive behaviors in mice. DARU J. Pharm. Sci. 2020, 28, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D.R.; Gorbach, S.L. Lactobacillus GG: Bacteriology and clinical applications. Gastroenterol. Clin. N. Am. 2005, 34, 483–498. [Google Scholar] [CrossRef]
- Ji, Y.S.; Kim, H.N.; Park, H.J.; Lee, J.E.; Yeo, S.Y.; Yang, J.S.; Park, S.Y.; Yoon, H.S.; Cho, G.S.; Franz, C.M.; et al. Modulation of the murine microbiome with a concomitant anti-obesity effect by Lactobacillus rhamnosus GG and Lactobacillus sakei NR28. Benef. Microbes. 2012, 3, 13–22. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, K.Y.; Kim, B.; Kim, E.; Hyun, C.K. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem. Biophys. Res. Commun. 2013, 431, 258–263. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar]
- Høydal, M.A.; Wisløff, U.; Kemi, O.J.; Ellingsen, Ø. Running speed and maximal oxygen uptake in rats and mice: Practical implications for exercise training. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 753–760. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.; Kovacsics, C. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Human Press: Totowa, NJ, USA, 2009. [Google Scholar]
- Stanford, S.C. The open field test: Reinventing the wheel. J. Psychopharmacol. 2007, 21, 134–136. [Google Scholar] [CrossRef]
- Samson, A.L.; Ju, L.; Kim, H.A.; Zhang, S.R.; Lee, J.A.; Sturgeon, S.A.; Sobey, C.G.; Jackson, S.P.; Schoenwaelder, S.M. MouseMove: An open source program for semi-automated analysis of movement and cognitive testing in rodents. Sci. Rep. 2015, 5, 16171. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, S.; Singh, D. Apigenin reverses behavioural impairments and cognitive decline in kindled mice via CREB-BDNF upregulation in the hippocampus. Nutr. Neurosci. 2020, 23, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Johnson, N.J.T. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol. Biochem. Behav. 1995, 52, 297–303. [Google Scholar] [CrossRef]
- Cevik, O.S.; Sahin, L.; Tamer, L. Long term treadmill exercise performed to chronic social isolated rats regulate anxiety behavior without improving learning. Life. Sci. 2018, 200, 126–133. [Google Scholar] [CrossRef]
- Maniam, J.; Antoniadis, C.P.; Le, V.; Morris, M.J. A diet high in fat and sugar reverses anxiety-like behaviour induced by limited nesting in male rats: Impacts on hippocampal markers. Psychoneuroendocrinology 2016, 68, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ohland, C.L.; Pankiv, E.; Baker, G.; Madsen, K.L. Western diet-induced anxiolytic effects in mice are associated with alterations in tryptophan metabolism. Nutr. Neurosci. 2016, 19, 337–345. [Google Scholar] [CrossRef]
- Lee, H.C.; Lo, Y.C.; Yu, S.C.; Tung, T.H.; Lin, I.H.; Huang, S.Y. Degree of lipid saturation affects depressive-like behaviour and gut microbiota in mice. Int. J. Food. Sci. Nutr. 2020, 71, 440–452. [Google Scholar] [CrossRef]
- Shin, A.C.; MohanKumar, S.M.; Sirivelu, M.P.; Claycombe, K.J.; Haywood, J.R.; Fink, G.D.; MohanKumar, P. Chronic exposure to a high-fat diet affects stress axis function differentially in diet-induced obese and diet-resistant rats. Int. J. Obes. 2010, 34, 1218–1226. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Zhou, J.; Zhang, J.; Li, M.; Qiu, J.; Jia, Z.; Zhang, R. Effects and HPA axis related mechanism of Kaixin-San and Danggui-Shaoyao-San on glucose and lipid metabolism in chronic stress rats with high-fat diet. Zhong. Yao. Cai. 2015, 38, 1919–1924. [Google Scholar]
- Tomiyama, A.J. Stress and obesity. Annu. Rev. Psychol. 2019, 70, 703–718. [Google Scholar] [CrossRef]
- Beaudry, J.L.; Dunford, E.C.; Leclair, E.; Mandel, E.R.; Peckett, A.J.; Haas, T.L.; Riddell, M.C. Voluntary exercise improves metabolic profile in high-fat fed glucocorticoid-treated rats. J. Appl. Physiol. 2015, 118, 1331–1343. [Google Scholar] [CrossRef]
- Ebada, M.E.; Kendall, D.A.; Pardon, M.-C. Corticosterone and dopamine D2/D3 receptors mediate the motivation for voluntary wheel running in C57BL/6J mice. Behav. Brain. Res. 2016, 311, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Campeau, S.; Nyhuis, T.; Sasse, S.; Kryskow, E.; Herlihy, L.; Masini, C.; Babb, J.; Greenwood, B.; Fleshner, M.; Day, H. Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. J. Neuroendocrinol. 2010, 22, 872–888. [Google Scholar] [PubMed]
- Salam, J.N.; Fox, J.H.; DeTroy, E.M.; Guignon, M.H.; Wohl, D.F.; Falls, W.A. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav. Brain. Res. 2009, 197, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, P.R.; Fulk, L.J.; Hand, G.A.; Wilson, M.A. The effects of chronic treadmill and wheel running on behavior in rats. Brain. Res. 2004, 1019, 84–96. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Feldon, J.; Alleva, E.; Cirulli, F.; Yee, B.K. The role of voluntary exercise in enriched rearing: A behavioral analysis. Behav. Neurosci. 2006, 120, 787. [Google Scholar] [CrossRef]
- Hicks, J.A.; Hatzidis, A.; Arruda, N.L.; Gelineau, R.R.; De Pina, I.M.; Adams, K.W.; Seggio, J.A. Voluntary wheel-running attenuates insulin and weight gain and affects anxiety-like behaviors in C57BL6/J mice exposed to a high-fat diet. Behav. Brain. Res. 2016, 310, 1–10. [Google Scholar] [CrossRef]
- Mansur, R.B.; Brietzke, E.; McIntyre, R.S. Is there a “metabolic-mood syndrome”? A review of the relationship between obesity and mood disorders. Neurosci. Biobehav. Rev. 2015, 52, 89–104. [Google Scholar] [CrossRef]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef]
- Myles, E.M. Probiotic Effects on Adult Anxiety and Systemic Inflammation after Exposure to Western Diet; Dalhouse University: Halifax, NS, Canada, 2019. [Google Scholar]
- Gareau, M.G.; Jury, J.; MacQueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007, 56, 1522–1528. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Théodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J. Neurogastroenterol. Motil. 2018, 24, 138. [Google Scholar] [CrossRef] [PubMed]
- Minami, J.; Iwabuchi, N.; Tanaka, M.; Yamauchi, K.; Xiao, J.-z.; Abe, F.; Sakane, N. Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: A randomized, double-blind, placebo-controlled trial. Biosci. Microbiota. Food Health 2018, 37, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Szulińska, M.; Łoniewski, I.; Van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-dependent effects of multispecies probiotic supplementation on the lipopolysaccharide (LPS) level and cardiometabolic profile in obese postmenopausal women: A 12-week randomized clinical trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Gøbel, R.J.; Larsen, N.; Jakobsen, M.; Mølgaard, C.; Michaelsen, K.F. Probiotics to Adolescents With Obesity: Effects on Inflammation and Metabolic Syndrome. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 673–678. [Google Scholar] [CrossRef]
- Jones, R.B.; Alderete, T.L.; Martin, A.A.; Geary, B.A.; Hwang, D.H.; Palmer, S.L.; Goran, M.I. Probiotic supplementation increases obesity with no detectable effects on liver fat or gut microbiota in obese Hispanic adolescents: A 16-week, randomized, placebo-controlled trial. Pediatr. Obes. 2018, 13, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Angelakis, E.; Paul, M.; Armougom, F.; Leibovici, L.; Raoult, D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb. Pathog. 2012, 53, 100–108. [Google Scholar] [CrossRef]
- Ballini, A.; Gnoni, A.; De Vito, D.; Dipalma, G.; Cantore, S.; Gargiulo Isacco, C.; Saini, R.; Santacroce, L.; Topi, S.; Scarano, A.; et al. Effect of probiotics on the occurrence of nutrition absorption capacities in healthy children: A randomized double-blinded placebo-controlled pilot study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8645–8657. [Google Scholar] [PubMed]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef] [PubMed]

| Groups | Statistics | ||||||
|---|---|---|---|---|---|---|---|
| Con (Mean ± SD) | HFD (Mean ± SD) | HP (Mean ± SD) | HT (Mean±SD) | HTP (Mean ± SD) | F | p | |
| Corticosterone (ng/m) | 165.10 ± 15.26 | 262.83 ± 48.39 | 147.38 ± 23.96 | 151.65 ± 33.76 | 132.62 ± 13.43 | 18.23 | <0.001 |
| Wall time (s) | 139.16 ± 21.7 | 98.20 ± 43.37 | 160.08 ± 33.32 | 134.07 ± 29.40 | 158.30 ± 18.38 | 2.26 | 0.092 |
| Total distance (s) | 222.43 ± 98.64 | 82.10 ± 33.20 | 284.30 ± 70.78 | 475.05 ± 130.40 | 870.31 ± 89.00 | 68.10 | <0.001 |
| Corner time (s) | 144.50 ± 31.42 | 248.68 ± 56.95 | 168.83 ± 44.34 | 151.27 ± 83.53 | 123.00 ± 53.10 | 4.22 | 0.010 |
| Overall speed (cm/s) | 3.32 ± 1.26 | 1.39 ± 0.44 | 3.71 ± 0.36 | 4.56 ± 0.36 | 6.35 ± 0.67 | 42.83 | <0.001 |
| Center time (s) | 4.36 ± 1.17 | 3.62 ± 1.50 | 5.88 ± 1.96 | 4.54 ± 0.92 | 6.98 ± 0.89 | 5.24 | 0.002 |
| % OAE (%) | 25.90 ± 12.12 | 2.26 ± 3.46 | 19.05 ± 7.06 | 30.58 ± 5.86 | 57.58 ± 7.96 | 41.97 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foroozan, P.; Koushkie Jahromi, M.; Nemati, J.; Sepehri, H.; Safari, M.A.; Brand, S. Probiotic Supplementation and High-Intensity Interval Training Modify Anxiety-Like Behaviors and Corticosterone in High-Fat Diet-Induced Obesity Mice. Nutrients 2021, 13, 1762. https://doi.org/10.3390/nu13061762
Foroozan P, Koushkie Jahromi M, Nemati J, Sepehri H, Safari MA, Brand S. Probiotic Supplementation and High-Intensity Interval Training Modify Anxiety-Like Behaviors and Corticosterone in High-Fat Diet-Induced Obesity Mice. Nutrients. 2021; 13(6):1762. https://doi.org/10.3390/nu13061762
Chicago/Turabian StyleForoozan, Parisa, Maryam Koushkie Jahromi, Javad Nemati, Hosein Sepehri, Mohammad Amin Safari, and Serge Brand. 2021. "Probiotic Supplementation and High-Intensity Interval Training Modify Anxiety-Like Behaviors and Corticosterone in High-Fat Diet-Induced Obesity Mice" Nutrients 13, no. 6: 1762. https://doi.org/10.3390/nu13061762
APA StyleForoozan, P., Koushkie Jahromi, M., Nemati, J., Sepehri, H., Safari, M. A., & Brand, S. (2021). Probiotic Supplementation and High-Intensity Interval Training Modify Anxiety-Like Behaviors and Corticosterone in High-Fat Diet-Induced Obesity Mice. Nutrients, 13(6), 1762. https://doi.org/10.3390/nu13061762








